Microbes that cause infections amongst insects leading to death or serious disabilities are known as entomopathogens. Entomopathogenic bacteria and fungi are quite frequently found in soils and insect cadavers. The first step in utilizing these microbes as biopesticides is to isolate them, and several culture media and insect baiting procedures have been tested in this direction. In this work, the authors review the current techniques that have been developed so far, in the last five decades, and display brief protocols which can be adopted for the isolations of these entomopathogens. Among bacteria, this study focuses on
Serratia
spp. and bacteria from the class Bacilli. Among fungi, the review focuses those from the order Hypocreales, for example, genera
Beauveria
,
Clonostachys
,
Lecanicillium
,
Metarhizium
, and
Purpureocillium
. The authors chose these groups of entomopathogenic bacteria and fungi based on their importance in the microbial biopesticide market.
- Beauveria,Metarhizium,Hypocreales,Bacillus thuringiensis,Serratia
- Beauveria
- Metarhizium
- Hypocreales
- Bacillus thuringiensis
- Serratia
1. Isolation of Entomopathogenic Bacteria
Entomopathogenic bacteria are commonly found in soils. Hence, isolating insect-pathogenic strains is quite important. Different bacterial groups, such as symbionts of entomopathogenic nematode (EPN)
Heterorhabditis
spp. and
Steinernema
spp., i.e.,
Photorhabdus
spp. and
Xenorhabdus
spp., and others, such as
Yersinia entomophaga
,
Pseudomonas entomophila
, and
Chromobacterium spp., exhibit entomopathogenicity [18].
spp., exhibit entomopathogenicity [1].
Entomopathogenic nematode symbiotic bacteria are isolated by dropping an insect’s hemolymph onto a nutrient bromothymol blue (0.0025% (
w/v
)) triphenyltetrazolium chloride (0.004% (
w/v)) agar (NBTA) and incubating the streaked plate at 25 °C, and continuously subculturing until the uniform colonies are obtained [19].
)) agar (NBTA) and incubating the streaked plate at 25 °C, and continuously subculturing until the uniform colonies are obtained [2].
Yersinia entomophaga
is isolated by culturing the hemolymph of diseased larvae of New Zealand grass grub,
Costelytra zealandica
White (Coleoptera: Scarabaeidae), onto Luria-Bertani (LB) agar, followed by growth on Caprylate-thallous agar (CTA) (
, Medium 1) and Deoxyribonuclease (DNase)-Toluidine Blue agar (
Appendix A, Medium 2), and no hemolysis on Columbia horse blood agar (Columbia agar + 5% horse blood) or Columbia sheep blood agar (Columbia agar + 5% sheep blood) [20]. Isolating
, Medium 2), and no hemolysis on Columbia horse blood agar (Columbia agar + 5% horse blood) or Columbia sheep blood agar (Columbia agar + 5% sheep blood) [3]. Isolating
P. entomophila
is rather tricky as the bacterium needs to elicit the systemic expression of Diptericin, an antimicrobial peptide in
Drosophila, after ingestion. However, the bacterial culture can be maintained on LB media [21]. Bacterial isolates from insects belonging to
, after ingestion. However, the bacterial culture can be maintained on LB media [4]. Bacterial isolates from insects belonging to
Chromobacterium exhibit violet pigment when cultured on L-agar [22]. However, EPB that are most commonly used as commercial biopesticides are further discussed in the review.
exhibit violet pigment when cultured on L-agar [5]. However, EPB that are most commonly used as commercial biopesticides are further discussed in the review.
1.1. Milky Disease-Causing Paenibacillus spp.
Paenibacillus popilliae
and
Paenibacillus lentimorbus are obligate pathogens of scarabs (Coleoptera) as they require the host for the growth and sporulation. In soils, they are present as endospores. These bacteria can be isolated from the hemolymph, and the methodologies may vary depending on the bacterial species. The protocols listed below have been described by Stahly et al., and more details of these protocols have been reported by Koppenhöfer et al. [23,24,25].
are obligate pathogens of scarabs (Coleoptera) as they require the host for the growth and sporulation. In soils, they are present as endospores. These bacteria can be isolated from the hemolymph, and the methodologies may vary depending on the bacterial species. The protocols listed below have been described by Stahly et al., and more details of these protocols have been reported by Koppenhöfer et al. [6][7][8].
- (a)
-
Disinfect the surface of the larvae of grubs (Coleoptera) with 0.5% (
(a) Disinfect the surface of the larvae of grubs (Coleoptera) with 0.5% (
- v
/
- v) sodium hypochlorite (NaOCl).
- (b)
-
Pinch the cadaver using a sterilized needle and collect the emerging drops in sterilized water.
- (c)
-
Culture the dilutions of the drops on St. Julian medium (J-Medium) (
) sodium hypochlorite (NaOCl).
(b) Pinch the cadaver using a sterilized needle and collect the emerging drops in sterilized water.
(c) Culture the dilutions of the drops on St. Julian medium (J-Medium) (
- Appendix A, Medium 1) [26], or Mueller-Hinton broth, yeast extract, potassium phosphate, glucose, and pyruvate (MYPGP) (
, Medium 1) [9], or Mueller-Hinton broth, yeast extract, potassium phosphate, glucose, and pyruvate (MYPGP) (
, Medium 2) agar [10].
Note: To enhance the germination of the vegetative cells, using 0.1% (
w
/
v) tryptone solution is recommended during bacterial dilutions [26]. For spores, it is advisable to heat them for 15 min in a 1 M calcium chloride solution (pH 7.0) at 60 °C, and suspend them in the hemolymph of the cabbage looper
) tryptone solution is recommended during bacterial dilutions [9]. For spores, it is advisable to heat them for 15 min in a 1 M calcium chloride solution (pH 7.0) at 60 °C, and suspend them in the hemolymph of the cabbage looper
Trichoplusia ni Hübner (Lepidoptera: Noctuidae) and in tyrosine at an alkaline pH. Another way to improve the germination is to heat the spores at 75 °C for 30 min and then apply pressure using a French press [28].
Hübner (Lepidoptera: Noctuidae) and in tyrosine at an alkaline pH. Another way to improve the germination is to heat the spores at 75 °C for 30 min and then apply pressure using a French press [11].
1.2. Amber Disease-Causing Serratia spp.
Serratia
spp. are quite frequently isolated from soils, and some of them, being saprophytes, can also be isolated from insect cadavers. Therefore, to enhance the growth of insect pathogenic
Serratia
spp. such as
Serratia entomophila
,
Serratia proteamaculans
, and
Serratia marcescens, a methodology based on a selective agar medium has been described by O’Callaghan and Jackson [30].
, a methodology based on a selective agar medium has been described by O’Callaghan and Jackson [12].
- (a)
-
Soil inoculums or hemolymph of the diseased larvae can be isolated on Caprylate-thallous agar (CTA) (
(a) Soil inoculums or hemolymph of the diseased larvae can be isolated on Caprylate-thallous agar (CTA) (
- (b)
-
Culturing is done by pulling and separating the anterior end of the cadavers. The gut contents are then cultured on CTA plates.
- (c)
, Medium 3) [13].
(b) Culturing is done by pulling and separating the anterior end of the cadavers. The gut contents are then cultured on CTA plates.
(c)
- Serratia marcescens
produces colonies that are red in color. Cream-colured bacterial colonies formed on CTA can then be transferred into different selective media for the identification of
- (d)
-
The production of a halo on a Deoxyribonuclease (DNase)-Toluidine Blue agar (
spp. [12].
(d) The production of a halo on a Deoxyribonuclease (DNase)-Toluidine Blue agar (
, Medium 4) when incubated at 30 °C for 24 h, indicates the presence of
spp. [14]. Thereafter, the production of blue or green colonies on adonitol agar (
, Medium 5) confirms
- S. proteamaculans
. The formation of yellow colonies on adonitol agar hints the presence of
- S. entomophila
, which can be confirmed by the growth on itaconate agar (
- Appendix A, Medium 6) at 30 °C after 96 h [25]. Further molecular approaches targeting specific DNA regions can distinguish pathogenic strains from the non-pathogenic ones.
, Medium 6) at 30 °C after 96 h [15]. Further molecular approaches targeting specific DNA regions can distinguish pathogenic strains from the non-pathogenic ones.
