Cervical cancer is the fourth most common cancer among women worldwide. Though several natural products have been reported regarding their efficacies against cervical cancer, there has been no review article that categorized them according to their anti-cancer mechanisms. In this study, anti-cancerous natural products against cervical cancer were collected using Pubmed (including Medline) and google scholar, published within three years. Their mechanisms were categorized as induction of apoptosis, inhibition of angiogenesis, inhibition of metastasis, reduction of resistance, and regulation of miRNAs. A total of 64 natural products suppressed cervical cancer. Among them,
Penicillium sclerotiorum
extracts from
Cassia fistula
L., ethanol extracts from
Bauhinia variegate candida
, thymoquinone obtained from
Nigella sativa
, lipid-soluble extracts of
Pinellia pedatisecta
Schott., and 1′S-1′-acetoxychavicol extracted from
Alpinia conchigera
have been shown to have multi-effects against cervical cancer. In conclusion, natural products could be attractive candidates for novel anti-cancer drugs.
- cervical cancer,dietary natural products,apoptosis,angiogenesis,metastasis,resistance,microRNA
- cervical cancer
- dietary natural products
- apoptosis
- angiogenesis
- metastasis
- resistance
- microRNA
1. Introduction
Cervical cancer, which is a cancer arising from the cervix, is characterized by abnormal vaginal bleeding, vaginal discharge, pelvic pain, or pain during sexual intercourse [1]. Currently, cervical cancer is the fourth most common cancer among women in the world [2]. According to Globocan 2018, the prevalence rate of cervical cancer is 3.2% of all cancers. The main treatments for cervical cancer are surgery such as pelvic lymphadenectomy and radical hysterectomy, radiotherapy, and chemotherapy [3]. Another therapy is targeted therapy, which regulates epidermal growth factor receptor (EGFR) [4,5] and cyclooxygenase-2 (COX-2) [6,7] for treating cervical carcinoma. However, these treatments showed possible side effects and complications: Surgery could cause bleeding, damage to the organs around the surgery, and a risk of clots in the deep veins of the legs, radiotherapy could yield menopause, infertility, discomfort, or pain with intercourse, and the side effects of chemotherapy may affect not only cancer cells but also rapidly dividing cells in systems of the whole body [8,9]. Moreover, the drugs that are usually prescribed for cervical cancer showed several side effects and drug resistance [10]. Cisplatin, which is one of the most effective anticancer drugs, has resistance capacity by a self-defense mechanism [11]. 5-fluorouracil (5-FU) is also reported for resistance and side effects when it comes to cervical cancer patients [12]. Thus, we have focused on discovering a new potent treatment for cervical cancer from natural products.
Cervical cancer, which is a cancer arising from the cervix, is characterized by abnormal vaginal bleeding, vaginal discharge, pelvic pain, or pain during sexual intercourse [1]. Currently, cervical cancer is the fourth most common cancer among women in the world [2]. According to Globocan 2018, the prevalence rate of cervical cancer is 3.2% of all cancers. The main treatments for cervical cancer are surgery such as pelvic lymphadenectomy and radical hysterectomy, radiotherapy, and chemotherapy [3]. Another therapy is targeted therapy, which regulates epidermal growth factor receptor (EGFR) [4][5] and cyclooxygenase-2 (COX-2) [6][7] for treating cervical carcinoma. However, these treatments showed possible side effects and complications: Surgery could cause bleeding, damage to the organs around the surgery, and a risk of clots in the deep veins of the legs, radiotherapy could yield menopause, infertility, discomfort, or pain with intercourse, and the side effects of chemotherapy may affect not only cancer cells but also rapidly dividing cells in systems of the whole body [8][9]. Moreover, the drugs that are usually prescribed for cervical cancer showed several side effects and drug resistance [10]. Cisplatin, which is one of the most effective anticancer drugs, has resistance capacity by a self-defense mechanism [11]. 5-fluorouracil (5-FU) is also reported for resistance and side effects when it comes to cervical cancer patients [12]. Thus, we have focused on discovering a new potent treatment for cervical cancer from natural products.
Natural products extracted from living organisms including plants and animals have several active ingredients, which are reported to be attractive alternatives to chemotherapeutic drugs or suitable for combined use with chemotherapeutic drugs [13,14]. For example, purified flaxseed hydrolysate (PFH), extracted from Lignan, induces apoptosis and inhibits angiogenesis and metastasis on HeLa cells [15]. Thymoquinone from
Natural products extracted from living organisms including plants and animals have several active ingredients, which are reported to be attractive alternatives to chemotherapeutic drugs or suitable for combined use with chemotherapeutic drugs [13][14]. For example, purified flaxseed hydrolysate (PFH), extracted from Lignan, induces apoptosis and inhibits angiogenesis and metastasis on HeLa cells [15]. Thymoquinone from
Nigella sativa
also showed apoptotic effect and anti-proliferation in SiHa and CaSki cells. Such natural products include
Bauhinia variegate candida
ethanol extracts, Praeruptorin-B, and well-known tea.
MicroRNA (miRNA, miR) are involved in the pathological development and metastasis of cancer [16,17]. Several natural products showed an anti-cancer effect by regulation of cancer-related miRNAs. Our team reported that
MicroRNA (miRNA, miR) are involved in the pathological development and metastasis of cancer [16][17]. Several natural products showed an anti-cancer effect by regulation of cancer-related miRNAs. Our team reported that
Spatholobus suberectus Dunn extract induces apoptosis by regulation of miR-657/activating transcription factor 2 (ATF2) in U266, U937 cells [18]. Another natural product,
Dunn extract induces apoptosis by regulation of miR-657/activating transcription factor 2 (ATF2) in U266, U937 cells [18]. Another natural product,
Salvia miltiorrhiza
, showed an anti-cancer effect via regulation of miR-216b [19]. 1′S-1′-acetoxychavicol acetate (ACA) from
Alpinia conchigera has been reported to induce apoptosis on SiHa and CaSki cells by targeting SMAD4 and miR-210. Targeting miRNAs with natural products could be a promising strategy for cervical cancer [20]. However, there have been no studies that organize the mechanisms, efficacy, and concentration of natural products for cervical cancer in last five years. In this present study, we aim to review the nonclinical studies about the anti-cancer mechanisms of natural products. The natural products were organized by their mechanisms including apoptosis, anti-metastasis, anti-angiogenesis, resistance, and microRNA regulation.
has been reported to induce apoptosis on SiHa and CaSki cells by targeting SMAD4 and miR-210. Targeting miRNAs with natural products could be a promising strategy for cervical cancer [20]. However, there have been no studies that organize the mechanisms, efficacy, and concentration of natural products for cervical cancer in last five years. In this present study, we aim to review the nonclinical studies about the anti-cancer mechanisms of natural products. The natural products were organized by their mechanisms including apoptosis, anti-metastasis, anti-angiogenesis, resistance, and microRNA regulation.
2. Results
2.1. Apoptosis
Apoptosis is a unique form of cell death and is an important process that regulates the homeostasis of cell survival [21]. Apoptosis eliminates potentially cancerous cells and this process is caused by atrophy of cells, synthesis of new proteins, and cell suicide genes; also, it has a great influence on the malignant phenotype [22]. For this reason, apoptosis is used as an anticancer mechanism for cancer research. A total of 47 studies have been performed to elucidate apoptosis-mediated anti-cancer pathway of natural products in Hela and SiHa cells. A total of 54 natural products were reviewed.
2.1.1. Compounds
Among the natural products, 35 compounds showed an apoptotic effect against cervical cancer (
). The chemical structures of the compounds are shown in
.
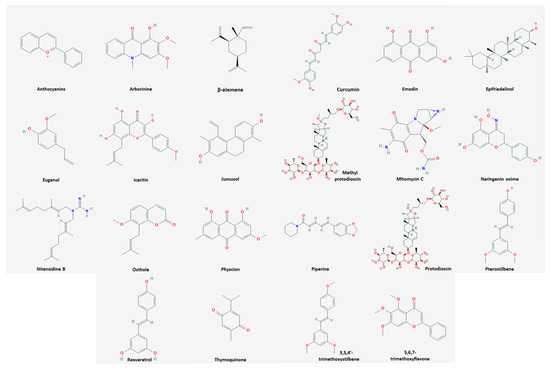
Figure 1.
Chemical structures of compounds derived from natural products inducing apoptosis.
Table 1.
Apoptosis inducing natural products-compounds.
| Classification | Compound | Source | Cell Line/ Animal Model |
Dose; Duration | Efficacy | Mechanism | Reference |
|---|
| Etc. | Acylhydrazone | HeLa |
]. Hexadecanoic acid, oleic acid, and benzoic acid were the major active parts in this treatment. Through controlling mechanisms, it could induce cell cycle arrest and inhibit angiogenesis at a dose of 7.75 µg/mL. Seifaddinipour et al. reported that ethyl acetate extracts isolated from
L. [60]. Hexadecanoic acid, oleic acid, and benzoic acid were the major active parts in this treatment. Through controlling mechanisms, it could induce cell cycle arrest and inhibit angiogenesis at a dose of 7.75 µg/mL. Seifaddinipour et al. reported that ethyl acetate extracts isolated from
Pistacia vera L. downregulated TNF, Bcl-2, IAP, and TRAF in CaSki cells [78]. Through the mechanisms, it induced apoptosis and inhibited angiogenesis. The efficient dose was 81.17 ± 2.87 µg/mL, dealing with a timeframe of 72 h.
L. downregulated TNF, Bcl-2, IAP, and TRAF in CaSki cells [74]. Through the mechanisms, it induced apoptosis and inhibited angiogenesis. The efficient dose was 81.17 ± 2.87 µg/mL, dealing with a timeframe of 72 h.
Table 3.
Angiogenesis inhibiting natural products.
| Classification | Compound/ Extract |
Source | Cell Line/ Animal Model |
Dose; Duration | Efficacy | Mechanism | Reference |
|---|
| 2.21 µM; 48 h |
| Inhibition of cancer activity | [ | 23 | ] | |
| Plant | Anthocyanins | Root tubers and leaves of | ||
| Ipomoea batatas | ||||
| HeLa | 100, 200 µg/mL; 48 h | Induction of apoptosis, cell cycle arrest | ↑CFP/YFP |
A total of four natural products exhibited anti-angiogenesis, and the mechanisms were very diverse. MMP performs a complex and important role in cancer growth and metastasis [79], and two substances inhibit it. Bcl-2 was inhibited by two substances, and in addition, it showed an anti-angiogenesis effect through a mechanism that inhibits factors such as VEGF, TNF, and IAP. In the study using
A total of four natural products exhibited anti-angiogenesis, and the mechanisms were very diverse. MMP performs a complex and important role in cancer growth and metastasis [75], and two substances inhibit it. Bcl-2 was inhibited by two substances, and in addition, it showed an anti-angiogenesis effect through a mechanism that inhibits factors such as VEGF, TNF, and IAP. In the study using
Penicillium sclerotiorum, the concentration of the substance (7.75 µg/mL) was significantly lower than that of other anti-angiogenesis studies [63]. By increasing the expression of Bax, p53, and Apaf-1 and inhibiting Bcl-2, it was shown to induce not only anti-angiogenesis, but also cell cycle arrest and apoptosis at doses of 5 µg/mL, 25 µg/mL, and 50 µg/mL.
