Phytoestrogens are compounds derived from plants that have a similar structure to human sex hormones.
- phytoestrogens
- cancer prevention
- antioxidant
1. Introduction
Phytoestrogens are naturally occurring compounds in plants and are characterized by a close structural similarity to estrogens. This allows them to act as weak estrogenic factors and interfere with hormonal signaling. Several reports suggest that phytoestrogens may have a positive effect on the prevention of menopausal symptoms, type 2 diabetes, cardiovascular disease, obesity, and cancer. These health benefits are presumably linked to their anti-inflammatory, anti-tumoral, anti-allergic, antioxidant, anti-thrombotic, and hepatoprotective properties [1]. The interest in phytoestrogens and cancer began after the observation that the consumption of soy and soy-derived foods was correlated with a decreased incidence of breast [2], ovarian [3], and prostate cancer [4]. In fact, the levels of genistein, the main soy isoflavonoid, and other phytoestrogens in plasma are inversely correlated to the risk of developing several types of cancer [5][6][7][8][5,6,7,8].
Phytoestrogens have been extensively tested in vitro and in vivo as anti-cancer treatments, although they have also been studied as adjuvant treatments to improve the response to chemotherapy, hormonotherapy, and radiotherapy. However, some studies alert that phytoestrogen consumption may interfere with cancer treatments and be harmful to patients.
2. Phytoestrogen Structure and Classification
Even though phytoestrogens are a large and heterogenic group, all of them are characterized by a phenolic ring and two hydroxyl groups (Figure 1), which are crucial for the binding to the estrogen receptors (ER). The agonist or antagonist properties of phytoestrogens depend on their phenolic group [1]. Based on their structure, phytoestrogens are classified into three main classes, which include flavonoids, lignans, and stilbenes [9][10][9,10].
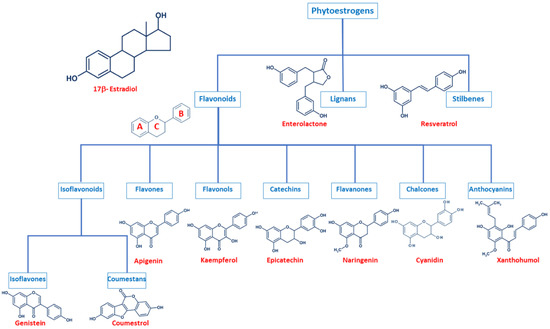
Figure 1. Comparison of the chemical structure of the different classes of phytoestrogens and 17β-estradiol.
2.1. Flavonoids
Flavonoids present the typical structure C6-C3-C6, with two aromatic rings (benzene A and B) joined together by a chain of 3 carbons cycled through an atom of oxygen (Figure 1) [11]. Flavonoids are commonly divided into several sub-classes, based on the connection position of the B and C rings, as well as the degree of saturation, oxidation, and hydroxylation of the C ring (Figure 1). This subclassification includes isoflavonoids (isoflavones and coumestans), flavones, flavonols, flavan-3-ols (or catechins), flavanones, chalcones, and anthocyanins [1][11][12][13][14][1,11,12,13,14].
Isoflavonoids are compounds derived from plant metabolism, and their structure consists of a 3-phenylchroman skeleton. They are also divided into two major groups, isoflavones, and coumestans. Isoflavones are flavonoids in which the B ring is linked to the heterocyclic ring at the C3 instead of the C2 position (Figure 1) [15]. Genistein and daidzein constitute up to 90% of isoflavones found in soybeans [16], and formononetin and biochanin A are mainly found in red clover. Isoflavones can be found in their free form or in their esterified forms [15]. Coumestans, the other subclass of isoflavonoids, have a 1-benzoxolo(3,2-c)chromen-6-one structure formed by a benzoxole fused with a chromen-2-one. One of the most studied coumestans is coumestrol, considered an endocrine disruptor, as it has the potential to bind to both ERs with similar affinity as estradiol, affecting the estrogenic signaling cascade [17]. Although the estrogenic activity of coumestrol is weaker than that of estradiol, it is 30 to 100 times greater than that of other isoflavones [18], due to the position of its two hydroxy groups, which match estradiol. This chemical structure also gives coumestrol the ability to inhibit aromatase and 3α-hydroxysteroid dehydrogenase [19], which are involved in the synthesis of steroid hormones [20].
