Cervical cancer is the fourth most common cancer among women worldwide. Though several natural products have been reported regarding their efficacies against cervical cancer, there has been no review article that categorized them according to their anti-cancer mechanisms. In this study, anti-cancerous natural products against cervical cancer were collected using Pubmed (including Medline) and google scholar, published within three years. Their mechanisms were categorized as induction of apoptosis, inhibition of angiogenesis, inhibition of metastasis, reduction of resistance, and regulation of miRNAs. A total of 64 natural products suppressed cervical cancer. Among them,
Penicillium sclerotiorum
extracts from
Cassia fistula
L., ethanol extracts from
Bauhinia variegate candida
, thymoquinone obtained from
Nigella sativa
, lipid-soluble extracts of
Pinellia pedatisecta
Schott., and 1′S-1′-acetoxychavicol extracted from
Alpinia conchigera
have been shown to have multi-effects against cervical cancer. In conclusion, natural products could be attractive candidates for novel anti-cancer drugs.
- cervical cancer
- dietary natural products
- apoptosis
- angiogenesis
- metastasis
- resistance
- microRNA
- cervical cancer,dietary natural products,apoptosis,angiogenesis,metastasis,resistance,microRNA
1. Introduction
Cervical cancer, which is a cancer arising from the cervix, is characterized by abnormal vaginal bleeding, vaginal discharge, pelvic pain, or pain during sexual intercourse [1]. Currently, cervical cancer is the fourth most common cancer among women in the world [2]. According to Globocan 2018, the prevalence rate of cervical cancer is 3.2% of all cancers. The main treatments for cervical cancer are surgery such as pelvic lymphadenectomy and radical hysterectomy, radiotherapy, and chemotherapy [3]. Another therapy is targeted therapy, which regulates epidermal growth factor receptor (EGFR) [4][5] and cyclooxygenase-2 (COX-2) [6][7] for treating cervical carcinoma. However, these treatments showed possible side effects and complications: Surgery could cause bleeding, damage to the organs around the surgery, and a risk of clots in the deep veins of the legs, radiotherapy could yield menopause, infertility, discomfort, or pain with intercourse, and the side effects of chemotherapy may affect not only cancer cells but also rapidly dividing cells in systems of the whole body [8][9]. Moreover, the drugs that are usually prescribed for cervical cancer showed several side effects and drug resistance [10]. Cisplatin, which is one of the most effective anticancer drugs, has resistance capacity by a self-defense mechanism [11]. 5-fluorouracil (5-FU) is also reported for resistance and side effects when it comes to cervical cancer patients [12]. Thus, we have focused on discovering a new potent treatment for cervical cancer from natural products.
Cervical cancer, which is a cancer arising from the cervix, is characterized by abnormal vaginal bleeding, vaginal discharge, pelvic pain, or pain during sexual intercourse [1]. Currently, cervical cancer is the fourth most common cancer among women in the world [2]. According to Globocan 2018, the prevalence rate of cervical cancer is 3.2% of all cancers. The main treatments for cervical cancer are surgery such as pelvic lymphadenectomy and radical hysterectomy, radiotherapy, and chemotherapy [3]. Another therapy is targeted therapy, which regulates epidermal growth factor receptor (EGFR) [4,5] and cyclooxygenase-2 (COX-2) [6,7] for treating cervical carcinoma. However, these treatments showed possible side effects and complications: Surgery could cause bleeding, damage to the organs around the surgery, and a risk of clots in the deep veins of the legs, radiotherapy could yield menopause, infertility, discomfort, or pain with intercourse, and the side effects of chemotherapy may affect not only cancer cells but also rapidly dividing cells in systems of the whole body [8,9]. Moreover, the drugs that are usually prescribed for cervical cancer showed several side effects and drug resistance [10]. Cisplatin, which is one of the most effective anticancer drugs, has resistance capacity by a self-defense mechanism [11]. 5-fluorouracil (5-FU) is also reported for resistance and side effects when it comes to cervical cancer patients [12]. Thus, we have focused on discovering a new potent treatment for cervical cancer from natural products.
Natural products extracted from living organisms including plants and animals have several active ingredients, which are reported to be attractive alternatives to chemotherapeutic drugs or suitable for combined use with chemotherapeutic drugs [13][14]. For example, purified flaxseed hydrolysate (PFH), extracted from Lignan, induces apoptosis and inhibits angiogenesis and metastasis on HeLa cells [15]. Thymoquinone from
Natural products extracted from living organisms including plants and animals have several active ingredients, which are reported to be attractive alternatives to chemotherapeutic drugs or suitable for combined use with chemotherapeutic drugs [13,14]. For example, purified flaxseed hydrolysate (PFH), extracted from Lignan, induces apoptosis and inhibits angiogenesis and metastasis on HeLa cells [15]. Thymoquinone from
Nigella sativa
also showed apoptotic effect and anti-proliferation in SiHa and CaSki cells. Such natural products include
Bauhinia variegate candida
ethanol extracts, Praeruptorin-B, and well-known tea.