, the concentration of the substance (7.75 µg/mL) was significantly lower than that of other anti-angiogenesis studies [60]. By increasing the expression of Bax, p53, and Apaf-1 and inhibiting Bcl-2, it was shown to induce not only anti-angiogenesis, but also cell cycle arrest and apoptosis at doses of 5 µg/mL, 25 µg/mL, and 50 µg/mL.
2.3. Anti-Metastasis
Metastasis is the propagation of transformed cells from the organ of origin to other parts of the body and the successive proliferation of tumor colonies [80]. The mechanism includes complicated processes, such as cancer cell detachment from extracellular matrix, migration, invasion, and extravasation to the circulation, and most cancer patients die from metastasis rather than primary tumors [81]. Six natural products including EGCG inhibited metastasis (
Metastasis is the propagation of transformed cells from the organ of origin to other parts of the body and the successive proliferation of tumor colonies [76]. The mechanism includes complicated processes, such as cancer cell detachment from extracellular matrix, migration, invasion, and extravasation to the circulation, and most cancer patients die from metastasis rather than primary tumors [77]. Six natural products including EGCG inhibited metastasis (
). The chemical structures of compounds are shown in
.
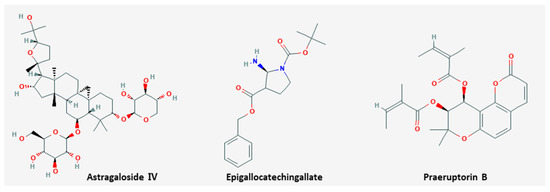
Figure 2.
Chemical structures of compounds derived from natural products inhibiting metastasis.
Table 4.
Metastasis inhibiting natural products.
| Classification | Compound/ Extract |
Source | Cell Line/ Animal Model |
Dose; Duration | Efficacy | Mechanism | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fruit | PfLP | Praecitrullus fistulosus | HeLa Swiss Albino mice |
In vitro: 50 µg/mL; 24 h | |||||||||||
| Plant | Astragaloside IV | Radix Astragali | SiHa | In vivo: 10 mg/kg | Induction of apoptosis Inhibition of angiogenesis |
↓ MMP-2, -9 | 200 µg/mL; 24 h | Inhibition of cell metastasis | ↑ E-cadherin [77][73] |
||||||
| ↓ p38, PI3K | [ | 82 | ] | [ | 78 | ||||||||||
| [ | 24 | ] | |||||||||||||
| ] | Plant | Purified flaxseed hydrolysate | Lignan | HeLa | 17.4 µg/mL; 48 h | Induction of apoptosis Inhibition of angiogenesis and metastasis |
↑ caspase-3 ↓ MMP-2, VEGF |
[15] | |||||||
| Plant | Arborinine | ||||||||||||||
| Glycosmis parva | |||||||||||||||
| HeLa | |||||||||||||||
| Fungus | |||||||||||||||
| Plant | Epigallocatechingallate | Green tea | HeLa | 50 µg/mL; 48 h | Inhibition of cell metastasis and proliferation Induction of apoptosis |
↓ MMP-2, -9, VEGF | [83][79] | ||||||||
| 110 μg/mL; 24 h | Induction of apoptosis | Inhibition of migration | ↑caspase-3, -7 ↓Bcl2-L1 |
||||||||||||
| Ethyl acetate extract | Penicillium sclerotiorum | HeLa | 7.75 µg/mL; 24 h | Induction of cell cycle arrest and apoptosis | Inhibition of angiogenesis |
↑ Bax, p53, Apaf-1 ↓ Bcl-2 | |||||||||
| [ | 25 | ] | |||||||||||||
| Plant | Praeruptorin B | [ | Peucedanum praeruptorum Dunn. | 63 | ] | [ | HeLa, SiHa60] | 40, 60 µM; 24 h | Inhibition of cell metastasis | ↓ NF-κB, MMP-2, -9 | |||||
| Plant | β-elemene | ||||||||||||||
| Ethanol extract | Curcuma zedoaria | ||||||||||||||
| SiHa | 30, 40, 50 μg/mL; | 24, 48, 72 h |
Inhibition of proliferation and migration Induction of cell cycle arrest and apoptosis |
||||||||||||
| Bauhinia variegate candida | |||||||||||||||
| ↑p15, p53, Bax | |||||||||||||||
| HeLa | 15 μg/mL; 24 h | ||||||||||||||
| [ | 84 | ] | [ | 80 | ] | ||||||||||
| Plant | |||||||||||||||
| ↓cyclin D1, Bcl-2, MMP-2, -9, | β-catenin, TCF7, c-Myc | ||||||||||||||
| Ethyl acetate extract | Pistacia vera | L. | Inhibition of cell viability and migration | Induction of apoptosis | |||||||||||
| [ | 26 | ] | |||||||||||||
| ↑c-caspase-3, -8, RIP, TNF-R1 | ↓MMP-2, MMP-9 | [ | 58 | ] | |||||||||||
| Seed | CaSki | [ | 55 | ] | |||||||||||
| 81.17 ± 2.87 µg/mL; 72 h | Induction of apoptosis | Inhibition of angiogenesis | Thymoquinone | ↓ TNF, Bcl-2, IAP, TRAF | [ | 78][74] | Nigella sativa | CaSki, HeLa | 5 µM; 24 h | Induction of apoptosis, migration and invasion | ↑ E-cadherin ↓ Twist1, Zeb1 |
[46] | |||
| Plant | Copper oxide nanoparticles | ||||||||||||||
| Azadirachta indica, Hibiscus rosa-sinensis, Murraya koenigii, Moringa oleifera, Tamarindus indica | |||||||||||||||
| Plant | |||||||||||||||
| HeLa | |||||||||||||||
| Ethanol extract | Botryidiopsidaceae | species | HeLa | ||||||||||||
| 2, 5, 10, 25, 50, 100 μg/mL; 48 h | Inhibition of oxidative stress | Induction of apoptosis | |||||||||||||
| 6.25, 12.5, 25, 50 μg/mL; 24 h | |||||||||||||||
| Inhibition of oxidative stress and migration | Induction of apoptosis | ↑p53, c-caspase-3 | ↓Bcl-2 | ||||||||||||
| [ | |||||||||||||||
| [ | 59 | ||||||||||||||
| Plant | ] | [ | 56 | ] | |||||||||||
| Ethanol extract | Bauhinia variegata candida | ||||||||||||||
| 27 | ] | ||||||||||||||
| HeLa | 25 µg/mL; 24 h | Inhibition of cell viability, migration and invasion | ↓ MMP-2, -9 | [ | 58 | ] | [55] | ||||||||
| Plant | |||||||||||||||
| Plant | |||||||||||||||
| Curcumin | |||||||||||||||
| Ethanol extract | Curcuma longa | ||||||||||||||
| Chloromonas | species | ||||||||||||||
| HeLa, | C57BL/6, BALB/c | In vitro: 2 μg/mL; 48 h | |||||||||||||
| HeLa | |||||||||||||||
| In vivo: 25 mg/kg | |||||||||||||||
| 12.5, 25 μg/mL; 24, 72 h | |||||||||||||||
| Induction of apoptosis and cell cycle arrest | |||||||||||||||
| Inhibition of oxidative stress | Induction of apoptosis | ||||||||||||||
| ↑p53, cytochrome c, PARP, | caspase-3, -7, -9 | ↓Bcl-2, NF-κB | [ | 28] | |||||||||||
| Plant | ↑c-caspase-3, p53 | ↓Bcl-2 | [ | Ethanol extract | Terminalia catappa | HeLa, SiHa | 25, 50, 75 µg/mL; 24 h60][57] | ||||||||
| Plant | Emodin | ||||||||||||||
| Rhamnus sphaerosperma | |||||||||||||||
| var. | |||||||||||||||
| pubescens | |||||||||||||||
| SiHa, C33A | 46.3, 92.8, 185 μg/mL; | 6, 12, 24 h |
Induction of, apoptosis | ↓NO-, O | |||||||||||
| 2 | |||||||||||||||
| -, HOCl/OCl-, p-Akt | [ | 29 | ] | ||||||||||||
| Plant | Epifriedelinol | ||||||||||||||
| Aster tataricus | |||||||||||||||
| , | |||||||||||||||
| Vitex peduncularis | |||||||||||||||
| Wall. | HeLa | 50, 100, 250, 500, 1000 μg/mL; 72 h | Induction of apoptosis | ↑caspase-3, -8, -9 ↓Bcl-2, -xL, survivin |
[30] | ||||||||||
| Inhibition of cell metastasis | ↓ MMP-9, ERK1/2 | [ | 85 | ] | [ | 81 | ] | Plant | Ethanol extract | Dendrobium chrysanthum | HeLa, Swiss albino mice |
In vitro: 450 μg/mL; 24 h In vivo: 50, 100 mg/kg |
Induction of apoptosis | ↑Bax, p53 ↓Bcl-2 |
[61][58] |
| Plant | Ethanol extract | Rhamnus sphaerosperma var. pubescens | SiHa, C33A | 25, 50, 100 μg/mL; 6, 12, 24 h |
Induction of apoptosis | ↓HOCl/OCl-, p-Akt | [29] | ||||||||
| Plant | Eugenol | ||||||||||||||
| Syzygium aromaticum | |||||||||||||||
| HeLa, SiHa | 12.5, 25 µM; 24, 48 h | Induction of apoptosis | ↑Bax, PARP, caspase-3, ROS ↓Bcl-2, XIAP |
[31] | |||||||||||
| Plant | Ethyl acetate extract | Gynura formosana Kitam. | HeLa | 30 μg/mL; 72 h | Inhibition of proliferation | ↑ LC3-II/LC3-I, ↓P62/GAPDH, MCM7/GAPDH |
[62][59] | ||||||||
| Plant | Icaritin | ||||||||||||||
| Epimedium | |||||||||||||||
| HeLa, SiHa | HeLa: 12.5, 25 µM; | 24, 48, 72 h SiHa: 17, 34 µM; 24, 48, 72 h |
Induction of apoptosis Inhibition of proliferation |
↑ROS, Bax, c-caspase-3, -9 ↓Bcl-2, XIAP |
[32] | ||||||||||
| Fungus | Ethyl acetate extract | Penicillium sclerotiorum | HeLa | 5, 25, 50 μg/mL; 24 h | Induction of apoptosis and cell cycle arrest | ↑Bax, p53, Apaf-1 ↓Bcl-2 |