2. Isolation of Entomopathogenic Fungi
Fungal entomopathogens can directly be isolated from insect cadavers in the case of visible mycosis [35]. Moreover, they can also be isolated from soils or phylloplane as they spend a considerable part of their life as saprophytes in soils or as plant endophytes. However, to our knowledge, their survival as soil saprophytes has not been proven yet [4,5,6,7,8,35,36]. In either case, the material can be cultured directly onto a medium selective for an EPF or the material can be baited with an infection-sensitive insect [37]. In case of the isolation of EPF as endophyte, proper disinfection of the material is needed. Nonetheless, different antibacterial and fungal saprophyte-inhibiting chemicals are added in the selective medium, as per the research interest. Here, different culture media used to isolate fungal entomopathogens, especially those belonging to the order Hypocreales are discussed.
Fungal entomopathogens can directly be isolated from insect cadavers in the case of visible mycosis [16]. Moreover, they can also be isolated from soils or phylloplane as they spend a considerable part of their life as saprophytes in soils or as plant endophytes. However, to our knowledge, their survival as soil saprophytes has not been proven yet [17][18][19][20][21][16][22]. In either case, the material can be cultured directly onto a medium selective for an EPF or the material can be baited with an infection-sensitive insect [23]. In case of the isolation of EPF as endophyte, proper disinfection of the material is needed. Nonetheless, different antibacterial and fungal saprophyte-inhibiting chemicals are added in the selective medium, as per the research interest. Here, different culture media used to isolate fungal entomopathogens, especially those belonging to the order Hypocreales are discussed.
2.1. Isolations from Naturally Mycosed Insect Cadavers
This method is applied to study the natural EPF infections in the fields as it relies on the collection of the dead insects from the fields. The protocol described below is similar to that employed by Sharma et al. [7].
- (a)
-
Insect cadavers are brought to the laboratory as separate entities in sterile tubes.
- (b)
-
Insects are observed under a stereomicroscope (40×) for probable mycosis.
- (c)
-
In case of a visible mycosis, the insects are surface sterilized using 70% ethanol or 1% NaOCl, for 3 min, followed by 3 distinct washes with 100 mL of sterilized water. Then, the sporulating EPF from the insect cadaver is plated directly.
- (d)
-
Cadavers are then cultured on a selective medium at 22 °C for up to 3 weeks, depending on the time taken by the fungi for germination and proliferation. In case of no germination, the cadavers can be homogenized and plated on the selective medium. Details of the different selective medium are provided later in the text.
- (e)
-
Obtained fungi are subcultured on potato dextrose agar (PDA) (
This method is applied to study the natural EPF infections in the fields as it relies on the collection of the dead insects from the fields. The protocol described below is similar to that employed by Sharma et al. [20].
(a) Insect cadavers are brought to the laboratory as separate entities in sterile tubes.
(b) Insects are observed under a stereomicroscope (40×) for probable mycosis.
(c) In case of a visible mycosis, the insects are surface sterilized using 70% ethanol or 1% NaOCl, for 3 min, followed by 3 distinct washes with 100 mL of sterilized water. Then, the sporulating EPF from the insect cadaver is plated directly.
(d) Cadavers are then cultured on a selective medium at 22 °C for up to 3 weeks, depending on the time taken by the fungi for germination and proliferation. In case of no germination, the cadavers can be homogenized and plated on the selective medium. Details of the different selective medium are provided later in the text.
(e) Obtained fungi are subcultured on potato dextrose agar (PDA) (
, Medium 8) or Sabouraud dextrose agar (SDA) (
- Appendix A, Medium 9) until pure culture is obtained.
- (f)
-
Fungi are identified by comparing morphological characteristics using light microscopy (400×), described in several fungal identification keys, such as Domsch et al. [38] and Humber [39].
- (g)
-
Molecular identifications can be done by extracting the DNA and performing PCR for the amplification and subsequent sequencing of the nuclear internal transcribed spacer (nrITS) region of the fungal nuclear ribosomal DNA, as described in Yurkov et al. [40].
, Medium 9) until pure culture is obtained.
(f) Fungi are identified by comparing morphological characteristics using light microscopy (400×), described in several fungal identification keys, such as Domsch et al. [24] and Humber [25].
(g) Molecular identifications can be done by extracting the DNA and performing PCR for the amplification and subsequent sequencing of the nuclear internal transcribed spacer (nrITS) region of the fungal nuclear ribosomal DNA, as described in Yurkov et al. [6].
2.2. Isolations from Soils
2.2.1. Soil Suspension Culture
This method is generally used to isolate a particular EPF genus of interest using different concentrations of the soil inoculums. To ensure correct isolation, the isolated EPF should also be characterized morphologically and molecularly, as described in Section 3.1. Here the authors discuss various selective media used, especially those which are useful for the isolation of the hypocrealean fungi pertaining to their dominance in fungi-based microbial pesticide market.
This method is generally used to isolate a particular EPF genus of interest using different concentrations of the soil inoculums. To ensure correct isolation, the isolated EPF should also be characterized morphologically and molecularly. Here the authors discuss various selective media used, especially those which are useful for the isolation of the hypocrealean fungi pertaining to their dominance in fungi-based microbial pesticide market.
Metarhizium spp.
Isolating EPF has always been challenged by the contamination from saprophytic fungi. In this direction, Veen and Ferron [49] suggested using dodine (
spp.
Isolating EPF has always been challenged by the contamination from saprophytic fungi. In this direction, Veen and Ferron [7] suggested using dodine (
N
-dodecylguanidine monoacetate) to inhibit the growth of saprophytes and developed Veen’s semi-selective medium to accomplish this (
Appendix A, Medium 12). Later, Chase et al. [50] and Sneh [51] also used dodine in their studies. However, Liu et al. [52] reported that the higher quantities of dodine can be inhibitory to EPF and suggested using only 10 µg/mL dodine (
, Medium 12). Later, Chase et al. [8] and Sneh [26] also used dodine in their studies. However, Liu et al. [27] reported that the higher quantities of dodine can be inhibitory to EPF and suggested using only 10 µg/mL dodine (
Appendix A, Medium 12). Later, Rangel et al. [53] cautioned against the use of dodine and showed the even 0.006% (
, Medium 12). Later, Rangel et al. [28] cautioned against the use of dodine and showed the even 0.006% (
w/v
) dodine in PDAY can completely inhibit
Metarhizium acridum
. This led to the development of CTC medium, which is made by the addition of 0.05% (
w/v
) chloramphenicol, 0.0001% (
w/v
) thiabendazole, and 0.025% (
w/v) cycloheximide in PDAY [54] (
) cycloheximide in PDAY [29] (
Appendix A, Medium 13). However, a recent study by Hernández-Domínguez et al. [55] suggested the use of CTC medium, along with other dodine-containing mediums, for better
, Medium 13). However, a recent study by Hernández-Domínguez et al. [30] suggested the use of CTC medium, along with other dodine-containing mediums, for better
Metarhizium recoveries. Posadas et al. [47] demonstrated that OM-CTAB is effective in isolating EPF while inhibiting saprophytes. Moreover, this negated the dependency on dodine, as it is not easily available in some countries.
recoveries. Posadas et al. [31] demonstrated that OM-CTAB is effective in isolating EPF while inhibiting saprophytes. Moreover, this negated the dependency on dodine, as it is not easily available in some countries.
Beauveria
spp.
Beauveria
spp., e.g.,
Beauveria bassiana
sensu lato (s.l.) and
Beauveria pseudobassiana, can be easily isolated using oatmeal dodine agar (ODA), as described by Chase et al. [50] (
, can be easily isolated using oatmeal dodine agar (ODA), as described by Chase et al. [8] (
Appendix A, Medium 14). This medium has also been used in recent studies [56,57,58,59]. Another medium, i.e., Sabouraud-2-glucose agar (S2GA), was made by Strasser et al. [60] (
, Medium 14). This medium has also been used in recent studies [32][33][34][35]. Another medium, i.e., Sabouraud-2-glucose agar (S2GA), was made by Strasser et al. [36] (
, Medium 15) for the isolation of
Beauveria brongniartii
, and was successfully used in studies concerning
B. brongniartii [61,62,63]. However, many recent studies have used S2GA, with slight modifications, to isolate
[37][38][39]. However, many recent studies have used S2GA, with slight modifications, to isolate
B. bassiana s.l. [64,65]. A dodine-free alternative in isolating
s.l. [40][41]. A dodine-free alternative in isolating
B. bassiana s.l. is OM-CTAB [47]. Moreover, Ramírez-Rodríguez and Sánchez-Peña [66] suggested using PDAY with CTAB (0.015% or 0.03% (
s.l. is OM-CTAB [31]. Moreover, Ramírez-Rodríguez and Sánchez-Peña [42] suggested using PDAY with CTAB (0.015% or 0.03% (
w/v
)) and any of the antibacterial compounds, i.e., dihydrostreptomycin, oxytetracycline, or doxycycline, to isolate
Beauveria
while inhibiting fungal saprophytes.