MicroRNA (miRNA, miR) are involved in the pathological development and metastasis of cancer [16][17]. Several natural products showed an anti-cancer effect by regulation of cancer-related miRNAs. Our team reported that
MicroRNA (miRNA, miR) are involved in the pathological development and metastasis of cancer [16,17]. Several natural products showed an anti-cancer effect by regulation of cancer-related miRNAs. Our team reported that
Spatholobus suberectus Dunn extract induces apoptosis by regulation of miR-657/activating transcription factor 2 (ATF2) in U266, U937 cells [18]. Another natural product,
Dunn extract induces apoptosis by regulation of miR-657/activating transcription factor 2 (ATF2) in U266, U937 cells [18]. Another natural product,
Salvia miltiorrhiza
, showed an anti-cancer effect via regulation of miR-216b [19]. 1′S-1′-acetoxychavicol acetate (ACA) from
Alpinia conchigera has been reported to induce apoptosis on SiHa and CaSki cells by targeting SMAD4 and miR-210. Targeting miRNAs with natural products could be a promising strategy for cervical cancer [20]. However, there have been no studies that organize the mechanisms, efficacy, and concentration of natural products for cervical cancer in last five years. In this present study, we aim to review the nonclinical studies about the anti-cancer mechanisms of natural products. The natural products were organized by their mechanisms including apoptosis, anti-metastasis, anti-angiogenesis, resistance, and microRNA regulation.
has been reported to induce apoptosis on SiHa and CaSki cells by targeting SMAD4 and miR-210. Targeting miRNAs with natural products could be a promising strategy for cervical cancer [20]. However, there have been no studies that organize the mechanisms, efficacy, and concentration of natural products for cervical cancer in last five years. In this present study, we aim to review the nonclinical studies about the anti-cancer mechanisms of natural products. The natural products were organized by their mechanisms including apoptosis, anti-metastasis, anti-angiogenesis, resistance, and microRNA regulation.
2. Results
2.1. Apoptosis
Apoptosis is a unique form of cell death and is an important process that regulates the homeostasis of cell survival [21]. Apoptosis eliminates potentially cancerous cells and this process is caused by atrophy of cells, synthesis of new proteins, and cell suicide genes; also, it has a great influence on the malignant phenotype [22]. For this reason, apoptosis is used as an anticancer mechanism for cancer research. A total of 47 studies have been performed to elucidate apoptosis-mediated anti-cancer pathway of natural products in Hela and SiHa cells. A total of 54 natural products were reviewed.
2.1.1. Compounds
Among the natural products, 35 compounds showed an apoptotic effect against cervical cancer (
). The chemical structures of the compounds are shown in
.
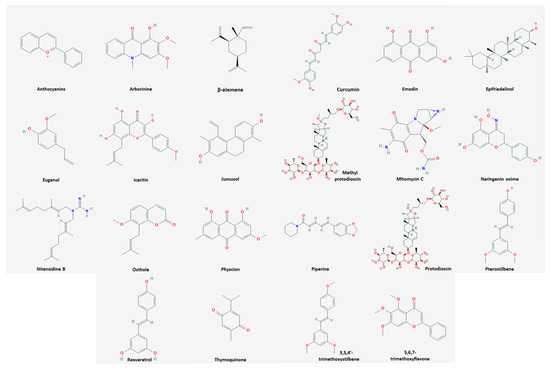
Figure 1.
Chemical structures of compounds derived from natural products inducing apoptosis.
Table 1.
Apoptosis inducing natural products-compounds.
| Classification | Compound | Source | Cell Line/ Animal Model |
Dose; Duration | Efficacy | Mechanism | Reference |
|---|
| Etc. | Acylhydrazone | HeLa | 2.21 µM; 48 h | Inhibition of cancer activity |
Pistacia vera L. downregulated TNF, Bcl-2, IAP, and TRAF in CaSki cells [74]. Through the mechanisms, it induced apoptosis and inhibited angiogenesis. The efficient dose was 81.17 ± 2.87 µg/mL, dealing with a timeframe of 72 h.