[63][60] | ||||||||
| Plant | Juncusol | ||||||||||||||
| Juncus inflexu | |||||||||||||||
| Plant | Ethyl acetate extract | Streptomyces | |||||||||||||
| HeLa, SiHa, CaSki | |||||||||||||||
| species | |||||||||||||||
| 1, 3, 10, 30 µM; | 24, 48, 72 h | ||||||||||||||
| SiHa | |||||||||||||||
| Induction of apoptosis | Inhibition of proliferation | ↑caspase-3, -8, -9 | ↓EGFR, tubulin polymerization | [33] | |||||||||||
| 20, 40, 60 μg/mL; 24 h | Induction of apoptosis and autophagy | ↑caspase-3, -9, Bax, LC3-Ⅱ | ↓PARP, LC3-Ⅰ, Beclin1, p62 | [ | 64 | ][61] | |||||||||
| Plant | Methyl protodioscin | Rhizoma of | |||||||||||||
| Polygonatum sibiricum | |||||||||||||||
| HeLa | 18.31, 40, 49 µM; 24 h | Induction of apoptosis and cell cycle arrest Inhibition of proliferation |
↑ ROS | [34] | |||||||||||
| Plant | Extract | Blueberry | SiHa | 50 mg/mL; 24 h with 4 Gy radiotherapy |
Enhancement of radiotherapy | ↑p53 ↓cyclin D, E, p21, survivin |
[65][62] | ||||||||
| Plant | Mitomycin C | Ginger, Frankincense | HeLa | 10 µg/mL; 24 h | Induction of apoptosis | ||||||||||
| Plant | Lipid-soluble extract | Pinellia pedatisecta Schott. | HPV+TC-1, C57BL/6 | ||||||||||||
| Inhibition of proliferation | |||||||||||||||
| In vitro: 500 μg/mL; | 72, 120 h | In vivo: 10, 20 mg/kg | Induction of cell cycle arrest and apoptosis | ↑ β-catenin, c-Myc, cyclin D1, PPAR1 ↓Th2, Th17 | |||||||||||
| [ | 35 | ] | |||||||||||||
| [ | 66 | ] | [ | 63 | ] | ||||||||||
| Etc. | Naringenin oxime | HeLa, SiHa | HeLa: 12, 24 µM; 24 h SiHa: 18, 36 µM; 24 h |
Induction of apoptosis Inhibition of proliferation |
↑caspase-3 | ||||||||||
| Plant | |||||||||||||||
| [ | 36 | ||||||||||||||
| Methanol extract | Allium atroviolaceum | HeLa | |||||||||||||
| ] | |||||||||||||||
| 20, 40, 60, 80, 100 μg/mL; | 24, 48, 72 h | Induction of cell cycle arrest | ↑caspase-3, -5, -9 | ↓Bcl-2, CDK1, p53 | [67][64] | ||||||||||
| Naringenin oxime ether | |||||||||||||||
| Plant | Methanol extract | Corylus avellane L. | HeLa | 250, 500 μg/mL; 24 h | Inhibition of oxidative stress Induction of apoptosis |
↑caspase-3 ↓PARP-1 |
[68][65] | ||||||||
| Plant | Nitensidine B | Leaves of | |||||||||||||
| Pterogyne nitens | |||||||||||||||
| Tul. | HPV16, SiHa | 30, 60, 120 µM; | |||||||||||||
| Plant | Methanol extract | ||||||||||||||
| 6, 12, 24 h | |||||||||||||||
| Cyperus rotundus | HeLa | 25, 50, 100 μg/mL; | |||||||||||||
| Induction of apoptosis | |||||||||||||||
| 24, 48 h | |||||||||||||||
| ↑caspase-3, -7 | ↓aldolase A, alpha-enolase, pyruvate kinase, glyceraldehyde 3-p-dehydrogenase | [ | 37 | ||||||||||||
| Induction of apoptosis | |||||||||||||||
| ] | |||||||||||||||
| [ | 69 | ] | [ | ||||||||||||
| Plant | Notoginsenoside R7 | ||||||||||||||
| Panax notoginseng | |||||||||||||||
| HeLa, | BALB/c | In vitro: 5, 10, 20, 40 μM; 24, 36, 48 h In vivo: 5, 10 mg/kg |
Induction of apoptosis Inhibition of proliferation |
↑Bax, p-PTEN, Akt ↓Bcl-2, -xL, caspase-3, -9, raptor |
[38] | ||||||||||
| 66 | ] | ||||||||||||||
| Plant | Methanol extract | Polyalthia longifolia | HeLa | 22 μg/mL; 6, 12, 24, 36 h | Induction of apoptosis | ↑Bax, BAD, caspase-3, p21, p53 ↓Bcl-2 |
[70][67] | ||||||||
| Plant | Osthole | ||||||||||||||
| Cnidiummonnieri | |||||||||||||||
| (L.) Cusson | HeLa, SiHa, | C-33A, CaSki |
40, 80, 120, 160, 200, 240 µM; 24, 48 h | Induction of apoptosis Inhibition of proliferation |
↑Bax, c-caspase-3, -9 proteins, E-cadherin, H2AX | ||||||||||
| Plant | |||||||||||||||
| ↓Bcl-2, MMP-2, -9, β-catenin, vimentin, N-cadherin, IKKα, | |||||||||||||||
| Methanol extract | Pyrrosia piloselloides | ||||||||||||||
| HeLa, | |||||||||||||||
| p-IKKα, p65, p-p65, p50, NF-κB | [ | 39 | ] | ||||||||||||
| 16.25 μg/mL; 24, 48, 72 h | Inhibition of proliferation | [ | 71 | ] | [ | 68] | |||||||||
| Plant | Physcion | ||||||||||||||
| Rhamnus sphaerosperma | |||||||||||||||
| var. | |||||||||||||||
| pubescens | |||||||||||||||
| SiHa, C33A | 43.8, 87.5, 175 μg/mL; | 6, 12, 24 h |
Induction of apoptosis | ↓HOCl/OCl-, p-Akt | [29] | ||||||||||
| Plant | Methanol extract | Teucrium mascatense | HeLa | 25, 50, 125, 250 μg/mL; 72 h | Induction of apoptosis Inhibition of proliferation |
↑c-caspase-7, -8, -9, PARP | [72][69] | ||||||||
| Plant | Phyto-synthesis of silver nanoparticles | Garlic, Green tea, Turmeric | HeLa | 2, 5, 10, 25, 50, 100 μg/mL; 48 h | Induction of apoptosis | ↓free radical | [40] | ||||||||
| Plant | Piperine | ||||||||||||||
| Piper nigrum | |||||||||||||||
| L. | HeLa, PTX | 50 µM; 6, 24, 72 h with paclitaxel |
Induction of apoptosis | ↑Bax, Bcl-2, c-PARP, caspase-3 ↓p-Akt, Mcl-1 |
[41] | ||||||||||
| Plant | Prenylflavonoids C1 | ||||||||||||||
| Mallotus conspurcatus | |||||||||||||||
| HeLa | 30 μM; 24 h | Induction of apoptosis | ↑EGFP, ROS, Bcl-2, cytochrome c, Apaf-1, caspase-3, -9 ↓c-Myc, hTERT |
[42] | |||||||||||
| Prenylflavonoids C5 | 10 μM; 24 h | ||||||||||||||
| Plant | Protodioscin | ||||||||||||||
| Dioscoreae rhizome | |||||||||||||||
| HeLa, C33A | 4 μM; 24, 48 h | Induction of apoptosis and mitochondrial dysfunction | ↑JNK, p38, PERK, ATF4, Bax, caspase-3, -8, -9, PARP ↓Bcl-2 |
[43] | |||||||||||
| Etc. | Pterostilbene | HPV E6, TC1, C57Bl/6 |
In vitro: 30 µM; 48 h In vivo: 1 mM; 5 days |
Induction of cell cycle arrest | ↑caspase-3 ↓PCNA, VEGF |
[44] | |||||||||
| Resveratrol | |||||||||||||||
| Plant | Tf-CT-ME | ||||||||||||||
| Tripterygium wilfordii | |||||||||||||||
| HeLa | 0.5, 1, 2 µg/mL; 24 h | Induction of cell cycle arrest and apoptosis Inhibition of proliferation |
↑c-caspase-3 ↓Bcl-2/Bax |
[45] | |||||||||||
| Seed | Thymoquinone | ||||||||||||||
| Nigella sativa | |||||||||||||||
| SiHa, CaSki | 10, 20, 40 μM; | 24, 36, 48 h |
Inhibition of migration and invasion | ↑Bax, E-cadherin ↓Bcl-2, Twist1, vimentin |
[46] | ||||||||||
| Plant | Triphala | ||||||||||||||
| Terminalia chebula | |||||||||||||||
| Retz., | |||||||||||||||
| Terminalia bellerica | |||||||||||||||
| (Gaertn) Roxb., | |||||||||||||||
| Phyllanthus emblica | |||||||||||||||
| Linn. | HeLa | 25-150 μg/mL; 48 h | Induction of apoptosis | ↑ERK, p53 ↓c-Myc, cyclin D1, p-Akt, p-NF-κB, p56, p-p44/42, MAPK |
[47] | ||||||||||
| Plant | 1′S-1′-acetoxychavicol acetate | ||||||||||||||
| Alpinia conchigera | |||||||||||||||
| CaSki, SiHa | 20, 30 μM; 6, 12, 48 h | Induction of apoptosis | ↑RSU1, GAPDH | [48] | |||||||||||
| Plant | 2D of oleanolic acid and glycyrrhetinic acid | ||||||||||||||
| Ligustri Lucidi Fructus, Glycyrrhiza uralensis | |||||||||||||||
| HeLa | 2, 4 µM; 24, 48 h | Induction of apoptosis Inhibition of proliferation |
↑ROS | [49] | |||||||||||
| 3O of oleanolic acid and glycyrrhetinic acid | 1, 2 μM; 48 h | ||||||||||||||
| Etc. | 3,5,4′-trimethoxystilbene | HeLa | 10 µM; 48 h | Induction of apoptosis | [50] | ||||||||||
| 5,6,7-trimethoxyflavone | |||||||||||||||
| Plant | 5′- | ||||||||||||||
| epi | |||||||||||||||
| -SPA-6952A | |||||||||||||||
| Streptomyces diastatochromogenes | |||||||||||||||
| HeLa | 2, 4, 8, 16 µg/mL; 24 h | Induction of apoptosis and cell cycle arrest Inhibition of proliferation | ↑Bax/Bcl-2, cytochrome c, caspase-3, -9, c-PARP, p53 ↓MMP |
[51] |