Purpureocillium
spp.
Purpureocillium
spp., i.e.,
Purpureocillium lilacinum
and
Purpureocillium lavendulum, can easily be isolated using an agar medium containing sodium chloride, benomyl, pentachloronitrobenzene, and Tergitol [67,68] (
, can easily be isolated using an agar medium containing sodium chloride, benomyl, pentachloronitrobenzene, and Tergitol [43][44] (
, Medium 16).
Lecanicillium
spp.
A
Lecanicillium-selective medium (LSM) was developed by Kope et al. [69]. OM agar with 0.05% (
-selective medium (LSM) was developed by Kope et al. [45]. OM agar with 0.05% (
w/v
) chloramphenicol and 0.05% (
w/v) CTAB can also be used, as described recently by Xie et al. [70] (
) CTAB can also be used, as described recently by Xie et al. [46] (
, Medium 17).
Clonostachys
spp.
Clonostachys
spp., e.g.,
Clonostachys rosea
f.
rosea
, is reported entomopathogenic and can be isolated frequently from soils. Culture medium such as DRBCA is highly effective in isolating
Clonostachys spp., at least in the case of the isolations from cadavers [7].
spp., at least in the case of the isolations from cadavers [20].
2.2.2. Insect Baiting
This method is arguably the most commonly used method for EPF isolation, as the bait insect specifically selects entomopathogens from other saprobes in the soils [35,71,72], although surface sterilization of the insect cadavers is needed to avoid occasional contaminations by saprophytic fungi.
This method is arguably the most commonly used method for EPF isolation, as the bait insect specifically selects entomopathogens from other saprobes in the soils [16][47][48], although surface sterilization of the insect cadavers is needed to avoid occasional contaminations by saprophytic fungi.
Galleria
-Bait Method or
Tenebrio
-Bait Method
The use of
Galleria mellonella
Linnaeus (Lepidoptera: Pyralidae) for isolating EPF from soil or the “
Galleria-bait method” was first described by Zimmermann [73]. Since then, it has been used for EPF isolations in many studies [74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91].
-bait method” was first described by Zimmermann [49]. Since then, it has been used for EPF isolations in many studies [50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67].
Tenebrio molitor
Linnaeus (
Coleoptera: Tenebrionidae) has also been used as a bait insect in some studies [92,93,94]. Some previous studies have noticed that insect baiting is more sensitive in isolating EPF than culturing soil suspensions on selective medium [61,62,95,96]. Other studies have also used insect baiting along with soil suspension cultures [57,97,98,99,100]. Although insect baiting is a widely accepted method for EPF isolation, it should be used with caution as some lines of insect baits, such as the dark (melanic) morphs of
) has also been used as a bait insect in some studies [68][69][70]. Some previous studies have noticed that insect baiting is more sensitive in isolating EPF than culturing soil suspensions on selective medium [37][38][71][72]. Other studies have also used insect baiting along with soil suspension cultures [33][73][74][75][76]. Although insect baiting is a widely accepted method for EPF isolation, it should be used with caution as some lines of insect baits, such as the dark (melanic) morphs of
G. mellonella
, are more resistant to
B. bassiana
s.l., and this trait has also been observed in
T. molitor
for
s.l. [77][78]. Similarly, immune-suppressed
G. mellonella were found to be highly (~200 times) susceptible to EPF, which can lead to the isolation of a diverse set of EPF from soils, although saprophytic fungi may not induce any insect mortality [103].
were found to be highly (~200 times) susceptible to EPF, which can lead to the isolation of a diverse set of EPF from soils, although saprophytic fungi may not induce any insect mortality [79].
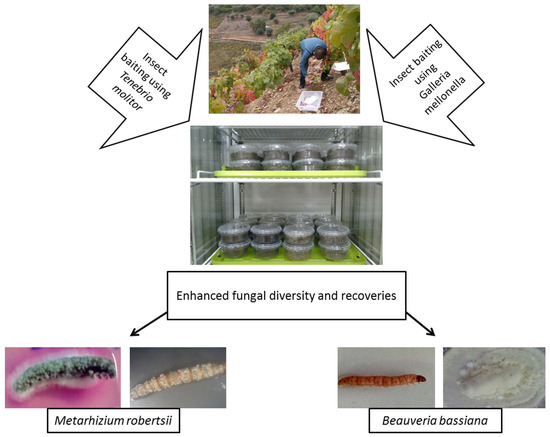
Galleria-Tenebrio
-Bait Method
As bait insects can be sensitive to infection by one particular EPF genus, some studies have used both
G. mellonella
and
T. molitor to isolate EPF, either in part or throughout their whole experiment [7,104,105,106,107]. Recently, Sharma et al. [7] suggested using the “
to isolate EPF, either in part or throughout their whole experiment [20][80][81][82][83]. Recently, Sharma et al. [20] suggested using the “
Galleria-Tenebrio
-bait method” to avoid any underestimation of EPF abundance and diversity, as it was found that
G. mellonella
and
T. molitor
were significantly more sensitive toward the infections by
B. bassiana
s.l. and
M. robertsii,
respectively. This method is described in
.
2.3. Isolation from Phyllosphere
S
Some studies have also isolated EPF from the phylloplane and other parts of the plant phyllosphere, as these fungi can also be present as plant epiphytes or endophytes [84]. Meyling et al. suggested a leaf imprinting methodology where the leaf is cultured onto a selective agar medium [40]. Petri dishes with partitions are used and the upper (adaxial), and the lower (abaxial) surface of the leaf are pressed on the separate sides of the Petri plate. Henceforth, the same leaf is put on a paper sheet and photocopied to estimate its surface area using image analysis software at a later stage. The petri plates are incubated in the dark at 23 °C to count fungal colony-forming units (CFUs) [40]. Surface sterilization is quite important in isolating hypocrealean fungi as endophytes. This can be done by dipping the plant part in either 70% ethanol and/or 1–5% NaOCl for 3 min. In the case of the leaves, the petiole can be first kept out of the sanitizer to avoid the chemical reaching inside the leaf, and then it can be cut to culture the sterilized part of the leaf on either of the selective mediums described above. It is always recommended to sanitize the intact plant part and then cut it into pieces for further culturing, as this avoids the sterilization of the endophytic fungi [85]. Different studies have isolated EPF from the phyllosphere, such as bark and branch samples [32][86] and leaves [35][87].
2.4. Molecular Identifications of the Isolated Entomopathogenic Fungi
After obtaining a single spore fungal culture on a PDA or SDA (Appendix A; Medium 8 and/or 9), the species can be resolved or identified by amplifying the regions of nuclear ribosomal DNA, such as nrITS, large (28S) subunit (nrLSU), or small (18S) subunit (nrSSU). Another, nuclear ribosomal DNA region, i.e., the intergenic spacer region between nrSSU and nrLSU or IGS, has also been used to understand Beauveria and Metarhizium speciation [87][88][89][90]. The resolution of the molecular identification can be increased by amplifying other nuclear DNA regions of interest, e.g., for Bloc for Beauveria [87][88][89] and the 5′ intron-containing region of translation elongation factor 1-alpha subunit (5′-tef1α) for Metarhizium [90][91]. Other nuclear DNA markers, such as the regions of the gene encoding for the largest subunit of RNA polymerase II (rpb1), the second largest subunit of RNA polymerase II (rpb2); β-tublin (β-tub), and the coding region of Tef1-α, can also be employed, in general, for any EPF [92][93].
Moreover, in the last decades, researchers have been constantly developing and validating the use of several microsatellite markers for the genotyping of Beauveria [69][89][94][95][96][97] and Metarhizium [98][99] isolates. For example, Oulevey et al. [99] described 18 small single repeats or microsatellite marker sets for Metarhizium, i.e., Ma145, Ma325, Ma307, Ma2049, Ma2054, Ma2055, Ma2056, Ma2057, Ma2060, Ma2063, Ma2069, Ma2070, Ma2077, Ma2089, Ma2283, Ma2287, Ma2292, and Ma2296. Similarly, Meyling et al. [69] and Goble et al. [97] validated the use of 17 to 18 microsatellite marker sets for Beauveria, i.e., Ba06, Ba08, and Ba12-Ba29. This methodology enables enhanced resolution among very closely related isolates which may otherwise be rendered as clones. Recently, Kepler and Rehner [93] developed primers for the amplification and sequencing of nuclear intergenic spacer markers for the resolution of Metarhizium isolates, i.e., BTIGS, MzFG543, MzFG546, MzIGS2, MzIGS3, MzIGS5, and MzIGS7, and Kepler et al. [75] successfully validated the use of MzIGS3 and MzFG543 on the Metarhizium isolated from agricultural soils.