L. downregulated TNF, Bcl-2, IAP, and TRAF in CaSki cells [78]. Through the mechanisms, it induced apoptosis and inhibited angiogenesis. The efficient dose was 81.17 ± 2.87 µg/mL, dealing with a timeframe of 72 h.
Table 3.
Angiogenesis inhibiting natural products.
| Classification | Compound/ Extract |
Source | Cell Line/ Animal Model |
Dose; Duration | Efficacy | Mechanism | Reference | |||
|---|---|---|---|---|---|---|---|---|---|---|
| [ | 23 | ] | ||||||||
| 15 μg/mL; 24 h | ||||||||||
| Fruit | PfLP | Praecitrullus fistulosus | HeLa Swiss Albino mice |
In vitro: 50 µg/mL; 24 h In vivo: 10 mg/kg |
Induction of apoptosis | |||||
| Plant | Anthocyanins | Root tubers and leaves of | ||||||||
| Ipomoea batatas | ||||||||||
| HeLa | 100, 200 µg/mL; 48 h | Induction of apoptosis, cell cycle arrest | ↑CFP/YFP | [24] | ||||||
| Plant | Arborinine | |||||||||
| Glycosmis parva | ||||||||||
| HeLa | 110 μg/mL; 24 h | Induction of apoptosis Inhibition of migration |
↑caspase-3, -7 ↓Bcl2-L1 |
[25] | ||||||
| Plant | β-elemene | |||||||||
| Curcuma zedoaria | ||||||||||
| SiHa | 30, 40, 50 μg/mL; | 24, 48, 72 h |
Inhibition of proliferation and migration Induction of cell cycle arrest and apoptosis |
Inhibition of cell viability and migration Induction of apoptosis↑p15, p53, Bax ↓cyclin D1, Bcl-2, MMP-2, -9, β-catenin, TCF7, c-Myc |
↑c-caspase-3, -8, RIP, TNF-R1 [26] |
|||||
| ↓MMP-2, MMP-9 | [ | 55 | ] | [ | 58 | Plant | Copper oxide nanoparticles | |||
| Azadirachta indica, Hibiscus rosa-sinensis, Murraya koenigii, Moringa oleifera, Tamarindus indica | ||||||||||
| HeLa | 2, 5, 10, 25, 50, 100 μg/mL; 48 h | Inhibition of oxidative stress Induction of apoptosis |
[27] | |||||||
| ] | ||||||||||
| Plant | Ethanol extract | Botryidiopsidaceae species | HeLa | 6.25, 12.5, 25, 50 μg/mL; 24 h | Inhibition of oxidative stress and migration Induction of apoptosis |
↑p53, c-caspase-3 ↓Bcl-2 |
[56][59] | Plant | Curcumin | |
| Curcuma longa | ||||||||||
| HeLa, | C57BL/6, BALB/c | In vitro: 2 μg/mL; 48 h In vivo: 25 mg/kg |
Induction of apoptosis and cell cycle arrest | ↑p53, cytochrome c, PARP, caspase-3, -7, -9 ↓Bcl-2, NF-κB |
||||||
| Plant | Ethanol extract | Chloromonas species | HeLa | 12.5, 25 μg/mL; 24, 72 h | Inhibition of oxidative stress Induction of apoptosis |
↑c-caspase-3, p53 | [28] | |||
| ↓Bcl-2 | [ | 57 | ] | [ | 60 | ] | ||||
| Plant | Ethanol extract | Dendrobium chrysanthum | HeLa, Swiss albino mice |
In vitro: 450 μg/mL; 24 h In vivo: 50, 100 mg/kg |
Induction of apoptosis | ↑Bax, p53 ↓Bcl-2 |
[58][61] | |||
| Inhibition of angiogenesis | ↓ MMP-2, -9 | [ | 73 | ] | [ | 77 | ] | |||
| Plant | Purified flaxseed hydrolysate | Lignan | HeLa | 17.4 µg/mL; 48 h | Induction of apoptosis Inhibition of angiogenesis and metastasis |
↑ caspase-3 ↓ MMP-2, VEGF |
[15] | |||
| Fungus | Ethyl acetate extract | Penicillium sclerotiorum | HeLa | 7.75 µg/mL; 24 h | Induction of cell cycle arrest and apoptosis Inhibition of angiogenesis |
↑ Bax, p53, Apaf-1 ↓ Bcl-2 |
[60][ | Plant | Emodin | |
| Rhamnus sphaerosperma | ||||||||||
| var. | ||||||||||
| pubescens | ||||||||||