Cyan fluorescent protein (CFP); yellow fluorescent protein (YFP); Bcl-2-like1 (Bcl2-L1); Bcl-2 associated X protein (Bax); B-cell lymphoma 2 (Bcl-2); matrix metalloproteinase (MMP); transcription factor (TCF); cellular myelocytomatosis oncogene (c-Myc); cytochrome complex (cytochrome c); poly (ADP-ribose) polymerase (PARP); nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB); phospho-Akt (p-Akt); B-cell lymphoma-extra large (Bcl-xL); reactive oxygen species (ROS); x-linked inhibitor of apoptosis protein (XIAP); cleaved caspase (c-caspase); epidermal growth factor receptor (EGFR); 3-phosphate-dehydrogenase (3-p-dehydrogenase); phospho-phosphatase and tensin homolog (p-PTEN); epithelial cadherin (E-cadherin); H2A histone family member X (H2AX); neural cadherin (N-cadherin); inhibitor of NF-κB kinase α (IKKα); phospho- IKKα (p-IKKα); phospho-p65 (p-p65); cleaved PARP (c-PARP); myeloid cell leukemia sequence 1 (Mcl-1); enhanced green fluorescent protein (EGFP); apoptotic protease activating factor 1 (Apaf-1); human telomerase reverse transcriptase (hTERT); c-Jun N-terminal kinases (JNK); protein kinase RNA-like endoplasmic reticulum kinase (PERK); activating transcription factor 4 (ATF4); proliferating cell nuclear antigen (PCNA); vascular endothelial growth factor (VEGF); transferrin-modified microemulsion carrying coix seed oil and tripterine (Tf-CT-ME); extracellular signal-regulated kinases (ERK); phospho- NF-κB (p-NF-κB); phospho-p44 (p-p44); mitogen-activated protein kinase (MAPK); Ras suppressor protein 1 (RSU1); glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
2.1.2. Extracts
The 19 natural extracts have been found to induce cell death in cervical cancer (
). Swanepoel et al. showed that aqueous extracts of
Anemone nemorosa caused a delay in the early mitosis phase of the cell cycle [55]. Apoptosis was confirmed through fluorescent staining with annexin V-FITC. The treatment with IC
caused a delay in the early mitosis phase of the cell cycle [52]. Apoptosis was confirmed through fluorescent staining with annexin V-FITC. The treatment with IC
50
concentration of 20.33 ± 2.480 µg/mL elevated the expression level of PS translocation, c-caspase-3, -8, and ROS in 24 h. In 48 h, however, the level of MMP and ROS were suppressed. The result of this study indicated that induction of apoptosis and autophagy and inhibition of proliferation were induced in the pathway. Aril extracts isolated from
Strelitzia nicolai induced apoptosis and inhibited oxidant in HeLa cells at a concentration of 250 μg/mL for an incubation time of 24 h, 48 h, and 72 h [56]. The aril extracts decreased cell viability by 52% and induced apoptosis in HeLa cells. Additionally, aril extracts produced a higher radical scavenging activity than the bilirubin standard. Ethanol extracts from
induced apoptosis and inhibited oxidant in HeLa cells at a concentration of 250 μg/mL for an incubation time of 24 h, 48 h, and 72 h [53]. The aril extracts decreased cell viability by 52% and induced apoptosis in HeLa cells. Additionally, aril extracts produced a higher radical scavenging activity than the bilirubin standard. Ethanol extracts from
Astragalus membranaceus
,
Angelica gigas
, and
Trichosanthes kirilowii upregulated c-caspase-3, -8, and PARP-1 and downregulated Bax, cyclin D, CDK2, CDK4, CDK6, and p27 in HeLa cells at doses of 100 μg/mL, 200 μg/mL, and 400 μg/mL for 24 h and 48 h [57]. This result suggested that induction of apoptosis, cell cycle arrest, and reduction of cell viability are involved in the pathway. However, ethanol extracts did not affect the intrinsic mitochondria-mediated apoptosis pathway in HeLa cells. Expression of c-caspase-3, -8, RIP, and TNF-R1 were increased and MMP-2 and-9 were decreased in HeLa cells by treatment of ethanol extracts from
upregulated c-caspase-3, -8, and PARP-1 and downregulated Bax, cyclin D, CDK2, CDK4, CDK6, and p27 in HeLa cells at doses of 100 μg/mL, 200 μg/mL, and 400 μg/mL for 24 h and 48 h [54]. This result suggested that induction of apoptosis, cell cycle arrest, and reduction of cell viability are involved in the pathway. However, ethanol extracts did not affect the intrinsic mitochondria-mediated apoptosis pathway in HeLa cells. Expression of c-caspase-3, -8, RIP, and TNF-R1 were increased and MMP-2 and-9 were decreased in HeLa cells by treatment of ethanol extracts from
Bauhinia variegate candida at dose of 15 μg/mL for an incubation time of 24 h [58]. Controlling mechanisms, it could reduce cell viability and inhibit migration and induct apoptosis. In conclusion, it contained components with potential tumor-selective cytotoxic action. Ethanol extracts isolated from
at dose of 15 μg/mL for an incubation time of 24 h [55]. Controlling mechanisms, it could reduce cell viability and inhibit migration and induct apoptosis. In conclusion, it contained components with potential tumor-selective cytotoxic action. Ethanol extracts isolated from
Botryidiopsidaceae species induced apoptosis and inhibited oxidant, proliferation, migration, and invasion in HeLa cells [59]. It upregulated p53 and c-caspase-3 and downregulated Bcl-2 at doses of 6.25 μg/mL, 12.5 μg/mL, 25 μg/mL, and 50 μg/mL for an incubation time of 24 h. In conclusion, inhibitory effects of ethanol extracts on migration and invasion might occur via the modulation of genes related to the processes of cellular invasion and migration. Ethanol extracts from
species induced apoptosis and inhibited oxidant, proliferation, migration, and invasion in HeLa cells [56]. It upregulated p53 and c-caspase-3 and downregulated Bcl-2 at doses of 6.25 μg/mL, 12.5 μg/mL, 25 μg/mL, and 50 μg/mL for an incubation time of 24 h. In conclusion, inhibitory effects of ethanol extracts on migration and invasion might occur via the modulation of genes related to the processes of cellular invasion and migration. Ethanol extracts from
Chloromonas species upregulated c-caspase-3 and p53 and downregulated Bcl-2 in HeLa cells at dose of 12.5 μg/mL and 25 μg/mL for an incubation time of 24 h and 72 h [60]. The result meant that ethanol extracts dealt with cancer cells by increasing the pro-apoptotic protein and reducing the anti-apoptotic protein. It suggested that induction of apoptosis through the modulation of apoptosis associated genes and inhibition of proliferation and oxidant were involved in the pathway. Ethanol extracts from
species upregulated c-caspase-3 and p53 and downregulated Bcl-2 in HeLa cells at dose of 12.5 μg/mL and 25 μg/mL for an incubation time of 24 h and 72 h [57]. The result meant that ethanol extracts dealt with cancer cells by increasing the pro-apoptotic protein and reducing the anti-apoptotic protein. It suggested that induction of apoptosis through the modulation of apoptosis associated genes and inhibition of proliferation and oxidant were involved in the pathway. Ethanol extracts from
Dendrobium chrysanthum upregulated Bax, and p53 and downregulated Bcl-2 in HeLa cells at the density of 450 μg/mL for an incubation time of 24 h [61]. This suggested that induction of apoptosis, DNA fragmentation, and ROS-altered cell morphology were involved in the pathway. In addition, the anticancer potential of the
upregulated Bax, and p53 and downregulated Bcl-2 in HeLa cells at the density of 450 μg/mL for an incubation time of 24 h [58]. This suggested that induction of apoptosis, DNA fragmentation, and ROS-altered cell morphology were involved in the pathway. In addition, the anticancer potential of the
Dendrobium chrysanthum
was mediated through p53-dependent apoptosis. In vivo, antitumor activity exhibited a significant increase in the life span of Dalton’s lymphoma-bearing mice with significant decrease in abdominal size along with reduced tumor ascites. Ethanol extracts from
Rhamnus sphaerosperma
var.