Flavones are another type of flavonoids with a double bond between C2 and C3 (Figure 1). Furthermore, the C3 position does not have any substitution, and C4 is oxidized [21][22][21,22]. Luteolin and apigenin are the main compounds in this group. Due to its structure, luteolin is a strong inhibitor of xanthine oxidase, one of the main sources of ROS production [23]. On the other hand, apigenin is thought to protect cells against oxidative damage by enhancing mitochondrial function [23]. Furthermore, flavones may induce cell cycle arrest and DNA damage in some cell types, and specifically, apigenin may trigger apoptosis by inducing the activity of p38 kinase [24].
Flavonols are characterized by a 3-hydroxyflavone skeleton and are classified by the position of their phenolic group (Figure 1). Quercetin and kaempferol are the most predominant flavonols in plants [14]. Catechins or flavanols are mainly found in tea, vinegar, peach, and pome fruits. Epicatechin is an abundant polyphenol in unfermented cocoa beans, and it is thought to be responsible for the main health effects of cocoa. Another widely studied catechin is epigallocatechin gallate (EGCG), which is formed by the ester of epigallocatechin and gallic acid and is present in green tea. Both catechins have been associated with antioxidant and chemopreventive effects in several cell lines [25][26][25,26].
Flavanones are found in all citric fruits, and their chemical structure differs from flavones in the saturation of the C ring, with a saturated double bond between positions 2 and 3 (Figure 1) [12]. Naringenin is the most studied flavanone and contributes to limit lipid peroxidation and protein carbonylation by increasing antioxidant defenses [27]. Isoxanthohumol and 8-prenylnaringenin are also flavanones found in hop (Humulus lupulus), and they are also widely studied for their anti-cancer effects [28]. 8-prenylnaringenin has been identified as the most potent phytoestrogen and binds to both ERs [29], and inhibits aromatase [30]. Chalcones also belong to the flavonoids class and have a common 1,3-diaryl-2-propen-1-one skeleton, named chalconoid (Figure 1). The most studied chalcone is xanthohumol, also present in hop plants, and it possesses antibacterial and anti-cancer effects [31]. Finally, anthocyanins are the most abundant flavonoids in fruits and vegetables [32]. They are formed by a flavylium cation (2-phenylbenzopyrilium), which links hydroxyl (-OH) and/or methoxyl (-OCH3) groups. Various anthocyanins have been described, and mainly six are found in vegetables and fruits: pelargonidin, cyaniding, delphinidin, petunidin, peonidin, and malvidin [33].
2.2. Lignans
Lignans are another class of phytoestrogens commonly found in grains, nuts, coffee and tea, cocoa, flaxseed, and some fruits [34]. The chemical structure of lignans consists of two phenylpropane groups linked by a β-β’ bond, which is formed by a C-C bond between the central atoms of their side chains (position 8 or β) (Figure 1) [35][36][37][38][35,36,37,38]. Some studies report that these phytoestrogens are capable of mimicking the antioxidant effects of some hormones without any associated deleterious effects [35][39][40][35,39,40]. Importantly, gut bacteria are responsible for the metabolization of lignans and produce enterodiol and enterolactone. Thus, the beneficial health effects of lignans may be conditioned to each individual’s microbiota [41].
