Peripheral artery disease (PAD) is caused by atherosclerosis in the lower extremities, which leads to a spectrum of life-altering symptomatology, including claudication, ischemic rest pain, and gangrene requiring limb amputation.
- myopathy
- peripheral vascular disease
- myopathy,peripheral vascular disease
1. Introduction
Peripheral artery disease (PAD) is a common manifestation of atherosclerosis that affects more than 200 million people worldwide and is the third leading cause of cardiovascular mortality [1]. PAD is caused by atherosclerotic narrowing or occlusion of blood vessels in the lower extremities that leads to a spectrum of life-altering symptomatology, including claudication, ischemic rest pain, and gangrene requiring limb amputation. Morbidity and mortality associated with PAD have increased over the last decade despite an increase in the number of lower extremity vascular procedures during that time. Current treatments for PAD are focused solely on re-establishing blood flow to the limb, implying that blood flow is the definitive factor that determines pathobiology. Unfortunately, failure rates of endovascular and revascularization procedures remain unacceptably high [2][3] [2,3] and numerous cell- and gene-based vascular therapies have failed to demonstrate efficacy in clinical trials [4][5][6][7][4,5,6,7]. The low success of vascular-focused therapies implies that other non-vascular tissues must contribute to PAD pathobiology. Although atherosclerotic PAD manifests in the vascular system, there are significant consequences to skeletal muscle that result in impaired muscle health/function and exercise intolerance [8]. Several large clinical studies in PAD patients have demonstrated that muscle function/exercise capacity is the strongest predictor of morbidity/mortality [9][10][11][12][13][14][15][9,10,11,12,13,14,15]. Previous reports have documented evidence of skeletal muscle myopathies in PAD patients [16][17][16,17], although its potential role in clinical pathology is not well understood. In this review, we analyze the evidence that PAD is associated with a skeletal muscle myopathy, including altered mitochondrial function and oxidative stress (Figure 1); discuss the risk factors that contribute to skeletal muscle myopathy; and review emerging therapeutic approaches that aim to mitigate these myopathic symptoms and have potential to improve outcomes for PAD patients.
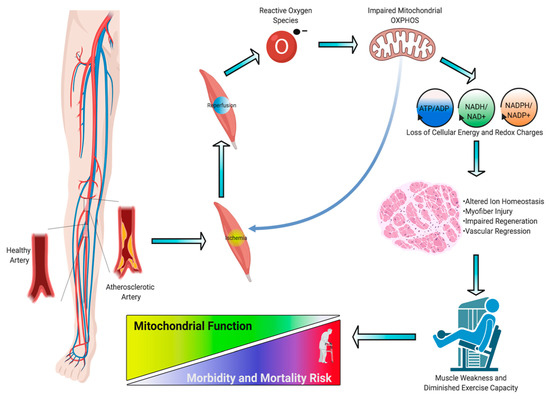
Figure 1.
2. Pathogenesis, Diagnosis, and Treatment of PAD
Before embarking upon an analysis of the role of skeletal muscle in PAD pathobiology, a brief discussion of pathogenesis, diagnosis, and treatment of PAD is required. The prevalence of PAD is estimated to be 1–4% in the general population; however, among selected subgroups of elderly patients, rates exceeding 20% have been reported [18]. Due to the frequent association of PAD with cardiovascular risk factors, such as smoking, diabetes, hypertension, dyslipidemia, and increasing age, approximately 200 million patients worldwide have some form of PAD [19]. The pathogenesis of PAD has been linked to several causes, including subendothelial dysfunction, aberrant platelet activity, hyperlipidemia, tobacco exposure, as well as a myriad of other immunologic and inflammatory factors [20][21][22][23][24][25][26]. The protean clinical presentation is underscored by subintimal accumulation of lipid and fibrous materials that form a fixed arterial stenosis and/or occlusion. Classically, lower extremity atherosclerotic occlusive disease involves either the aortoiliac, femoral-popliteal, or tibial vessels, and certain subpopulations, like diabetic patients and/or subjects with renal dysfunction, present with more distal disease [27][28][29]. The clinical manifestation of PAD occurs along a spectrum with some patients being asymptomatic while others describe disabling intermittent claudication (IC). Notably, 5–10% of patients have chronic limb-threatening ischemia (CLTI) and have significantly elevated risk of major limb amputation, adverse cardiac events, and mortality compared to age-matched controls [30][31][32]. The characterization of PAD severity is predominantly dependent upon the constellation of symptoms, with non-invasive studies corroborating the presence of disease. Reproducible exertional lower extremity muscle cramping that is relieved by rest and associated with a fixed arterial stenosis/occlusion is the hallmark description of vasculogenic claudication. In contrast, CLTI may present as rest pain or tissue loss, which classically involves the forefoot. Initial evaluation includes a thorough history and physical with ankle-brachial index (ABI) assessment to confirm the diagnosis.
For patients with claudication, risk of major limb amputation is < 1% per year, but the 5-year major adverse cardiovascular event risk including death approached 30% [33][34]. Therefore, due to the non-limb-threatening nature of this PAD presentation, clinical practice guidelines and peer reviewed evidence strongly advocates that first-line therapy be medical, including smoking cessation, an exercise walking program, hypertension and blood glucose control, daily antiplatelet and statin therapy, as well as a trial of cilostazol [35][36][37]. Subjects that are compliant with initial medical management but have refractory symptoms generally undergo further imaging (e.g., computed tomographic arteriogram, magnetic resonance arteriogram, segmental doppler, and/or pulse volume recording) to delineate the anatomic extent of disease and identify potential treatment options. Most commonly, patients with claudication present with single-level aorto-iliac or superficial femoral artery atherosclerotic occlusive disease and revascularization is usually based upon endovascular strategies if anatomically eligible. However, long-segment occlusive disease is optimally treated with construction of an arterial bypass. Successful outcomes of endovascular or open surgical remediation are determined by improvement in absolute walking distance, patient quality of life, and augmented ABIs.