pubescens (EERs) reduced activation of HOCl/OCl- and p-Akt at 25 μg/mL, 50 μg/mL, and 100 μg/mL for 6 h, 12 h, and 24 h for the treatment of SiHa and C33A cells [29]. The result showed that EERs were related to the induction of cell cytotoxicity, apoptosis, oxidative stress, and DNA damage. Ma et al. reported that EAEG, ethyl acetate extracts of
(EERs) reduced activation of HOCl/OCl- and p-Akt at 25 μg/mL, 50 μg/mL, and 100 μg/mL for 6 h, 12 h, and 24 h for the treatment of SiHa and C33A cells [29]. The result showed that EERs were related to the induction of cell cytotoxicity, apoptosis, oxidative stress, and DNA damage. Ma et al. reported that EAEG, ethyl acetate extracts of
Gynura formosana Kitam. leaves, exhibited antioxidant, anti-inflammatory, and autophagy-mediated inhibition of cell proliferation activity on HeLa cells [62]. The increased levels of LC3-II/LC3-I and decreased levels of P62/GAPDH and MCM7/GAPDH were observed at a concentration of 30 µg/mL for 72 h. Kuriakose et al. reported that ethyl acetate extracts of
Kitam. leaves, exhibited antioxidant, anti-inflammatory, and autophagy-mediated inhibition of cell proliferation activity on HeLa cells [59]. The increased levels of LC3-II/LC3-I and decreased levels of P62/GAPDH and MCM7/GAPDH were observed at a concentration of 30 µg/mL for 72 h. Kuriakose et al. reported that ethyl acetate extracts of
Penicillium sclerotiorum
, isolated from
Cassia fistula L., had cytotoxic activity [63]. At doses of 5 μg/mL, 25 μg/mL, and 50 μg/mL for 24 h, it arrested cells at S and G2/M phase of the cell cycle in a dose-dependent manner. Annexin V/propidium iodide double staining showed apoptosis more than necrosis. Moreover, the decreased Bcl-2 and increased Bax, p53, and Apaf-1 support apoptotic cell death. Ethyl acetate extracts from
L., had cytotoxic activity [60]. At doses of 5 μg/mL, 25 μg/mL, and 50 μg/mL for 24 h, it arrested cells at S and G2/M phase of the cell cycle in a dose-dependent manner. Annexin V/propidium iodide double staining showed apoptosis more than necrosis. Moreover, the decreased Bcl-2 and increased Bax, p53, and Apaf-1 support apoptotic cell death. Ethyl acetate extracts from
Streptomyces species upregulated caspase-3, -9, Bax, and LC3-II and downregulated PARP, LC3-I, Beclin1, and p62 in SiHa cells at concentrations of 20 μg/mL, 40 μg/mL, and 60 μg/mL for 24 h [64]. Half of the SiHa cells underwent death at a concentration of 20 μg/mL. This induced altered cell morphology and apoptosis, autophagy, and inhibited proliferation in the pathway. Consequently, ethyl acetate extracts-induced Bax-dependent mitochondrial permeabilization plays the role of an initiator of intrinsic pathway of apoptosis. Expression of p53 was increased and cyclin D, E, p21, and survivin were decreased in SiHa cells by treatment of blueberry extracts at the density of 50 mg/mL for an incubation time of 24 h with 4 Gy radiotherapy [65]. Through the mechanisms, it enhanced radiotherapy. Lipid-soluble extracts from
species upregulated caspase-3, -9, Bax, and LC3-II and downregulated PARP, LC3-I, Beclin1, and p62 in SiHa cells at concentrations of 20 μg/mL, 40 μg/mL, and 60 μg/mL for 24 h [61]. Half of the SiHa cells underwent death at a concentration of 20 μg/mL. This induced altered cell morphology and apoptosis, autophagy, and inhibited proliferation in the pathway. Consequently, ethyl acetate extracts-induced Bax-dependent mitochondrial permeabilization plays the role of an initiator of intrinsic pathway of apoptosis. Expression of p53 was increased and cyclin D, E, p21, and survivin were decreased in SiHa cells by treatment of blueberry extracts at the density of 50 mg/mL for an incubation time of 24 h with 4 Gy radiotherapy [62]. Through the mechanisms, it enhanced radiotherapy. Lipid-soluble extracts from
Pinellia pedatisecta Schott. upregulated β-catenin, c-Myc, cyclin D1, PPAR1, and downregulated Th2 and Th17 in HPV and TC-1 cells at a dose of 500 μg/mL dealing with 72 h [66]. This result suggested that induction of apoptosis and cell cycle arrest were involved in the pathway. The subset proportion of Th1 cells increased significantly and both Th2 cells and Th17 cells decreased profoundly. In vivo, T lymphocyte infiltration in tumor-burdened mice was enhanced with treatment. Methanol extracts from
Schott. upregulated β-catenin, c-Myc, cyclin D1, PPAR1, and downregulated Th2 and Th17 in HPV and TC-1 cells at a dose of 500 μg/mL dealing with 72 h [63]. This result suggested that induction of apoptosis and cell cycle arrest were involved in the pathway. The subset proportion of Th1 cells increased significantly and both Th2 cells and Th17 cells decreased profoundly. In vivo, T lymphocyte infiltration in tumor-burdened mice was enhanced with treatment. Methanol extracts from
Allium atroviolaceum upregulated caspase-3, -5, and -9 and downregulated Bcl-2, CDK1, and p53 in HeLa cells at concentrations of 20 μg/mL, 40 μg/mL, 60 μg/mL, 80 μg/mL, and 100 μg/mL for 24 h, 48 h, and 72 h [67]. The efficacy was the best at 72 h. Controlling the mechanisms, it inhibited cell growth and proliferation, induced cell cycle arrest, and reduced cell viability. The level of caspase-3 was increased and the level of PARP-1 was decreased by methanol extracts from
upregulated caspase-3, -5, and -9 and downregulated Bcl-2, CDK1, and p53 in HeLa cells at concentrations of 20 μg/mL, 40 μg/mL, 60 μg/mL, 80 μg/mL, and 100 μg/mL for 24 h, 48 h, and 72 h [64]. The efficacy was the best at 72 h. Controlling the mechanisms, it inhibited cell growth and proliferation, induced cell cycle arrest, and reduced cell viability. The level of caspase-3 was increased and the level of PARP-1 was decreased by methanol extracts from
Corylus avellane in HeLa cells at dose of 250 μg/mL and 500 μg/mL for 24 h [68]. The expression of cleaved forms of caspase-3 and PARP-1 suggested that the extracts induced apoptosis through caspase-3 activation in HeLa cells. In conclusion, this result suggested that reduction of cell viability, induction of apoptosis, inhibition of oxidant and proliferation were involved in the pathway. Methanol extracts isolated from
in HeLa cells at dose of 250 μg/mL and 500 μg/mL for 24 h [65]. The expression of cleaved forms of caspase-3 and PARP-1 suggested that the extracts induced apoptosis through caspase-3 activation in HeLa cells. In conclusion, this result suggested that reduction of cell viability, induction of apoptosis, inhibition of oxidant and proliferation were involved in the pathway. Methanol extracts isolated from
Cyperus rotundus induced apoptosis and DNA fragmentation and inhibited migration in HeLa cells at doses of 25 μg/mL, 50 μg/mL, and 100 μg/mL for an incubation time of 24 h and 48 h [69]. Cytotoxic effects of methanol extracts on the tested cancer cell lines ranged from 4.52 ± 0.57 μg/mL
induced apoptosis and DNA fragmentation and inhibited migration in HeLa cells at doses of 25 μg/mL, 50 μg/mL, and 100 μg/mL for an incubation time of 24 h and 48 h [66]. Cytotoxic effects of methanol extracts on the tested cancer cell lines ranged from 4.52 ± 0.57 μg/mL
−1
to 9.85 ± 0.68 μg/mL
−1
. Moreover, methanol extracts showed anticancer and antimigration activity and it also induced nuclear fragmentation in HeLa cells. Expression of Bax, BAD, caspase-3, p21, and p53 were increased and Bcl-2 was decreased in HeLa cells by treatment of methanol extracts isolated from
Polyalthia longifolia at dose of 22 μg/mL for 6 h, 12 h, 24 h, 36 h [70]. Controlling the mechanisms, it altered cell morphology and induced apoptosis. Consequently, extracts produced distinctive porphological features of HeLa cell death that corresponds to apoptosis. Sul’ain et al. reported that the methanol extracts of
at dose of 22 μg/mL for 6 h, 12 h, 24 h, 36 h [67]. Controlling the mechanisms, it altered cell morphology and induced apoptosis. Consequently, extracts produced distinctive porphological features of HeLa cell death that corresponds to apoptosis. Sul’ain et al. reported that the methanol extracts of
Pyrrosia piloselloides showed antiproliferative effects on HeLa cells [71]. This efficacy was caused by treatment with an IC
showed antiproliferative effects on HeLa cells [68]. This efficacy was caused by treatment with an IC
50
of 16.25 μg/mL. Meanwhile,
Pyrrosia piloselloides
water extracts were without influence. Panicker et al. reported that methanol extracts from
Teucrium mascatense were shown to activate caspases and PARP on HeLa cells, following treatment with 25 µg/mL, 50 µg/mL, 125 µg/mL, and 250 µg/mL, for 72 h [72]. In addition, cell rounding, shrinkage, and detachment from other cells were shown by methanol extracts. This result suggested apoptosis and alteration of cell morphology were related to the pathway.
were shown to activate caspases and PARP on HeLa cells, following treatment with 25 µg/mL, 50 µg/mL, 125 µg/mL, and 250 µg/mL, for 72 h [69]. In addition, cell rounding, shrinkage, and detachment from other cells were shown by methanol extracts. This result suggested apoptosis and alteration of cell morphology were related to the pathway.
Table 2.
Apoptosis inducing natural products-extracts.
| Classification | Extract | Source | Cell Line/ Animal Model |
Dose; Duration | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|---|---|
| Plant | Aqueous extract | Anemone nemorosa | HeLa | 20.33 ± 2.480 μg/mL; 24, 48 h |
Induction of apoptosis Inhibition of proliferation |
↑PS translocation, c-caspase-3, -8, ROS (24 h) ↓MMP, ROS (48 h) |
[55][52] |
| Plant | Aril extract | Strelitzia nicolai | HeLa | 250 μg/mL; 24, 48, 72 h | Inhibition of oxidative stress Induction of apoptosis |
[56][53] | |
| Plant | Ethanol extract | Astragalus membranaceus, Angelica gigas, Trichosanthes kirilowii Maximowicz. |
HeLa | 100, 200, 400 μg/mL; 24, 48 h |
Induction of apoptosis and cell cycle arrest Inhibition of cell viability |
↑c-caspase-3, -8, PARP-1 ↓Bax, cyclin D, CDK2, CDK4, CDK6, p27 |
[57][54] |
| Plant |
Phosphatidylserine (PS); cyclin-dependant kinase (CDK); receptor-interacting protein (RIP); tumor necrosis factor receptor 1 (TNF-R1); light chain (LC); minichromosome maintenance protein complex (MCM); peroxisome proliferator-activated receptor 1 (PPAR1); T helper cell (Th); Bcl-2-associated death promoter (BAD).
3.2. Anti-angiogenesis
2.2. Anti-angiogenesis
Angiogenesis is a major cause in the development and metastasis of a variety of tumor types [74]. Local angiogenesis provides oxygen and essential nutrients to the growing tumor, supports tumor expansion and invasion into nearby normal tissue, and is essential for distant metastasis [75]. To be specific, in cervical cancer, angiogenesis plays a leading role in initiation, proliferation, and progression, and also relates to blocking p53 and stabilizing hypoxia-inducible factor-1α, which led to expression of VEGF [76]. So, preventing angiogenesis could be significant in treatment of the disease. Four natural products have this ability toward HeLa cells and CaSki cells (
Angiogenesis is a major cause in the development and metastasis of a variety of tumor types [70]. Local angiogenesis provides oxygen and essential nutrients to the growing tumor, supports tumor expansion and invasion into nearby normal tissue, and is essential for distant metastasis [71]. To be specific, in cervical cancer, angiogenesis plays a leading role in initiation, proliferation, and progression, and also relates to blocking p53 and stabilizing hypoxia-inducible factor-1α, which led to expression of VEGF [72]. So, preventing angiogenesis could be significant in treatment of the disease. Four natural products have this ability toward HeLa cells and CaSki cells (
). When HeLa cells were treated by
Praecitrullus fistulosus lectin protein (PfLP), which was grown and consumed in subtropical countries, MMP-2 and -9 became downregulated and it led to the induction of apoptosis and inhibition of angiogenesis [77]. This mechanism was efficient at a dose of 50 µg/mL with 24 h. In vivo, anticancer and anti-angiogenic properties of PfLP were observed at dose of 10 mg/kg on day 7, 9, and 11 after the tumor cell transplantation. Moreover, it did not show any side effects or secondary complications in the study. Purified Flaxseed hydrolysate (PFH), extracted from lignan, was shown to induce apoptosis and inhibit angiogenesis and metastasis on HeLa cells [15]. This process was caused by increasing caspase-3 and downregulating MMP-2 and VEGF. The efficient dose was 17.4 µg/mL, dealing with a timeframe of 48 h. These results suggest that PFH could be great treatment with its anticancer activity. Based on our findings, Kuriakose et al. reported that expression of Bax, p53, and Apaf-1 were increased and Bcl-2 was decreased in HeLa cells by treatment of ethyl acetate extracts of
lectin protein (PfLP), which was grown and consumed in subtropical countries, MMP-2 and -9 became downregulated and it led to the induction of apoptosis and inhibition of angiogenesis [73]. This mechanism was efficient at a dose of 50 µg/mL with 24 h. In vivo, anticancer and anti-angiogenic properties of PfLP were observed at dose of 10 mg/kg on day 7, 9, and 11 after the tumor cell transplantation. Moreover, it did not show any side effects or secondary complications in the study. Purified Flaxseed hydrolysate (PFH), extracted from lignan, was shown to induce apoptosis and inhibit angiogenesis and metastasis on HeLa cells [15]. This process was caused by increasing caspase-3 and downregulating MMP-2 and VEGF. The efficient dose was 17.4 µg/mL, dealing with a timeframe of 48 h. These results suggest that PFH could be great treatment with its anticancer activity. Based on our findings, Kuriakose et al. reported that expression of Bax, p53, and Apaf-1 were increased and Bcl-2 was decreased in HeLa cells by treatment of ethyl acetate extracts of
Penicillium sclerotiorum
from
Cassia fistula L. [63
Praecitrullus fistulosus lectin protein (PfLP); inhibitor of apoptosis protein (IAP); TNF receptor-associated factor (TRAF).