Appendix A
Come studies have also isolated EPF from the phylloplane and other parts of the plant phyllosphere, as these fungi can also be present as plant epiphytes or endophytes [41]. Meylon culture medingum et al. suggested a leaf imprinting methodology where the leaf is cultured onto a selective agar medium [64]. Peused for the isolatri dishes with partitions are used and the upper (adaxial), and the lower (abaxial) surface of the leaf are pressed on the separate sides of the Petri plate. Henceforth, the same leaf is put on a paper sheet and photocopied to estimate its surface area using image analysis software at a later stageon of entomopathogenic bacteria.
- (1) Caprylate-thallous agar (CTA).
The petri plates are incubated in the dark at 23 °C to count fungal colony-forming units (CFUs) [64]. Surface sterilization is quite is medium is mportant in isolating hypocrealean fungi as endophytes. This can be done by dipping the plant part in either 70% ethanol and/or 1–5% NaOCl for 3 min. In the case of the leaves, the petiole can be first kept out of the sanitizer to avoid the chemical reaching inside the leaf, and then it can be cut to culture the sterilized part of the leaf on either of the selectivede by mixing two solutions, i.e., A and B. Both these mediums described above. It is always recommended to sanitize the intact plant part and then cut it into pieces for further culturing, as this avoids the sterilization of the endophytic fungi [111]. Different stu should be autoclaved separately andies have isolated EPF from the phyllosphere, such as bark and branch samples [56,112] anadded leaves [59,113].
2.4. Molecular Identifications of the Isolated Entomopathogenic Fungi
Aftser obptaining a single spically.
-
(1a) Solution A
|
Reagents and Chemicals |
Chemical Formula (If Applicable) |
Quantity |
|
Monopotassium phosphate |
KH2PO4 |
0.68 g |
|
Magnesium sulfate heptahydrate |
MgSO4.7H2O |
0.3 g |
|
Dipotassium phosphate |
K2HPO4 |
0.15 g |
|
Thallium(I) sulphate |
Tl2SO4 |
0.25 g |
|
Yeast Extract |
1 g |
|
|
Calcium chloride |
CaCl2 |
0.1 g |
|
Caprylic (n-octanoic) acid |
CH3(CH2)6.COOH |
1.1 mL |
|
Trace element solution |
10 mL |
|
|
Distilled water |
H2O |
1 L |
Norte fungal culture on a PDA or SDA (Appendix A; Med: Thallium 8 and/or 9(I), as described in the Section 3.1, sulphathe species can be resolved or identified by amplifying the regions of nuclear ribosomal DNA, such as nrITS, large (28S) sis extremely toxic so it shoubunit (nrLSU), or smalld (18S) subunit (nrSSU). Anothber, nuclear ribosomal DNA region, i.e., the intergenic spacer region between nrSSU and nrLSU or IGS,used with caution. The haspH also been usshould be adjusted to understand Beauveria and Metarhizium speciation [113,114,115,116]7.2 The resolution of the molecular identification can beeither by increased by amplifying other nuclear DNA regions of interest, e.g., fing it using K2HPO4 or Bloc for Beauveria [113,114,115] and the 5′ intron-containing region of translation elongation fcreasing it is using KH2PO4.
Tractore 1-alpha subunit (5′-tef1α) for Metarhizium [116,117]. Other nuclear DNA markers, such as the regment solutions
| Reagents and Chemicals | Chemical Formula (If Applicable) | Quantity |
| Ferrous sulphate heptahydrate | FeSO4.7H2O | 0.055 g |
| Trihydrogen phosphate | H3PO4 | 1.96 g |
| Zinc sulphate heptahydrate | ZnSO4.7H2O | 0.0287 g |
| Manganese(II) sulphate monohydrate | MnSO4.H2O | 0.0223 g |
| Copper(II) sulphate pentahydrate | CuSO4.5H2O | 0.0025 g |
| Cobalt(II) nitrate hexahydrate | Co(NO3)2.6H2O | 0.003 g |
| Boric acid | H3BO3 | 0.0062 g |
| Distilled water | H2O | 1 L |
Nof the gene encoding for the largest subunit of RNA polymerase II (rpb1), the: Once made the trace ele smecond largest subunit of RNA polymerase II (rpb2); β-tnt solublin (β-tub), and the coding region of Tef1-α, on can also be employed, in general, for any EPF [118,119].
Mkept for moreover, in the last decades, researchers have been cths at 4 °C.
-
(1b) Solution B
|
Reagents and Chemicals |
Chemical Formula (If Applicable) |
Quantity |
|
Ammonium sulphate |
(NH4)2SO4 |
1.0 g |
|
Sodium chloride |
NaCl |
7.0 g |
|
Agar |
15 g | |
| Distilled water | H2O | 1 L |
Nonstantly developing and validating the use of several microsatellite markers for the genotyping of Beauveria [93,115,120,121,122,123]e: Adjust the pH to 7.3–7.5 and Metarhizium [124,125] isautolatesclave. For example, Oulevey et al. [125] plate culture, adescribed 18 small single repeats or microsatellite marker sets for Metarhizium, i.e., Ma145, Ma325, Ma307, Ma2049, Ma2054, Ma2055, Ma2056, Ma2057, Ma2060, Ma2063, Ma2069, Ma2070, Ma2077, Ma2089, Ma2283, Ma2287, Ma2292, and Ma2296. Simi20 g agar. Add glucose after autoclarly, Meylving et al. [93] and G
- (4) Mueller-Hinton broth, yeast extract, potassium phosphate, glucose and pyruvate (MYPGP) medium.
use of 17 to 18 microsatellite marker sets for Beauveria,t the pH to i7.e., Ba06, Ba08, and Ba12-Ba29. This methodology enables enhanced resolution among very closely related isolates which may otherwise be rendered as clones. Recently, Kepler and Rehner [119] 1 and autoclave. For plate culture, add 20 g agar. Adeveloped primers for the amplification and sequencing of nuclear intergenic spacer markers for the resolution of glucose after autoclaving.
- (5) Adonitol agar.
Note: Adjust the pH to 7.4 before adding bromothymol blue solution.
Bromothymol blue solution
|
Reagents and Chemicals |
Chemical Formula (If Applicable) |
Quantity |
This medium is easily available as powder and sold by the majority of the culture media suppliers.
|
Reagents and Chemicals |
Chemical Formula (If Applicable) |
Quantity |
Bromothymol blue |
C27H28Br2O5S |
|
Dichloran Rose-Bengal Chloramphenicol agar |
0.2 g |
|||
|
32.0 g |
Sodium hydroxide (0.1M) |
NaOH |
||
|
Distilled water |
H2O 5 mL |
|||
Distilled water |
H2O |
1 L |
- (2) Deoxyribonuclease (DNase)-Toluidine Blue agar.
1 L |
|
Reagents and Chemicals |
Chemical Formula (If Applicable) |
Quantity |
|
Deoxyribonuclease test agar |
37.8 g |
|
|
Toluidine blue 0.1% w/v solution |
NaCl |
90.0 ml |
|
L-arabinose |
C5H10O5 |
10.0 g |
|
Distilled water |
H2O |
900 mL |
- (3) St. Julian medium (J-medium).
| Reagents and Chemicals | Chemical Formula (If Applicable) | Quantity | |||
| Yeast extract | 15 g | ||||
| Tryptone | 5 g | ||||
| Dipotassium phosphate | K2HPO4 | 3 g | |||
| Glucose (sterilized by filtration) | C6H12O6 | 2.0 g | Reagents and Chemicals | Chemical Formula (If Applicable) | Quantity |
| Dipotassium phosphate | K2HPO4 | 3.0 g | |||
| Sodium pyruvate | C3H3O3Na | 1.0 g | |||
| Mueller-Hinton broth | 10.0 g | ||||
| Glucose (sterilized by filtration) | C6H12O6 | 2.0 g | |||
| Yeast Extract | 10.0 g | ||||
| Distilled water | 1 L |
Noblte et al. [123] vali: Adated the j
Distilled water |
H | 2O |
900 mL |
| Reagents and Chemicals | Chemical Formula (If Applicable) | Quantity |
| Sodium chloride | NaCl | 4.17 g |
| Adonitol | C5H12O5 | 5.0 g |
| Peptone | 8.33 g | |
| Bacto agar | 12.5 g | |
| Bromothymol blue solution | C27H28Br2O5S | 10 mL |
| Distilled water | H2O | 990 mL |
- (6) Itaconate agar.
| Reagents and Chemicals | Chemical Formula (If Applicable) | Quantity |
| Monopotassium phosphate | KH2PO4 | 3.0 g |
| Disodium phosphate | Na2HPO4 | 6.0 g |
| Sodium chloride | NaCl | 0.5 g |
| Ammonium chloride | NH4Cl | 1.0 g |
| Calcium chloride solution (sterilised) (0.01M) | CaCl2 | 10.0 mL |
| Magnesium sulfate heptahydrate (sterilised) (1M) | MgSO4.7H2O | 1.0 mL |
| Itaconic acid solution (filter sterilised) (20%) | C5H6O4 | 10 mL |
| Distilled water | H2O | 1 L |
Note: Adjust the pH to 7.0 before autoclaving.