| SiHa, C33A | 46.3, 92.8, 185 μg/mL; | 6, 12, 24 h |
Induction of, apoptosis | ↓NO-, O | ||||||
| 2 | ||||||||||
| -, HOCl/OCl-, p-Akt | [ | 29 | ] | |||||||
| Plant | Epifriedelinol | |||||||||
| Aster tataricus | ||||||||||
| , | ||||||||||
| Vitex peduncularis | ||||||||||
| Wall. | HeLa | 50, 100, 250, 500, 1000 μg/mL; 72 h | Induction of apoptosis | ↑caspase-3, -8, -9 ↓Bcl-2, -xL, survivin |
Plant[30] | |||||
| Ethanol extract | Rhamnus sphaerosperma | var. pubescens | SiHa, C33A | 25, 50, 100 μg/mL; 6, 12, 24 h |
Induction of apoptosis | ↓HOCl/OCl-, p-Akt | [29] | Plant | Eugenol | |
| Syzygium aromaticum | ||||||||||
| HeLa, SiHa | 12.5, 25 µM; 24, 48 h | |||||||||
| Plant | Ethyl acetate extract | Gynura formosana | Induction of apoptosis | Kitam. | ↑Bax, PARP, caspase-3, ROS ↓Bcl-2, XIAP |
HeLa | 30 μg/mL; 72 h | Inhibition of proliferation | ↑ LC3-II/LC3-I, ↓P62/GAPDH, MCM7/GAPDH[31 |
[59][62] |
| Fungus | Ethyl acetate extract | Penicillium sclerotiorum | HeLa | 5, 25, 50 μg/mL; 24 h |
79], and two substances inhibit it. Bcl-2 was inhibited by two substances, and in addition, it showed an anti-angiogenesis effect through a mechanism that inhibits factors such as VEGF, TNF, and IAP. In the study using
Penicillium sclerotiorum, the concentration of the substance (7.75 µg/mL) was significantly lower than that of other anti-angiogenesis studies [60]. By increasing the expression of Bax, p53, and Apaf-1 and inhibiting Bcl-2, it was shown to induce not only anti-angiogenesis, but also cell cycle arrest and apoptosis at doses of 5 µg/mL, 25 µg/mL, and 50 µg/mL.
, the concentration of the substance (7.75 µg/mL) was significantly lower than that of other anti-angiogenesis studies [63]. By increasing the expression of Bax, p53, and Apaf-1 and inhibiting Bcl-2, it was shown to induce not only anti-angiogenesis, but also cell cycle arrest and apoptosis at doses of 5 µg/mL, 25 µg/mL, and 50 µg/mL.
2.3. Anti-Metastasis
Metastasis is the propagation of transformed cells from the organ of origin to other parts of the body and the successive proliferation of tumor colonies [76]. The mechanism includes complicated processes, such as cancer cell detachment from extracellular matrix, migration, invasion, and extravasation to the circulation, and most cancer patients die from metastasis rather than primary tumors [77]. Six natural products including EGCG inhibited metastasis (
Metastasis is the propagation of transformed cells from the organ of origin to other parts of the body and the successive proliferation of tumor colonies [80]. The mechanism includes complicated processes, such as cancer cell detachment from extracellular matrix, migration, invasion, and extravasation to the circulation, and most cancer patients die from metastasis rather than primary tumors [81]. Six natural products including EGCG inhibited metastasis (
). The chemical structures of compounds are shown in
.
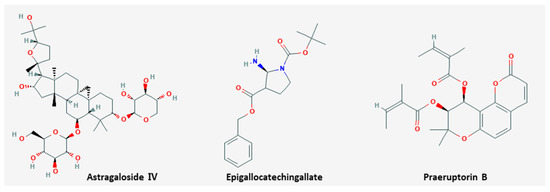
Figure 2.
Chemical structures of compounds derived from natural products inhibiting metastasis.
Table 4.
Metastasis inhibiting natural products.