Phosphoinositide 3-kinase (PI3K); Zinc finger E-box-binding homeobox 1 (Zeb1).
Expression of E-cadherin was increased and p38 and PI3K were decreased in SiHa cells by treatment of astragaloside IV from Radix Astragali [82]. Additionally, astragaloside IV demonstrated inhibition of cell metastasis on SiHa cell lines. The efficient dose was 200 µg/mL, dealing with a timeframe of 24 h. Expression of MMP-2, -9, and VEGF was decreased in HeLa cells by treatment of epigallocatechingallate (EGCG) from green tea [83]. Thus, it induced apoptosis at a dose of 50 µg/mL for 48 h. In addition, it inhibited cell metastasis and proliferation in cervical cancer cells by reducing VEGF, CDK2, and ERK1/2. Praeruptorin-B isolated from
Expression of E-cadherin was increased and p38 and PI3K were decreased in SiHa cells by treatment of astragaloside IV from Radix Astragali [78]. Additionally, astragaloside IV demonstrated inhibition of cell metastasis on SiHa cell lines. The efficient dose was 200 µg/mL, dealing with a timeframe of 24 h. Expression of MMP-2, -9, and VEGF was decreased in HeLa cells by treatment of epigallocatechingallate (EGCG) from green tea [79]. Thus, it induced apoptosis at a dose of 50 µg/mL for 48 h. In addition, it inhibited cell metastasis and proliferation in cervical cancer cells by reducing VEGF, CDK2, and ERK1/2. Praeruptorin-B isolated from
Peucedanum praeruptorum Dunn. downregulated NF-κB, MMP-2, and -9 in HeLa and SiHa cells [84]. Inhibition of cell metastasis could be observed following the treatment of praeruptorin-B at doses of 40 µg and 60 µg over 24 h. Moreover, it blocked Akt phosphorylation without affecting the MAPK pathway. These results suggested that praeruptorin-B could be good at anticancer activity, especially in cervical cancer cells. Thymoquinone from
Dunn. downregulated NF-κB, MMP-2, and -9 in HeLa and SiHa cells [80]. Inhibition of cell metastasis could be observed following the treatment of praeruptorin-B at doses of 40 µg and 60 µg over 24 h. Moreover, it blocked Akt phosphorylation without affecting the MAPK pathway. These results suggested that praeruptorin-B could be good at anticancer activity, especially in cervical cancer cells. Thymoquinone from
Nigella sativa was shown to induce apoptosis, migration, and invasion on CaSki and HeLa cells [46]. It was efficient when CaSki cells and HeLa cells were treated at a concentration of 5 µM for 24 h. Furthermore, it included the mechanism of increasing E-cadherin level and decreasing the level of Twist1 and Zeb1. Ethanol extracts from
was shown to induce apoptosis, migration, and invasion on CaSki and HeLa cells [46]. It was efficient when CaSki cells and HeLa cells were treated at a concentration of 5 µM for 24 h. Furthermore, it included the mechanism of increasing E-cadherin level and decreasing the level of Twist1 and Zeb1. Ethanol extracts from
Bauhinia variegata candida decreased the level of MMP-2 and -9 on HeLa cells [58]. This mechanism was caused when HeLa cells were treated at a concentration of 25 µg/mL for 24 h. Subsequently, it reduced cell viability, migration, and invasion. Lee et al. reported that the decline of MMP-9 and ERK1/2 was observed after the exposure of
decreased the level of MMP-2 and -9 on HeLa cells [55]. This mechanism was caused when HeLa cells were treated at a concentration of 25 µg/mL for 24 h. Subsequently, it reduced cell viability, migration, and invasion. Lee et al. reported that the decline of MMP-9 and ERK1/2 was observed after the exposure of
Terminalia catappa ethanol extracts on HeLa and SiHa cells [85]. This process was efficient when the dose was 25 µg/mL, 50 µg/mL, and 75 µg/mL, dealing with a timeframe of 24 h and it led to inhibition of cell metastasis. In conclusion, ethanol extracts blocked the MMP-9 through ERK1/2 pathway by anti-metastatic effects.
ethanol extracts on HeLa and SiHa cells [81]. This process was efficient when the dose was 25 µg/mL, 50 µg/mL, and 75 µg/mL, dealing with a timeframe of 24 h and it led to inhibition of cell metastasis. In conclusion, ethanol extracts blocked the MMP-9 through ERK1/2 pathway by anti-metastatic effects.
A total of six substances derived from natural products inhibited the metastasis of cervical cancer, and MMP control was the most important mechanism. Four substances regulated MMP, MMP-2 was inhibited in three substances, and MMP-9 was inhibited in four substances. E-cadherin is a protein remarkably related to tumor invasion, metastatic transmission, and poor patient prognosis [86]. It was derived from two substances, and in addition, it showed anti-metastasis action through inhibitory mechanisms such as p38, VEGF, and Twist1. In particular, ethanol extracts from
A total of six substances derived from natural products inhibited the metastasis of cervical cancer, and MMP control was the most important mechanism. Four substances regulated MMP, MMP-2 was inhibited in three substances, and MMP-9 was inhibited in four substances. E-cadherin is a protein remarkably related to tumor invasion, metastatic transmission, and poor patient prognosis [82]. It was derived from two substances, and in addition, it showed anti-metastasis action through inhibitory mechanisms such as p38, VEGF, and Twist1. In particular, ethanol extracts from
Bauhinia variegate candida exhibited multi-effect, inhibited migration and invasion at 25 µg/mL, and also induced apoptosis at 15 µg/mL [58]. However, the concentration of the natural product was so high (200 µg/mL) that there was a study involving cytotoxicity concerns [82].
exhibited multi-effect, inhibited migration and invasion at 25 µg/mL, and also induced apoptosis at 15 µg/mL [55]. However, the concentration of the natural product was so high (200 µg/mL) that there was a study involving cytotoxicity concerns [78].
References
- Urasa, M.; Darj, E. Knowledge of cervical cancer and screening practices of nurses at a regional hospital in Tanzania. Afr. Health Sci. 2011, 11, 48–57.
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Kim, J.Y.; Byun, S.J.; Kim, Y.S.; Nam, J.-H. Disease courses in patients with residual tumor following concurrent chemoradiotherapy for locally advanced cervical cancer. Gynecol. Oncol. 2017, 144, 34–39.
- Hertlein, M.L.; Lenhard, M.; Kirschenhofer, A.; Kahlert, S.; Mayr, D.; Burges, A.; Friese, K. Cetuximab monotherapy in advanced cervical cancer: A retrospective study with five patients. Arch. Gynecol. Obstet. 2011, 283, 109–113.
- Kurtz, J.; Hardy-Bessard, A.-C.; Deslandres, M.; Lavau-Denes, S.; Largillier, R.; Roemer-Becuwe, C.; Weber, B.; Guillemet, C.; Paraiso, D.; Pujade-Lauraine, E. Cetuximab, topotecan and cisplatin for the treatment of advanced cervical cancer: A phase II GINECO trial. Gynecol. Oncol. 2009, 113, 16–20.
- Gaffney, D.K.; Winter, K.; Dicker, A.P.; Miller, B.; Eifel, P.J.; Ryu, J.; Avizonis, V.; Fromm, M.; Greven, K. A Phase II study of acute toxicity for Celebrex™ (celecoxib) and chemoradiation in patients with locally advanced cervical cancer: Primary endpoint analysis of RTOG. Int. J. Radiat. Oncol. 2007, 67, 104–109.
- Herrera, F.G.; Chan, P.; Doll, C.; Milosevic, M.; Oza, A.; Syed, A.; Pintilie, M.; Levin, W.; Manchul, L.; Fyles, A. A prospective phase I–II trial of the cyclooxygenase-2 inhibitor celecoxib in patients with carcinoma of the cervix with biomarker assessment of the tumor microenvironment. Int. J. Radiat. Oncol. 2007, 67, 97–103.
- Broutet, N.; Eckert, L.; Ullrich, A.; Bloem, P. Comprehensive Cervical Cancer Control: A Guide to Essential Practice; World Health Organization: Geneva, Switzerland, 2014; pp. 1–378.
- Lee, J.; Jeong, M.I.; Kim, H.-R.; Park, H.; Moon, W.-K.; Kim, B. Plant Extracts as Possible Agents for Sequela of Cancer Therapies and Cachexia. Antioxidants 2020, 9, 836.
- Federico, C.; Sun, J.; Muz, B.; Alhallak, K.; Cosper, P.F.; Muhammad, N.; Jeske, A.; Hinger, A.; Markovina, S.; Grigsby, P.; et al. Localized Delivery of Cisplatin to Cervical Cancer Improves Its Therapeutic Efficacy and Minimizes Its Side-Effect Profile. Int. J. Radiat. Oncol. 2020.
- Shen, D.-W.; Pouliot, L.M.; Hall, M.D.; Gottesman, M.M. Cisplatin Resistance: A Cellular Self-Defense Mechanism Resulting from Multiple Epigenetic and Genetic Changes. Pharmacol. Rev. 2012, 64, 706–721.
- Sun, C.; Brown, A.J.; Jhingran, A.; Frumovitz, M.; Ramondetta, L.; Bodurka, D.C. Patient Preferences for Side Effects Associated With Cervical Cancer Treatment. Int. J. Gynecol. Cancer 2014, 24, 1077–1084.
- Momtazi-Borojeni, A.A.; Ghasemi, F.; Hesari, A.; Majeed, M.; Caraglia, M.; Sahebkar, A. Anti-Cancer and Radio-Sensitizing Effects of Curcumin in Nasopharyngeal Carcinoma. Curr. Pharm. Des. 2018, 24, 2121–2128.
- Nasreen, S.; Safeer, S.; Dar, K.K.; Andleeb, S.; Ejaz, M.; Khan, M.A.; Ali, S. Etiology of hepatocellular carcinoma and treatment through medicinal plants: A comprehensive review. Orient. Pharm. Exp. Med. 2018, 18, 187–197.
- Ezzat, S.M.; Shouman, S.A.; ElKhoely, A.; Attia, Y.M.; Elsesy, M.E.; El Senousy, A.S.; Choucry, M.A.; El Gayed, S.H.; El Sayed, A.A.; Sattar, E.A.; et al. Anticancer potentiality of lignan rich fraction of six Flaxseed cultivars. Sci. Rep. 2018, 8, 544.