- (7) Minimal Basal Salt (MBS) medium.
| Reagents and Chemicals | Chemical Formula (If Applicable) | Quantity |
| Monopotassium phosphate | KH2PO4 | 6.8 g |
| Magnesium sulfate heptahydrate | MgSO4.7H2O | 0.3 g |
| Manganese monohydrate sulphate | MnSO4.1H2O | 0.02 g |
| Ferric sulfate | Fe2(SO4)3 | 0.02 g |
| Zinc sulfate heptahydrate | ZnSO4.7H2O | 0.02 g |
| Calcium chloride | CaCl2 | 0.2 g |
| Tryptone | 10 g | |
| Yeast Extract | 2 g |
Note: Adjust the pH to 7.2 before autoclaving.
Common culture medium used for the isolation of entomopathogenic fungi.
- (8) Potato Dextrose agar (PDA)
| Reagents and Chemicals | Chemical formula (If Applicable) | Quantity |
| Potato dextrose agar | 39.0 g | |
| Distilled water | H2O | 1 L |
- (9) Sabouraud Dextrose agar (SDA)
| Reagents and Chemicals | Chemical Formula (if Applicable) | Quantity |
| Sabouraud dextrose agar | 65.0 g | |
| Distilled water | H2O | 1 L |
- (10) Oatmeal Cetyl Trimethyl Ammonium Bromide (OM-CTAB) agar.
| Reagents and Chemicals | Chemical Formula (If Applicable) | Quantity |
| Oatmeal (cooked in distilled water) | 20.0 g | |
| Cetyl trimethyl ammonium bromide (CTAB) | C19H42BrN | 0.6 g |
| Chloramphenicol | C11H12Cl2N2O5 | 0.5 g |
| Agar | 20 g | |
| Distilled water | H2O | To make upto 1L |
- (11) Dichloran Rose-Bengal Chloramphenicol agar (DRBCA).
- (12)
Metarhizium is
- Medium
| Reagents and Chemicals | Chemical Formula (If Applicable) | Quantity |
| Glucose | C6H12O6 | 10.0 g |
| Peptone | 10.0 g | |
| Oxgall | 15.0 g | |
| Agar | 35.0 g | |
| Dodine (N-dodecylguanidine monoacetate) | C15H33N3O2 | 10 mg |
| Cycloheximide | C15H23NO4 | 250 mg |
| Chloramphenicol | C11H12Cl2N2O5 | 500 mg |
| Distilled water | H2O | 1 L |
Note: Cyclohexamide is quite toxic and caution is needed while handling.
- (13) Chloramphenicol Thiabendazole Cycloheximide (CTC) medium.
| Reagents and Chemicals | Chemical Formula (If Applicable) | Quantity |
| Potato dextrose agar | 39.0 g | |
| Yeast extract | 0.5 g | |
| Chloramphenicol | C11H12Cl2N2O5 | 500 mg |
| Thiabendazole | C10H7N3S | 1 mg |
| Cycloheximide | C15H23NO4 | 250 mg |
| Distilled water | H2O | 1 L |
- (14) Oatmeal Dodine agar (ODA).
| Reagents and Chemicals | Chemical Formula (If Applicable) | Quantity |
| Oatmeal infusion | 20.0 g | |
| Dodine (N-dodecylguanidine monoacetate) | C15H33N3O2 | 550 mg |
| Chlortetracycline | C22H23ClN2O8 | 5 mg |
| Crystal violet | C25N3H30Cl | 10 mg |
| Agar | 20.0 g | |
| Distilled water | H2O | 1 L |
- (15) Sabouraud-2-Glucose agar (S2GA).
| Reagents and Chemicals | Chemical Formula (If Applicable) | Quantity |
| Glucose | C6H12O6 | 20.0 g |
| Peptone | 10.0 g | |
| Streptomycin sulphate | C42H84N14O36S3 | 600 mg |
| Tetracycline | C22H24N2O8 | 50 mg |
| Cycloheximide | C15H23NO4 | 50 mg |
| Dodine (N-dodecylguanidine monoacetate) | C15H33N3O2 | 100 mg |
| Agar | 12.0 g | |
| Distilled water | H2O | 1 L |
- (16) Purpureocillium lilacinum medium.
| Reagents and Chemicals | Chemical formula (If Applicable) | Quantity |
| Potato dextrose agar | 39.0 g | |
| Sodium chloride | NaCl | 10–30 g |
| Tergitol | 1 g | |
| Pentachloronitrobenzene | C6Cl5NO2 | 500 mg |
| Benomyl | C14H18N4O3 | 500 mg |
| Streptomycin sulphate | C42H84N14O36S3 | 100 mg |
| Chlortetracycline hydrochloride | C22H24Cl2N2O8 | 50 mg |
| Distilled water | H2O | 1 L |
- (17) Lecanicillium-specific medium.
| Reagents and Chemicals | Chemical Formula (If Applicable) | Quantity |
| L-sorbose | C6H12O6 | 2 g |
| L-asparagine | C4H8N2O3 | 2 g |
| Dipotassium phosphate | K2HPO4 | 1 g |
| Potassium chloride | KCl | 1 g |
| Magnesium sulfate heptahydrate | MgSO4.7H2O | 0.5 g |
| Ferric-sodium salt (FeNaEDTA) | C10H12N2O8FeNa | 0.01 g |
| Agar | 20 g | |
| Streptomycin sulphate | C42H84N14O36S3 | 0.3 g |
| Chlortetracycline hydrochloride | C22H24Cl2N2O8 | 0.05 g |
| Pentachloronitrobenzene | C6Cl5NO2 | 0.8 g |
| Borax | NaB4O7.10H2O | 1 g |
| Distilled water | 1 L |
Nolates, i.e., BTIGS, MzFG543, MzFG546, MzIGS2, MzIGS3, MzIGS5, and MzIGS7, and Kepler et al. [99] s: Adjust the pH to 4.0 uccessfully validated the use of MzIGS3 and MzFG543 on the Metarhizium iing 10% trihydrogen phosolphated (H3PO4) befrom agricultural soilsore autoclaving.
References
- Ruiu, L. Insect Pathogenic Bacteria in Integrated Pest Management. Insects 2015, 6, 352.
- Godjo, A.; Afouda, L.; Baimey, H.; Decraemer, W.; Willems, A. Molecular diversity of Photorhabdus and Xenorhabdus bacteria, symbionts of Heterorhabditis and Steinernema nematodes retrieved from soil in Benin. Arch. Microbiol. 2018, 200, 589–601.
- Hurst, M.R.H.; Becher, S.A.; Young, S.D.; Nelson, T.L.; Glare, T.R. Yersinia entomophaga sp. nov., isolated from the New Zealand grass grub Costelytra zealandica. Int. J. Syst. Evol. Microbiol. 2011, 61, 844–849.
- Vodovar, N.; Vinals, M.; Liehl, P.; Basset, A.; Degrouard, J.; Spellman, P.; Boccard, F.; Lemaitre, B. Drosophila host defense after oral infection by an entomopathogenic Pseudomonas species. Proc. Natl. Acad. Sci. USA 2005, 102, 11414–11419.
- Martin, P.A.W.; Hirose, E.; Aldrich, J.R. Toxicity of Chromobacterium subtsugae to southern green stink bug (Heteroptera: Pentatomidae) and corn rootworm (Coleoptera: Chrysomelidae). J. Econ. Entomol. 2007, 100, 680–684.