In contrast to claudication, PAD patients with CLTI require revascularization due to the significant risk of major limb amputation without restoration of limb perfusion [30]. An ankle pressure < 50mmHg, toe pressures < 30mmHg, monophasic Doppler waveforms, low-amplitude calf pulse volume recordings, transcutaneous oxygen pressure < 30mmHg, and/or tissue loss with any objective measurement of occlusive disease all strongly support the clinical diagnosis of CLTI [29]. Limb salvageability is determined using the Society for Vascular Surgery Wound, Ischemia, and foot Infection (WiFi) and Rutherford classification systems [38][39]. Tenets of revascularization include determination of the inflow vessel, the recipient outflow vessel, conduit choice, hemodynamic significance of the lesion, and characterization of the patient’s functional and physiological status. These variables inform the clinician to optimally select the unique arterial reconstruction strategy for each patient. Generally, CLTI patients present with more complex patterns of PAD, so the probability of a durable outcome with endovascular recanalization is less compared to bypass but many providers subscribe to an ‘endo first’ approach [40][41][42]. For subjects undergoing infrainguinal arterial bypass, use of single-segment ipsilateral saphenous vein and clinical stage of the CLTI presentation are the most important determinants of limb salvage [43][44]. Clinical success is highlighted by freedom from major adverse limb events, such as unplanned re-intervention, thrombosis free graft function, and/or major limb amputation.
3. Skeletal Muscle Pathology in PAD
The pathogenesis of PAD is undoubtedly vascular in nature with a primary causative factor involving atherosclerosis. However, the ensuing limb muscle pathology appears to be intimately linked to patient outcomes [10][11][13][14][15][45][46][47][48][49][10,11,13,14,15,45,46,47,48,49]. A large body of literature from clinical human studies reports that altered skeletal muscle phenotype is prevalent in ischemic limbs and that patients with PAD present with reduced exercise capacity and impaired lower extremity skeletal muscle function compared with age-matched healthy counterparts [49][50][51][52][53][49,50,51,52,53]. Longitudinal cohort studies determined that the exercise capacity and functional performance are strong predictors of mortality [15][54][15,54], implicating skeletal muscle health/function as an important clinical feature of PAD pathobiology.
Limb muscle pathology in PAD manifests as weakness and exercise intolerance. In addition, claudication and/or ischemic rest pain further reduce physical activity levels and negatively impact functional independence and quality of life [55]. The genesis of exercise intolerance in PAD is without doubt multifactorial [55][56][55,56]. Blockage of arteries that causes restricted blood flow to lower extremities is amongst the primary factors that negatively affects mobility and functional performance [57]. Further to limb perfusion limitations, walking performance in PAD patients is also related to muscle fiber size [48], and muscle size/strength is predictive of mortality [10][11][13][10,11,13]. Indeed, one of the most notable features of skeletal myopathy related to peripheral arterial insufficiency is lower extremity muscular atrophy. Data from computed tomographic (CT) imaging demonstrated that PAD patients have smaller calf muscle area with accumulated adipose tissue compared to the individuals without PAD [13][57][58][13,57,58]. Further investigation of morphology using histopathologic analysis revealed that a smaller cross-sectional area of both type I and II fibers as well as increased intramuscular collagen deposition [53][57][59][53,57,59]. The underlying mechanisms that explain these changes are complex and still require further examination, but a number of studies in human and animal studies suggest that impaired proteostasis (i.e., altered signaling pathways that regulate protein synthesis and degradation) plays a central role in muscle atrophy [60][61][60,61]. A dysfunctional peripheral nervous system, such as identified demyelination or focal denervation on the neuromuscular junction, will also likely stimulate the process of muscle atrophy and contribute to the weakness and functional impairment [62][63][62,63]. Skeletal muscle abnormalities also include capillary rarefaction and impaired microcirculation in PAD patients with IC and these findings are more obvious in people suffering from CLTI [59][64][65][59,64,65]. Considering the aspects of limited conduit artery blood flow and impaired endothelium-dependent vasodilation during physical activity in this population, there will probably be an increased reliance on the microcirculation to meet the metabolic demands of the contracting muscle. Therefore, novel strategies targeting skeletal muscle myopathy to reverse muscle atrophy [66][67] [66,67] that can be used in addition to those targeting capillary rarefaction [68][69] [68,69] hold great potential to produce greater and more sustainable clinical impact.
Interestingly, following progressive resistance training, PAD patients with intermittent claudication showed delayed onset of claudication time and improved maximum walking time during a graded treadmill test in addition to the increased leg muscle strength [68] [68]. These changes occurred without improving limb hemodynamics (ABI), suggesting the perfusion-independent beneficial impact of resistance training may arise from changes in skeletal muscle. In support of this concept, invasive revascularization in symptomatic PAD patients does not effectively normalize exercise performance [70][71][70,71], and acute pharmacological administration of sildenafil, which enhances limb perfusion, did not alter walking capacity [72]. Taken together, these observations indicate that factors other than lower extremity perfusion must play a significant role [8].