- Hong, B.; Li, J.; Huang, C.; Huang, T.; Zhang, M.; Huang, L. miR-300/FA2H affects gastric cancer cell proliferation and apoptosis. Open Med. 2020, 15, 882–889.
- Escuin, D.; López-Vilaró, L.; Bell, O.; Mora, J.; Moral, A.; Pérez, J.I.; Arqueros, C.; Cajal, T.R.Y.; Lerma, E.; Barnadas, A. MicroRNA-1291 Is Associated With Locoregional Metastases in Patients With Early-Stage Breast Cancer. Front. Genet. 2020, 11, 562114.
- Lim, H.J.; Park, M.N.; Kim, C.; Kang, B.; Song, H.-S.; Lee, H.; Kim, S.-H.; Shim, B.S.; Kim, B. MiR-657/ATF2 Signaling Pathway Has a Critical Role in Spatholobus suberectus Dunn Extract-Induced Apoptosis in U266 and U937 Cells. Cancers 2019, 11, 150.
- Kim, C.; Song, H.-S.; Park, H.; Kim, B. Activation of ER Stress-Dependent miR-216b Has a Critical Role in Salvia miltiorrhiza Ethanol-Extract-Induced Apoptosis in U266 and U937 Cells. Int. J. Mol. Sci. 2018, 19, 1240.
- Phuah, N.H.; Azmi, M.N.; Awang, K.; Nagoor, N.H. Down-Regulation of MicroRNA-210 Confers Sensitivity towards 1’S-1’-Acetoxychavicol Acetate (ACA) in Cervical Cancer Cells by Targeting SMAD. Mol. Cells 2017, 40, 291–298.
- Noh, S.; Choi, E.; Hwang, C.-H.; Jung, J.H.; Kim, S.-H.; Kim, B. Dietary Compounds for Targeting Prostate Cancer. Nutritients 2019, 11, 2401.
- Lowe, S.W.; Lin, A.W. Apoptosis in cancer. Carcinog. 2000, 21, 485–495.
- Li, F.-Y.; Wang, X.; Duan, W.-G.; Lin, G.-S. Synthesis and In Vitro Anticancer Activity of Novel Dehydroabietic Acid-Based Acylhydrazones. Molecules 2017, 22, 1087.
- Vishnu, V.R.; Renjith, R.S.; Mukherjee, A.; Anil, S.R.; Sreekumar, J.; Jyothi, A.N.; Alummoottil, J.N. Comparative Study on the Chemical Structure and In Vitro Antiproliferative Activity of Anthocyanins in Purple Root Tubers and Leaves of Sweet Potato (Ipomoea batatas). J. Agric. Food Chem. 2019, 67, 2467–2475.
- Piboonprai, K.; Khumkhrong, P.; Khongkow, M.; Yata, T.; Ruangrungsi, N.; Chansriniyom, C.; Iempridee, T. Anticancer activity of arborinine from Glycosmis parva leaf extract in human cervical cancer cells. Biochem. Biophys. Res. Commun. 2018, 500, 866–872.
- Wang, L.; Zhao, Y.; Wu, Q.; Guan, Y.; Wu, X. Therapeutic effects of β-elemene via attenuation of the Wnt/β-catenin signaling pathway in cervical cancer cells. Mol. Med. Rep. 2018, 17, 4299–4306.
- Rehana, D.; Mahendiran, D.; Kumar, R.S.; Rahiman, A.K. Evaluation of antioxidant and anticancer activity of copper oxide nanoparticles synthesized using medicinally important plant extracts. Biomed. Pharmacother. 2017, 89, 1067–1077.
- Wang, J.; Liu, Q.; Yang, L.; Xia, X.; Zhu, R.; Chen, S.; Wang, M.; Cheng, L.; Wu, X.; Wang, S. Curcumin-Loaded TPGS/F127/P123 Mixed Polymeric Micelles for Cervical Cancer Therapy: Formulation, Characterization, and In Vitro and In Vivo Evaluation. J. Biomed. Nanotechnol. 2017, 13, 1631–1646.
- Moreira, T.F.; Sorbo, J.M.; Souza, F.D.O.; Fernandes, B.C.; Ocampos, F.M.M.; De Oliveira, D.M.S.; Arcaro, C.A.; Assis, R.P.; Barison, A.; Miguel, O.G.; et al. Emodin, Physcion, and Crude Extract of Rhamnus sphaerosperma var. pubescens Induce Mixed Cell Death, Increase in Oxidative Stress, DNA Damage, and Inhibition of AKT in Cervical and Oral Squamous Carcinoma Cell Lines. Oxidative Med. Cell. Longev. 2018, 2018, 1–18.
- Yang, J.; Fa, J.; Li, B. Apoptosis induction of epifriedelinol on human cervical cancer cell line. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 80–86.
- Das, A.; Harshadha, K.; Dhinesh Kannan, S.; Hari Raj, K.; Jayaprakash, B. Evaluation of therapeutic potential of eugenol-a natural derivative of Syzygium aromaticum on cervical cancer. APJCP 2018, 19, 1977.
- Chen, X.; Song, L.; Hou, Y.; Li, F. Reactive oxygen species induced by icaritin promote DNA strand breaks and apoptosis in human cervical cancer cells. Oncol. Rep. 2018, 41, 765–778.
- Kuo, C.-Y.; Schelz, Z.; Tóth, B.; Vasas, A.; Ocsovszki, I.; Chang, F.-R.; Hohmann, J.; Zupkó, I.; Wang, H.-C. Investigation of natural phenanthrenes and the antiproliferative potential of juncusol in cervical cancer cell lines. Phytomedicine 2018, 58, 152770.
- Ma, Y.-L.; Zhang, Y.-S.; Zhang, F.; Zhang, Y.-Y.; Thakur, K.; Zhang, J.-G.; Wei, Z.-J. Methyl protodioscin from Polygonatum sibiricum inhibits cervical cancer through cell cycle arrest and apoptosis induction. Food Chem. Toxicol. 2019, 132, 110655.
- Al-Otaibi, W.A.; Alkhatib, M.H.; Wali, A.N. Cytotoxicity and apoptosis enhancement in breast and cervical cancer cells upon coadministration of mitomycin C and essential oils in nanoemulsion formulations. Biomed. Pharmacother. 2018, 106, 946–955.
- Latif, A.D.; Gonda, T.; Vágvölgyi, M.; Kúsz, N.; Kulmány, Á.; Ocsovszki, I.; Zomborszki, Z.P.; Zupkó, I.; Hunyadi, A. Synthesis and In Vitro Antitumor Activity of Naringenin Oxime and Oxime Ether Derivatives. Int. J. Mol. Sci. 2019, 20, 2184.
- Souza, F.D.O.; Sorbo, J.M.; Regasini, L.O.; Rosa, J.C.; Czernys, É.D.S.; Valente, V.; Moreira, T.F.; Navegante, G.; Fernandes, B.C.; Soares, C.P. Nitensidine B affects proteins of the glycolytic pathway and induces apoptosis in cervical carcinoma cells immortalized by HPV. Phytomedicine 2018, 48, 179–186.
- Li, L.; Sun, J.-X.; Wang, X.-Q.; Liu, X.-K.; Chen, X.-X.; Zhang, B.; He, Z.-D.; Liu, D.-Z.; Chen, L.-X.; Wang, L.-W.; et al. Notoginsenoside R7 suppresses cervical cancer via PI3K/PTEN/Akt/mTOR signaling. Oncotarget 2017, 8, 109487–109496.
- Che, Y.; Li, J.; Li, Z.; Li, J.; Wang, S.; Yan, Y.; Zou, K.; Zou, L. Osthole enhances antitumor activity and irradiation sensitivity of cervical cancer cells by suppressing ATM/NF-κB signaling. Oncol. Rep. 2018, 40, 737–747.
- Selvan, D.A.; Mahendiran, D.; Kumar, R.S.; Rahiman, A.K. Garlic, green tea and turmeric extracts-mediated green synthesis of silver nanoparticles: Phytochemical, antioxidant and in vitro cytotoxicity studies. J. Photochem. Photobiol. B Biol. 2018, 180, 243–252.
- Xie, Z.; Wei, Y.; Xu, J.; Lei, J.; Yu, J. Alkaloids from Piper nigrum Synergistically Enhanced the Effect of Paclitaxel against Paclitaxel-Resistant Cervical Cancer Cells through the Downregulation of Mcl-1. J. Agric. Food Chem. 2019, 67, 5159–5168.
- Zhang, Y.; Zhou, D.; Liu, W.; Li, C.; Hao, L.; Zhang, G.; Deng, S.; Yang, R.; Qin, J.K.; Li, J.; et al. Cytotoxic Activity and Related Mechanisms of Prenylflavonoids Isolated from Mallotus conspurcatus Croizat. Chem. Biodivers. 2019, 16, e1800465.
- Lin, C.-L.; Lee, C.-H.; Chen, C.-M.; Cheng, C.-W.; Chen, P.-N.; Ying, T.-H.; Hsieh, Y.-H. Protodioscin induces apoptosis through ROS-mediated endoplasmic reticulum stress via the JNK/p38 activation pathways in human cervical cancer cells. Cell. Physiol. Biochem. 2018, 46, 322–334.
- Chatterjee, K.; Mukherjee, S.; Vanmanen, J.; Banerjee, P.; Fata, J.E. Dietary Polyphenols, Resveratrol and Pterostilbene Exhibit Antitumor Activity on an HPV E6-Positive Cervical Cancer Model: An in vitro and in vivo Analysis. Front. Oncol. 2019, 9, 352.
- Chen, Y.; Qu, D.; Fu, R.; Guo, M.; Qin, Y.; Guo, J.; Chen, Y. A Tf-modified tripterine-loaded coix seed oil microemulsion enhances anti-cervical cancer treatment. Int. J. Nanomed. 2018, 13, 7275–7287.
- Li, J.; Khan, A.; Wei, C.; Cheng, J.; Chen, H.; Yang, L.; Ijaz, I.; Fu, J. Thymoquinone Inhibits the Migration and Invasive Characteristics of Cervical Cancer Cells SiHa and CaSki In Vitro by Targeting Epithelial to Mesenchymal Transition Associated Transcription Factors Twist1 and Zeb1. Molecules 2017, 22, 2105.
- Zhao, Y.; Wang, M.; Tsering, J.; Li, H.; Li, S.; Li, Y.; Liu, Y.; Hu, X. An Integrated Study on the Antitumor Effect and Mechanism of Triphala Against Gynecological Cancers Based on Network Pharmacological Prediction and In Vitro Experimental Validation. Integr. Cancer Ther. 2018, 17, 894–901.
- Phuah, N.H.; Azmi, M.N.; Awang, K.; Nagoor, N.H. Suppression of microRNA-629 enhances sensitivity of cervical cancer cells to 1′S-1′-acetoxychavicol acetate via regulating RSU1. OncoTargets Ther. 2017, 10, 1695–1705.