- Yurkov, A.; Guerreiro, M.A.; Sharma, L.; Carvalho, C.; Fonseca, Á. Correction: Multigene assessment of the species boundaries and sexual status of the basidiomycetous yeasts Cryptococcus flavescens and C. terrestris (Tremellales). PLoS ONE 2015, 10, e0126996.
- Veen, K.H.; Ferron, P. A selective medium for the isolation of Beauveria tenella and of Metarrhizium anisopliae. J. Invertebr. Pathol. 1966, 8, 268–269.
- Chase, A.R.; Osborne, L.S.; Ferguson, V.M. Selective isolation of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae from an artificial potting medium. Fla. Entomol. 1986, 69, 285–292.
- St. Julian, G.J.; Pridham, T.G.; Hall, H.H. Effect of diluents on viability of Popillia japonica Newman larvae, Bacillus popilliae Dutky, and Bacillus lentimorbus Dutky. J. Invertebr. Pathol. 1963, 5, 440–450.
- Dingman, D.W.; Stahly, D.P. Medium Promoting Sporulation of Bacillus larvae and Metabolism of Medium Components. Appl. Environ. Microbiol. 1983, 46, 860–869.
- Krieger, L.; Franken, E.; Schnetter, W. Bacillus popilliae var melolontha H1, a pathogen for the May beetles, Melolontha spp. In Proceedings of the 3rd International Workshop on Microbial Control of Soil Dwelling Pests, Lincoln, New Zealand, 21–23 February 1996; Jackson, T.A., Glare, T.R., Eds.; AgResearch: Lincoln, New Zealand, 1996; pp. 79–87.
- O’Callaghan, M.; Jackson, T.A. Isolation and enumeration of Serratia entomophila—a bacterial pathogen of the New Zealand grass grub, Costelytra zealandica. J. Appl. Bacteriol. 1993, 75, 307–314.
- Starr, M.P.; Grimont, P.A.; Grimont, F.; Starr, P.B. Caprylate-thallous agar medium for selectively isolating Serratia and its utility in the clinical laboratory. J. Clin. Microbiol. 1976, 4, 270.
- Berkowitz, D.M.; Lee, W.S. A selective medium for the isolation and identification of Serratia marcescens. In Abstracts of the Annual Meeting of the American Society for Microbiology; American Society for Microbiology: Washington, DC, USA, 1973; Volume 105.
- Koppenhöfer, A.M.; Jackson, T.; Klein, M.G. Bacteria for Use Against Soil-Inhabiting Insects; Academic Press: San Diego, CA, USA, 2012; pp. 129–149.
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as Biological Control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41.
- Sharma, L.; Gonçalves, F.; Oliveira, I.; Torres, L.; Marques, G. Insect-associated fungi from naturally mycosed vine mealybug Planococcus ficus (Signoret) (Hemiptera: Pseudococcidae). Biocontrol Sci. Technol. 2018, 28, 122–141.
- Sharma, L.; Marques, G. Fusarium, an Entomopathogen—A Myth or Reality? Pathogens 2018, 7, 93.
- Sharma, L.; Oliveira, I.; Raimundo, F.; Torres, L.; Marques, G. Soil chemical properties barely perturb the abundance of entomopathogenic Fusarium oxysporum: A case study using a generalized linear mixed model for microbial pathogen occurrence count data. Pathogens 2018, 7, 89.
- Sharma, L.; Oliveira, I.; Torres, L.; Marques, G. Entomopathogenic fungi in Portuguese vineyards soils: Suggesting a ‘Galleria-Tenebrio-bait method’ as bait-insects Galleria and Tenebrio significantly underestimate the respective recoveries of Metarhizium (robertsii) and Beauveria (bassiana). MycoKeys 2018, 38, 1–23.
- Sharma, L.; Bohra, N.; Singh, R.K.; Marques, G. Potential of Entomopathogenic Bacteria and Fungi. In Microbes for Sustainable Insect Pest Management: An Eco-friendly Approach—Volume 1; Khan, M.A., Ahmad, W., Eds.; Springer: Cham, Switzerland, 2019; pp. 115–149.
- Meza-Menchaca, T.; Singh, R.K.; Quiroz-Chávez, J.; García-Pérez, L.M.; Rodríguez-Mora, N.; Soto-Luna, M.; Gastélum-Contreras, G.; Vanzzini-Zago, V.; Sharma, L.; Quiroz-Figueroa, F.R. First demonstration of clinical Fusarium strains causing cross-kingdom infections from humans to plants. Microorganisms 2020, 8, 947.
- Carlos, C.G.F.; Sousa, S.; Salvação, J.; Sharma, L.; Soares, R.; Manso, J.; Nóbrega, M.; Lopes, A.; Soares, S.; Aranha, J.; et al. Environmentally safe strategies to control the European Grapevine Moth, Lobesia botrana (Den. & Schiff.) in the Douro Demarcated Region. Cienc. Tec. Vitivinic. 2013, 28, 1006–1011.
- Domsch, K.H.; Gams, W.; Anderson, T.H. Compendium of Soil Fungi, 2nd ed.; IHW-Verlag and Verlagsbuchhandlung: Eching, Germany, 2007.
- Humber, R.A. Chapter VI—Identification of entomopathogenic fungi. In Manual of Techniques in Invertebrate Pathology, 2nd Ed.; Lacey, L.A., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 151–187.
- Sneh, B. Isolation of Metarhizium anisopliae from insects on an improved selective medium based on wheat germ. J. Invertebr. Pathol. 1991, 58, 269–273.
- Liu, Z.Y.; Milner, R.J.; McRae, C.F.; Lutton, G.G. The use of dodine in selective media for the isolation of Metarhizium spp. from soil. J. Invertebr. Pathol. 1993, 62, 248–251.
- Rangel, D.E.N.; Dettenmaier, S.J.; Fernandes, É.K.K.; Roberts, D.W. Susceptibility of Metarhizium spp. and other entomopathogenic fungi to dodine-based selective media. Biocontrol. Sci. Technol. 2010, 20, 375–389.
- Fernandes, É.K.K.; Keyser, C.A.; Rangel, D.E.N.; Foster, R.N.; Roberts, D.W. CTC medium: A novel dodine-free selective medium for isolating entomopathogenic fungi, especially Metarhizium acridum, from soil. Biol. Control. 2010, 54, 197–205.
- Hernández-Domínguez, C.; Cerroblanco-Baxcajay, M.d.L.; Alvarado-Aragón, L.U.; Hernández-López, G.; Guzmán-Franco, A.W. Comparison of the relative efficacy of an insect baiting method and selective media for diversity studies of Metarhizium species in the soil. Biocontrol. Sci. Technol. 2016, 26, 707–717.
- Posadas, J.B.; Comerio, R.M.; Mini, J.I.; Nussenbaum, A.L.; Lecuona, R.E. A novel dodine-free selective medium based on the use of cetyl trimethyl ammonium bromide (CTAB) to isolate Beauveria bassiana, Metarhizium anisopliae sensu lato and Paecilomyces lilacinus from soil. Mycologia 2012, 104, 974–980.
- Ormond, E.L.; Thomas, A.P.; Pugh, P.J.; Pell, J.K.; Roy, H.E. A fungal pathogen in time and space: The population dynamics of Beauveria bassiana in a conifer forest. FEMS Microbiol. Ecol. 2010, 74, 146–154.
- Clifton, E.H.; Jaronski, S.T.; Hodgson, E.W.; Gassmann, A.J. Abundance of soil-borne entomopathogenic fungi in organic and conventional fields in the midwestern usa with an emphasis on the effect of herbicides and fungicides on fungal persistence. PLoS ONE 2015, 10, e0133613.
- Garrido-Jurado, I.; Fernandez-Bravo, M.; Campos, C.; Quesada-Moraga, E. Diversity of entomopathogenic Hypocreales in soil and phylloplanes of five Mediterranean cropping systems. J. Invertebr. Pathol. 2015, 130, 97–106.
- Clifton, E.H.; Jaronski, S.T.; Coates, B.S.; Hodgson, E.W.; Gassmann, A.J. Effects of endophytic entomopathogenic fungi on soybean aphid and identification of Metarhizium isolates from agricultural fields. PLoS ONE 2018, 13, e0194815.
- Strasser, H.; Forer, A.; Schinner, F. Development of media for the selective isolation and maintenance of Beauveria brongniartii. In Microbial Control of Soil Dwelling Pests; Jackson, T.A., Glare, T.R., Eds.; AgResearch: Lincoln, New Zealand, 1996; pp. 125–130.