2.3. Stilbenes
Finally, stilbenes are an important group of nonflavonoid phytoestrogens with a polyphenolic structure with a 1,2-diphenylethylene nucleus (Figure 1) [42]. The most studied stilbene is resveratrol, a compound with two phenolic rings connected by a styrene double bond. This compound can occur in trans- and cis-isoforms, being the trans-isoform the most predominant one [43]. Resveratrol is found in a wide variety of dietary foods, including grapes, wine, nuts, and berries [44][45][44,45], and in fact, is considered a key compound in the French Paradox [46]. Several in vitro and in vivo studies report that resveratrol has anti-cancer properties, as well as antioxidant, anti-aging, anti-inflammatory, and anti-pathogen effects [43][44][45][47][48][43,44,45,47,48].
3. Mechanism of Action of Phytoestrogens and Cancer Prevention
It has been established that phytoestrogens interact with ERs, activating the transcription of several target genes. This results in the increase of the levels of antioxidants enzymes, such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (Gpx), as well as an improvement of mitochondrial function [49][50][51][49,50,51]. Phytoestrogens also bind to G-protein-coupled estrogen receptor 1 (GPER/GPR30) and exert non-genomic effects [52][53][54] [52,53,54] (Figure 2).
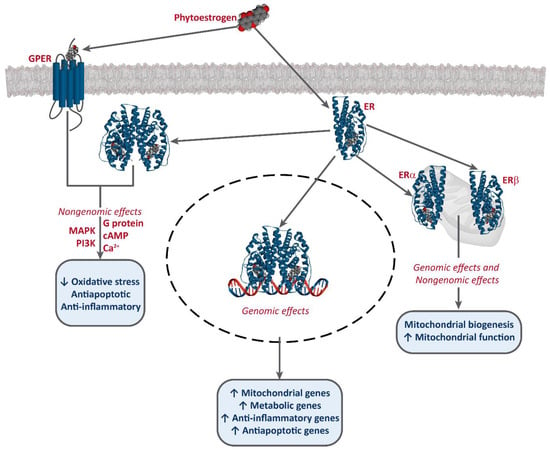
Figure 2.
Interaction of phytoestrogens with ERs and GPER and their described effects.
Phytoestrogens also have ER-independent effects. For instance, genistein and resveratrol can act as tyrosine kinase inhibitors, altering the activity of some downstream kinases [55]. Moreover, some studies also report an epigenetic mechanism for some phytoestrogens and the involvement of miRNA expression [56][57][58][56,57,58], and the modulation of chromatin structure [53][59][53,59]. However, they are mostly known as potent antioxidants, protecting cellular structures from ROS, such as epicatechin [25] [25] or lignans [35], although the in vivo effects of these are much greater, due to the conversion into their active metabolites, enterolactone and enterodiol [60]. Furthermore, some studies report that at high concentrations, phytoestrogens may have an oxidant effect and induce cell death. This effect has been described for several compounds, including genistein [61][62][63][64][61,62,63,64], resveratrol [65][66][65,66], and xanthohumol [67][68][69][67,68,69]. Resveratrol is one of the most studied phytoestrogens, and several clinical trials have been developed to test its potential as an anti-cancer treatment [44][47][70][44,47,70], and some analogs are being designed [42][43][42,43].
These mechanisms of action have been associated with the chemoprevention potential of phytoestrogens. For instance, in Asian populations, soy consumption correlates with a lower incidence of prostate and breast cancer, which has been attributed to the presence of genistein in soy [71]. Genistein has a higher affinity for ERβ (87%) than for ERα (4%), and ERβ has been reported to have a protective effect against malignant transformation. In this regard, the chemoprevention effect of genistein may depend on the ERα/ERβ ratio [15][72][73][74][15,72,73,74].
Other phytoestrogens have been less widely studied, although they also show potential as chemopreventive agents. Quercetin may reduce the incidence of esophageal and stomach cancers, although this protection has not been observed for lung cancer [14][75][76][77][14,75,76,77]. There is also evidence for mild protection against gastrointestinal cancers associated with the consumption of tea flavanols [78]. Also, most epidemiological studies about lignans suggest that their intake reduces the risk of premenopausal breast cancer and possibly of postmenopausal breast cancer [39].