- Wang, R.; Yang, W.; Fan, Y.; Dehaen, W.; Li, Y.; Li, H.-J.; Wang, W.; Zheng, Q.; Huai, Q.-Y. Design and synthesis of the novel oleanolic acid-cinnamic acid ester derivatives and glycyrrhetinic acid-cinnamic acid ester derivatives with cytotoxic properties. Bioorganic Chem. 2019, 88, 102951.
- Hassan, A.H.; Choi, E.; Yoon, Y.M.; Lee, K.W.; Yoo, S.Y.; Cho, M.C.; Yang, J.S.; Kim, H.I.; Hong, J.Y.; Shin, J.-S.; et al. Natural products hybrids: 3,5,4’-Trimethoxystilbene-5,6,7-trimethoxyflavone chimeric analogs as potential cytotoxic agents against diverse human cancer cells. Eur. J. Med. Chem. 2018, 161, 559–580.
- Fan, Y.; Zhang, Y.; Liu, Y.; Xu, W.; Yang, Y.; Hao, Y.; Tao, L. A natural product enhances apoptosis via mitochondria/caspase-mediated pathway in HeLa cells. J. Cell. Biochem. 2019, 120, 16811–16823.
- Swanepoel, B.; Venables, L.; Octavian-Tudorel, O.; Nitulescu, G.M.; Van De Venter, M. In Vitro Anti-proliferative Activity and Mechanism of Action of Anemone nemorosa. Int. J. Mol. Sci. 2019, 20, 1217.
- Dwarka, D.; Thaver, V.; Naidu, M.; Koorbanally, N.A.; Baijnath, A.H. In vitro chemo-preventative activity of strelitzia nicolai aril extract containing bilirubin. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 147–156.
- Lee, K.M.; Lee, K.; Choi, Y.K.; Choi, Y.J.; Seo, H.S.; Ko, S.G. SH003-induced G1 phase cell cycle arrest induces apoptosis in HeLa cervical cancer cells. Mol. Med. Rep. 2017, 16, 8237–8244.
- Dos Santos, K.M.; Gomes, I.N.F.; Silva-Oliveira, R.J.; Pinto, F.E.; Oliveira, B.G.; Chagas, R.C.R.; Romão, W.; Reis, R.M.; Ribeiro, R.I.M.D.A. Bauhinia variegata candida Fraction Induces Tumor Cell Death by Activation of Caspase-3, RIP, and TNF-R1 and Inhibits Cell Migration and Invasion In Vitro. BioMed Res. Int. 2018, 2018, 1–10.
- Suh, S.-S.; Kim, S.-M.; Kim, J.E.; Hong, J.-M.; Lee, S.G.; Youn, U.J.; Han, S.J.; Kim, I.C.; Kim, S. Anticancer activities of ethanol extract from the Antarctic freshwater microalga, Botryidiopsidaceae sp. BMC Complement. Altern. Med. 2017, 17, 509.
- Suh, S.-S.; Yang, E.J.; Lee, S.G.; Youn, U.J.; Han, S.J.; Kim, I.-C.; Kim, S. Bioactivities of ethanol extract from the Antarctic freshwater microalga, Chloromonas sp. Int. J. Med Sci. 2017, 14, 560–569.
- Prasad, R.; Rana, N.K.; Koch, B. Dendrobium chrysanthum ethanolic extract induces apoptosis via p53 up-regulation in HeLa cells and inhibits tumor progression in mice. J. Complement. Integr. Med. 2017, 14, 14.
- Ma, J.F.; Wei, P.F.; Guo, C.; Shi, Y.P.; Lv, Y.; Qiu, L.X.; Wen, L.P. The Ethyl Acetate Extract of Gynura formosana Kitam. Leaves Inhibited Cervical Cancer Cell Proliferation via Induction of Autophagy. BioMed Res. Int. 2018, 2018, 1–10.
- Kuriakose, G.C.; M, D.L.; Bp, A.; Rs, H.K.; Th, A.K.; Ananthaswamy, K.; Chelliah, J. Extract of Penicillium sclerotiorum an endophytic fungus isolated from Cassia fistula L. induces cell cycle arrest leading to apoptosis through mitochondrial membrane depolarization in human cervical cancer cells. Biomed. Pharmacother. 2018, 105, 1062–1071.
- Dan, V.M.; Muralikrishnan, B.; Sanawar, R.; S, V.J.; Burkul, B.B.; Srinivas, K.P.; Lekshmi, A.; Pradeep, N.S.; Dastager, S.G.; Santhakumari, B.; et al. Streptomyces sp. metabolite(s) promotes Bax mediated intrinsic apoptosis and autophagy involving inhibition of mTOR pathway in cervical cancer cell lines. Sci. Rep. 2018, 8, 2810.
- Davidson, K.T.; Zhu, Z.; Bai, Q.; Xiao, H.; Wakefield, M.R.; Fang, Y. Blueberry as a Potential Radiosensitizer for Treating Cervical Cancer. Pathol. Oncol. Res. 2019, 25, 81–88.
- Huang, H.; Zhang, M.; Yao, S.; Zhang, M.; Peng, J.; Guiling with the Pinellia Pedatisecta (PE) Advisory Group; Xu, C.-J.; Ye, Y.; Gui, S. Immune modulation of a lipid-soluble extract of Pinellia pedatisecta Schott in the tumor microenvironment of an HPV + tumor-burdened mouse model. J. Ethnopharmacol. 2018, 225, 103–115.
- Khazaei, S.; Ramachandran, V.; Hamid, R.A.; Esa, N.M.; Etemad, A.; Moradipoor, S.; Patimah, I. Flower extract of Allium atroviolaceum triggered apoptosis, activated caspase-3 and down-regulated antiapoptotic Bcl-2 gene in HeLa cancer cell line. Biomed. Pharmacother. 2017, 89, 1216–1226.
- Esposito, T.; Sansone, F.; Franceschelli, S.; Del Gaudio, P.; Picerno, P.; Aquino, R.P.; Mencherini, T. Hazelnut (Corylus avellana L.) Shells Extract: Phenolic Composition, Antioxidant Effect and Cytotoxic Activity on Human Cancer Cell Lines. Int. J. Mol. Sci. 2017, 18, 392.
- Mannarreddy, P.; Denis, M.; Munireddy, D.; Pandurangan, R.; Thangavelu, K.P.; Venkatesan, K. Cytotoxic effect of Cyperus rotundus rhizome extract on human cancer cell lines. Biomed. Pharmacother. 2017, 95, 1375–1387.
- Vijayarathna, S.; Chen, Y.; Kanwar, J.R.; Sasidharan, S. Standardized Polyalthia longifolia leaf extract (PLME) inhibits cell proliferation and promotes apoptosis: The anti-cancer study with various microscopy methods. Biomed. Pharmacother. 2017, 91, 366–377.
- Sul‘ain, M.D.; Fashihah Zakaria, M.F.J. Anti-Proliferative Effects of Methanol and Water Extracts of Pyrrosia piloselloides on the Hela Human Cervical Carcinoma Cell Line. APJCP 2019, 20, 185.
- Panicker, N.G.; Balhamar, S.O.M.S.; Akhlaq, S.; Qureshi, M.M.; Rizvi, T.S.; Al-Harrasi, A.; Hussain, J.; Mustafa, F. Identification and Characterization of the Caspase-Mediated Apoptotic Activity of Teucrium mascatense and an Isolated Compound in Human Cancer Cells. Molecules 2019, 24, 977.
- Ma, J.; Waxman, D.J. Combination of antiangiogenesis with chemotherapy for more effective cancer treatment. Mol. Cancer Ther. 2008, 7, 3670–3684.
- Rajabi, M.; Mousa, S.A. The Role of Angiogenesis in Cancer Treatment. Biomediences 2017, 5, 34.
- Tomao, S.; Tomao, F.; Rossi, L.; Zaccarelli, E.; Caruso, D.; Zoratto, F.; Panici, P.B.; Papa, A. Angiogenesis and antiangiogenic agents in cervical cancer. OncoTargets Ther. 2014, 7, 2237–2248.
- Shivamadhu, M.C.; Srinivas, B.K.; Jayarama, S.; Chandrashekaraiah, S.A. Anti-cancer and anti-angiogenic effects of partially purified lectin from Praecitrullus fistulosus fruit on in vitro and in vivo model. Biomed. Pharmacother. 2017, 96, 1299–1309.
- Seifaddinipour, M.; Farghadani, R.; Namvar, F.; Bin Mohamad, J.; Kadir, H.A. Cytotoxic Effects and Anti-Angiogenesis Potential of Pistachio (Pistacia vera L.) Hulls against MCF-7 Human Breast Cancer Cells. Molecules 2018, 23, 110.
- Foda, H.D.; Zucker, S. Matrix metalloproteinases in cancer invasion, metastasis and angiogenesis. Drug Discov. Today 2001, 6, 478–482.
- Weber, G.F. Why does cancer therapy lack effective anti-metastasis drugs? Cancer Lett. 2013, 328, 207–211.
- Chanvorachote, P.; Chamni, S.; Ninsontia, C.; Phiboonchaiyanan, P.P. Potential Anti-metastasis Natural Compounds for Lung Cancer. Anticancer Res. 2016, 36, 5707–5718.
- Zhang, L.; Zhou, J.; Qin, X.; Huang, H.; Nie, C. Astragaloside IV inhibits the invasion and metastasis of SiHa cervical cancer cells via the TGF-β1-mediated PI3K and MAPK pathways. Oncol. Rep. 2019, 41, 2975–2986.
- Wang, Y.-Q.; Lu, J.-L.; Liang, Y.-R.; Li, Q.-S. Suppressive Effects of EGCG on Cervical Cancer. Molecules 2018, 23, 2334.
- Hung, C.-Y.; Lee, C.-H.; Chiou, H.-L.; Lin, C.-L.; Chen, P.-N.; Lin, M.-T.; Hsieh, Y.-H.; Chou, M.-C. Praeruptorin-b inhibits 12-o-tetradecanoylphorbol-13-acetate-induced cell invasion by targeting akt/nf-kappab via matrix metalloproteinase-2/-9 expression in human cervical cancer cells. Cell Physiol. Biochem. 2019, 52, 1255–1266.
- Lee, C.-Y.; Yang, S.-F.; Wang, P.-H.; Su, C.-W.; Hsu, H.-F.; Tsai, H.-T.; Hsiao, Y.-H. Antimetastatic effects of Terminalia catappa leaf extracts on cervical cancer through the inhibition of matrix metalloprotein-9 and MAPK pathway. Environ. Toxicol. 2019, 34, 60–66.
- Onder, T.T.; Gupta, P.B.; Mani, S.A.; Yang, J.; Lander, E.S.; Weinberg, R.A. Loss of E-Cadherin Promotes Metastasis via Multiple Downstream Transcriptional Pathways. Cancer Res. 2008, 68, 3645–3654.