- Keller, S.; Kessler, P.; Schweizer, C. Distribution of insect pathogenic soil fungi in Switzerland with special reference to Beauveria brongniartii and Metharhizium anisopliae. BioControl 2003, 48, 307–319.
- Enkerli, J.; Widmer, F.; Keller, S. Long-term field persistence of Beauveria brongniartii strains applied as biocontrol agents against European cockchafer larvae in Switzerland. Biol. Control. 2004, 29, 115–123.
- Kessler, P.; Enkerl, J.; Schweize, C.; Keller, S. Survival of Beauveria brongniartii in the soil after application as a biocontrol agent against the European cockchafer Melolontha melolontha. BioControl 2004, 49, 563–581.
- Meyling, N.V.; Eilenberg, J. Isolation and characterisation of Beauveria bassiana isolates from phylloplanes of hedgerow vegetation. Mycol. Res. 2006, 110, 188–195.
- Świergiel, W.; Meyling, N.V.; Porcel, M.; Rämert, B. Soil application of Beauveria bassiana GHA against apple sawfly, Hoplocampa testudinea (Hymenoptera: Tenthredinidae): Field mortality and fungal persistence. Insect. Sci. 2016, 23, 854–868.
- Ramírez-Rodríguez, D.; Sánchez-Peña, S.R. Recovery of endophytic Beauveria bassiana on a culture medium based on cetyltrimethylammonium bromide. Biocontrol. Sci. Technol. 2016, 26, 570–575.
- Mitchell, D.J.; Kannwischer-Mitchell, M.E.; Dickson, D.W. A semi-selective medium for the isolation of Paecilomyces lilacinus from soil. J. Nematol. 1987, 19, 255–256.
- Goettel, M.S.; Inglis, G.D. Chapter V-3—Fungi: Hyphomycetes. In Manual of Techniques in Insect Pathology; Lacey, L.A., Ed.; Academic Press: London, UK, 1997; pp. 213–249.
- Kope, H.; Alfaro, R.; Lavallee, R. Virulence of the entomopathogenic fungus Lecanicillium (Deuteromycota: Hyphomycetes) to Pissodes strobi (Coleoptera: Curculionidae). Can. Entomol. 2006, 138, 253–262.
- Xie, M.; Zhang, Y.-J.; Peng, D.-L.; Zhou, J.; Zhang, X.-L.; Zhang, Z.-R.; Zhao, J.-J.; Wu, Y.-H. Persistence and Viability of Lecanicillium lecanii in Chinese Agricultural Soil. PLoS ONE 2015, 10, e0138337.
- Scheepmaker, J.W.A.; Butt, T.M. Natural and released inoculum levels of entomopathogenic fungal biocontrol agents in soil in relation to risk assessment and in accordance with EU regulations. Biocontrol. Sci. Technol. 2010, 20, 503–552.
- Vega, F.E.; Meyling, N.V.; Luangsa-ard, J.J.; Blackwell, M. Fungal Entomopathogens. In Insect Pathology, 2nd ed.; Vega, F.E., Kaya, H.K., Eds.; Academic Press Elsevier Inc.: San Diego, CA, USA, 2012; pp. 171–220.
- Zimmermann, G. The ‘Galleria bait method’ for detection of entomopathogenic fungi in soil. J. Appl. Entomol. 1986, 102, 213–215.
- Chandler, D.; Hay, D.; Reid, A.P. Sampling and occurrence of entomopathogenic fungi and nematodes in UK soils. Appl. Soil Ecol. 1997, 5, 133–141.
- Barker, C.W.; Barker, G.M. Generalist entomopathogens as biological indicators of deforestation and agricultural land use impacts on Waikato soils. N. Zeal. J. Ecol. 1998, 22, 189–196.
- Bidochka, M.J.; Kasperski, J.E.; Wild, G.A.M. Occurrence of the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana in soils from temperate and near-northern habitats. Can. J. Bot. 1998, 76, 1198–1204.
- Hummel, R.L.; Walgenbach, J.F.; Barbercheck, M.E.; Kennedy, G.G.; Hoyt, G.D.; Arellano, C. Effects of production practices on soil-borne entomopathogens in Western North Carolina vegetable systems. Environ. Entomol. 2002, 31, 84–91.
- Ali-Shtayeh, M.S.; Mara’i, A.-B.B.M.; Jamous, R.M. Distribution, occurrence and characterization of entomopathogenic fungi in agricultural soil in the Palestinian area. Mycopathologia 2003, 156, 235–244.
- Asensio, L.; Carbonell, T.; Lopez Jimenez, J.; López Llorca, L. Entomopathogenic fungi in soils from Alicante province. Span. J. Agric. Res. 2003, 1, 37–45.
- Meyling, N.V.; Eilenberg, J. Occurrence and distribution of soil borne entomopathogenic fungi within a single organic agroecosystem. Agric. Ecosyst. Environ. 2006, 113, 336–341.
- Quesada-Moraga, E.; Navas-Cortés, J.A.; Maranhao, E.A.A.; Ortiz-Urquiza, A.; Santiago-Álvarez, C. Factors affecting the occurrence and distribution of entomopathogenic fungi in natural and cultivated soils. Mycol. Res. 2007, 111, 947–966.
- Sun, B.-D.; Yu, H.-y.; Chen, A.J.; Liu, X.-Z. Insect-associated fungi in soils of field crops and orchards. Crop. Protect. 2008, 27, 1421–1426.
- Jabbour, R.; Barbercheck, M.E. Soil management effects on entomopathogenic fungi during the transition to organic agriculture in a feed grain rotation. Biol. Control. 2009, 51, 435–443.
- Sevim, A.; Demir, I.; Höfte, M.; Humber, R.A.; Demirbag, Z. Isolation and characterization of entomopathogenic fungi from hazelnut-growing region of Turkey. BioControl 2009, 55, 279–297.
- Fisher, J.J.; Rehner, S.A.; Bruck, D.J. Diversity of rhizosphere associated entomopathogenic fungi of perennial herbs, shrubs and coniferous trees. J. Invertebr. Pathol. 2011, 106, 289–295.
- Muñiz-Reyes, E.; Guzmán-Franco, A.W.; Sánchez-Escudero, J.; Nieto-Angel, R. Occurrence of entomopathogenic fungi in tejocote (Crataegus mexicana) orchard soils and their pathogenicity against Rhagoletis pomonella. J. Appl. Microbiol. 2014, 117, 1450–1462.
- Pérez-González, V.H.; Guzmán-Franco, A.W.; Alatorre-Rosas, R.; Hernández-López, J.; Hernández-López, A.; Carrillo-Benítez, M.G.; Baverstock, J. Specific diversity of the entomopathogenic fungi Beauveria and Metarhizium in Mexican agricultural soils. J. Invertebr. Pathol. 2014, 119, 54–61.
- Medo, J.; Michalko, J.; Medová, J.; Cagáň, Ľ. Phylogenetic structure and habitat associations of Beauveria species isolated from soils in Slovakia. J. Invertebr. Pathol. 2016, 140, 46–50.
- Fernández-Salas, A.; Alonso-Díaz, M.A.; Alonso-Morales, R.A.; Lezama-Gutiérrez, R.; Rodríguez-Rodríguez, J.C.; Cervantes-Chávez, J.A. Acaricidal activity of Metarhizium anisopliae isolated from paddocks in the Mexican tropics against two populations of the cattle tick Rhipicephalus microplus. Med. Vet. Entomol. 2017, 31, 36–43.
- Gan, H.; Wickings, K. Soil ecological responses to pest management in golf turf vary with management intensity, pesticide identity, and application program. Agric. Ecosyst. Environ. 2017, 246, 66–77.
- Kirubakaran, S.A.; Abdel-Megeed, A.; Senthil-Nathan, S. Virulence of selected indigenous Metarhizium pingshaense (Ascomycota: Hypocreales) isolates against the rice leaffolder, Cnaphalocrocis medinalis (Guenèe) (Lepidoptera: Pyralidae). Physiol. Mol. Plant. Pathol. 2018, 101, 105–115.
- Sánchez-Peña, S.R.; Lara, J.S.-J.; Medina, R.F. Occurrence of entomopathogenic fungi from agricultural and natural ecosystems in Saltillo, México, and their virulence towards thrips and whiteflies. J. Insect Sci. 2011, 11, 1–10.
- Steinwender, B.M.; Enkerli, J.; Widmer, F.; Eilenberg, J.; Thorup-Kristensen, K.; Meyling, N.V. Molecular diversity of the entomopathogenic fungal Metarhizium community within an agroecosystem. J. Invertebr. Pathol. 2014, 123, 6–12.
- Aguilera Sammaritano, J.A.; López Lastra, C.C.; Leclerque, A.; Vazquez, F.; Toro, M.E.; D’Alessandro, C.P.; Cuthbertson, A.G.S.; Lechner, B.E. Control of Bemisia tabaci by entomopathogenic fungi isolated from arid soils in Argentina. Biocontrol. Sci. Technol. 2016, 26, 1668–1682.
- Imoulan, A.; Alaoui, A.; El Meziane, A. Natural occurrence of soil-borne entomopathogenic fungi in the moroccan endemic forest of Argania spinosa and their pathogenicity to Ceratitis capitata. World J. Microbiol. Biotechnol. 2011, 27, 2619–2628.
- Keyser, C.A.; De Fine Licht, H.H.; Steinwender, B.M.; Meyling, N.V. Diversity within the entomopathogenic fungal species Metarhizium flavoviride associated with agricultural crops in Denmark. BMC Microbiol. 2015, 15, 249.
- Medo, J.; Cagáň, Ľ. Factors affecting the occurrence of entomopathogenic fungi in soils of Slovakia as revealed using two methods. Biol. Control. 2011, 59, 200–208.
- Tkaczuk, C.; Król, A.; Majchrowska-Safaryan, A.; Nicewicz, Ł. The occurrence of entomopathogenic fungi in soils from fields cultivated in a conventional and organic system. J. Ecol. Eng. 2014, 15, 137–144.
- Kepler, R.M.; Ugine, T.A.; Maul, J.E.; Cavigelli, M.A.; Rehner, S.A. Community composition and population genetics of insect pathogenic fungi in the genus Metarhizium from soils of a long-term agricultural research system. Environ. Microbiol. 2015, 17, 2791–2804.
- Hernández-Domínguez, C.; Guzmán-Franco, A.W. Species diversity and population dynamics of entomopathogenic fungal species in the genus Metarhizium—a spatiotemporal Study. Microb. Ecol. 2017, 74, 194–206.
- Barnes, A.I.; Siva-Jothy, M.T. DensitY-dependent prophylaxis in the mealworm beetle Tenebrio molitor L. (Coleoptera: Tenebrionidae): Cuticular melanization is an indicator of investment in immunity. Proc. R. Soc. Lond. B. Biol. Sci. 2000, 267, 177–182.
- Dubovskiy, I.M.; Whitten, M.M.A.; Kryukov, V.Y.; Yaroslavtseva, O.N.; Grizanova, E.V.; Greig, C.; Mukherjee, K.; Vilcinskas, A.; Mitkovets, P.V.; Glupov, V.V.; et al. More than a colour change: Insect melanism, disease resistance and fecundity. Proc. R. Soc. Lond. B. Biol. Sci. 2013, 280, 20130584.
- Kryukov, V.Y.; Tyurin, M.V.; Tomilova, O.G.; Yaroslavtseva, O.N.; Kryukova, N.A.; Duisembekov, B.A.; Tokarev, Y.S.; Glupov, V.V. Immunosuppression of insects by the venom of Habrobracon hebetor increases the sensitivity of bait method for the isolation of entomopathogenic fungi from soils. Biol. Bull. 2017, 44, 401–405.
- Vänninen, I. Distribution and occurrence of four entomopathogenic fungi in Finland: Effect of geographical location, habitat type and soil type. Mycol. Res. 1996, 100, 93–101.
- Hughes, W.O.H.; Thomsen, L.; Eilenberg, J.; Boomsma, J.J. Diversity of entomopathogenic fungi near leaf-cutting ant nests in a neotropical forest, with particular reference to Metarhizium anisopliae var. anisopliae. J. Invertebr. Pathol. 2004, 85, 46–53.
- Oddsdottir, E.S.; Nielsen, C.; Sen, R.; Harding, S.; Eilenberg, J.; Halldorsson, G. Distribution patterns of soil entomopathogenic and birch symbiotic ectomycorrhizal fungi across native woodlandand degraded habitats in Iceland. Icel. Agric. Sci. 2010, 23, 37–49.
- Meyling, N.V.; Schmidt, N.M.; Eilenberg, J. Occurrence and diversity of fungal entomopathogens in soils of low and high Arctic Greenland. Polar Biol. 2012, 35, 1439–1445.
- Inglis, G.D.; Enkerli, J.; Goettel, M.S. Chapter VII—Laboratory techniques used for entomopathogenic fungi: Hypocreales. In Manual of Techniques in Invertebrate Pathology, 2nd ed.; Lacey, L.A., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 189–253.
- Ownley, B.H.; Griffin, M.R.; Klingeman, W.E.; Gwinn, K.D.; Moulton, J.K.; Pereira, R.M. Beauveria bassiana: Endophytic colonization and plant disease control. J. Invertebr. Pathol. 2008, 98, 267–270.
- Nishi, O.; Sushida, H.; Higashi, Y.; Iida, Y. Epiphytic and endophytic colonisation of tomato plants by the entomopathogenic fungus Beauveria bassiana strain GHA. Mycology 2020, 1–9.
- Meyling, N.V.; Pilz, C.; Keller, S.; Widmer, F.; Enkerli, J. Diversity of Beauveria spp. isolates from pollen beetles Meligethes aeneus in Switzerland. J. Invertebr. Pathol. 2012, 109, 76–82.
- Rehner, S.A.; Posada, F.; Buckley, E.P.; Infante, F.; Castillo, A.; Vega, F.E. Phylogenetic origins of African and neotropical Beauveria bassiana s.l. pathogens of the coffee berry borer, Hypothenemus hampei. J. Invertebr. Pathol. 2006, 93, 11–21.
- Meyling, N.V.; Lubeck, M.; Buckley, E.P.; Eilenberg, J.; Rehner, S.A. Community composition, host range and genetic structure of the fungal entomopathogen Beauveria in adjoining agricultural and seminatural habitats. Mol. Ecol. 2009, 18, 1282–1293.
- Bischoff, J.F.; Rehner, S.A.; Humber, R.A. A multilocus phylogeny of the Metarhizium anisopliae lineage. Mycologia 2009, 101, 512–530.
- Rezende, J.M.; Zanardo, A.B.R.; da Silva Lopes, M.; Delalibera, I.; Rehner, S.A. Phylogenetic diversity of Brazilian Metarhizium associated with sugarcane agriculture. BioControl 2015, 60, 495–505.
- Spatafora, J.W.; Sung, G.H.; Sung, J.M.; Hywel-Jones, N.L.; White, J.F. Phylogenetic evidence for an animal pathogen origin of ergot and the grass endophytes. Mol. Ecol. 2007, 16, 1701–1711.
- Kepler, R.M.; Rehner, S.A. Genome-assisted development of nuclear intergenic sequence markers for entomopathogenic fungi of the Metarhizium anisopliae species complex. Mol. Ecol. Resour. 2013, 13, 210–217.
- Enkerli, J.; Widmer, F.; Gessler, C.; Keller, S. Strain-specific microsatellite markers in the entomopathogenic fungus Beauveria brongniartii. Mycol. Res. 2001, 105, 1079–1087.
- Rehner, S.A.; Buckley, E.P. Isolation and characterization of microsatellite loci from the entomopathogenic fungus Beauveria bassiana (Ascomycota: Hypocreales). Mol. Ecol. Notes 2003, 3, 409–411.
- Enkerli, J.; Widmer, F. Molecular ecology of fungal entomopathogens: Molecular genetic tools and their applications in population and fate studies. BioControl 2010, 55, 17–37.
- Goble, T.A.; Costet, L.; Robene, I.; Nibouche, S.; Rutherford, R.S.; Conlong, D.E.; Hill, M.P. Beauveria brongniartii on white grubs attacking sugarcane in South Africa. J. Invertebr. Pathol. 2012, 111, 225–236.
- Enkerli, J.; Kölliker, R.; Keller, S.; Widmer, F. Isolation and characterization of microsatellite markers from the entomopathogenic fungus Metarhizium anisopliae. Mol. Ecol. Notes 2005, 5, 384–386.
- Oulevey, C.; Widmer, F.; Kölliker, R.; Enkerli, J. An optimized microsatellite marker set for detection of Metarhizium anisopliae genotype diversity on field and regional scales. Mycol. Res. 2009, 113, 1016–1024.