| Classification | Compound/ Extract |
Source | Cell Line/ Animal Model |
Dose; Duration | Efficacy | Mechanism | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant | Astragaloside IV | Radix Astragali | SiHa | 200 µg/mL; 24 h | Inhibition of cell metastasis | ↑ E-cadherin ↓ p38, PI3K |
[78][82] | ||||||||
| Plant | Epigallocatechingallate | Green tea | HeLa | 50 µg/mL; 48 h | Inhibition of cell metastasis and proliferation Induction of apoptosis |
↓ MMP-2, -9, VEGF | [79][83] | ||||||||
| Plant | Praeruptorin B | Peucedanum praeruptorum Dunn. | HeLa, SiHa | 40, 60 µM; 24 h | Inhibition of cell metastasis | ↓ NF-κB, MMP-2, -9 | [80][84 | ||||||||
| ] | |||||||||||||||
| Induction of apoptosis and cell cycle arrest | |||||||||||||||
| ↑Bax, p53, Apaf-1 | |||||||||||||||
| ↓Bcl-2 | [ | 60 | ] | [ | 63 | ] | |||||||||
| ] | Plant | Ethyl acetate extract | Streptomyces species | SiHa | 20, 40, 60 μg/mL; 24 h | Induction of apoptosis and autophagy | ↑caspase-3, -9, Bax, LC3-Ⅱ ↓PARP, LC3-Ⅰ, Beclin1, p62 |
[61][64] | |||||||
| 63 | ] | ||||||||||||||
| Seed | Thymoquinone | Nigella sativa | CaSki, HeLa | 5 µM; 24 h | Induction of apoptosis, migration and invasion | ↑ E-cadherin ↓ Twist1, Zeb1 |
[46] | ||||||||
| Plant | Ethanol extract | Bauhinia variegata candida | HeLa | 25 µg/mL; 24 h | Inhibition of cell viability, migration and invasion | ↓ MMP-2, -9 | [55][58] | ||||||||
| Plant | Ethanol extract | Terminalia catappa | HeLa, SiHa | 25, 50, 75 µg/mL; 24 h | Inhibition of cell metastasis | ↓ MMP-9, ERK1/2 | [81][85] | Plant | Icaritin | ||||||
| Epimedium | |||||||||||||||
| HeLa, SiHa | HeLa: 12.5, 25 µM; | 24, 48, 72 h SiHa: 17, 34 µM; 24, 48, 72 h |
Induction of apoptosis Inhibition of proliferation |
↑ROS, Bax, c-caspase-3, -9 ↓Bcl-2, XIAP |
[32] | ||||||||||
| Plant | Juncusol | ||||||||||||||
| Juncus inflexu | |||||||||||||||
| HeLa, SiHa, CaSki | 1, 3, 10, 30 µM; | 24, 48, 72 h |
Induction of apoptosis Inhibition of proliferation |
↑caspase-3, -8, -9 ↓EGFR, tubulin polymerization |
[33] | ||||||||||
| Plant | Methyl protodioscin | Rhizoma of | |||||||||||||
| Polygonatum sibiricum | |||||||||||||||
| HeLa | 18.31, 40, 49 µM; 24 h | Induction of apoptosis and cell cycle arrest Inhibition of proliferation |
↑ ROS | Plant | Extract | Blueberry | SiHa | 50 mg/mL; 24 h with 4 Gy radiotherapy[34] |
|||||||
| Enhancement of radiotherapy | ↑p53 | ↓cyclin D, E, p21, survivin | [ | 62 | ] | [65] | Plant | Mitomycin C | Ginger, Frankincense | ||||||
| Plant | Lipid-soluble extract | HeLa | Pinellia pedatisecta10 µg/mL; 24 h | Schott. | HPVInduction of apoptosis Inhibition of proliferation |
+TC-1, C57BL/6 |
In vitro: 500 μg/mL; 72, 120 h In vivo: 10, 20 mg/kg[35] |
||||||||
| Induction of cell cycle arrest and apoptosis | ↑ β-catenin, c-Myc, cyclin D1, PPAR1 | ↓Th2, Th17 | [ | 63 | ] | [66] | Etc. | Naringenin oxime | HeLa, SiHa | HeLa: 12, 24 µM; 24 h SiHa: 18, 36 µM; 24 h |
Induction of apoptosis Inhibition of proliferation |
↑caspase-3 | [36] | ||
| Naringenin oxime ether | |||||||||||||||
| Plant | Nitensidine B | Leaves of | |||||||||||||
| Pterogyne nitens | |||||||||||||||
| Tul. | HPV16, SiHa | 30, 60, 120 µM; 6, 12, 24 h |
Induction of apoptosis | ↑caspase-3, -7 ↓aldolase A, alpha-enolase, pyruvate kinase, glyceraldehyde 3-p-dehydrogenase |
[37] | ||||||||||
| Plant | Notoginsenoside R7 | ||||||||||||||
| Panax notoginseng | |||||||||||||||
| HeLa, | BALB/c | In vitro: 5, 10, 20, 40 μM; 24, 36, 48 h In vivo: 5, 10 mg/kg |
Induction of apoptosis Inhibition of proliferation |
↑Bax, p-PTEN, Akt ↓Bcl-2, -xL, caspase-3, -9, raptor |
[38] | ||||||||||
| Plant | Osthole | ||||||||||||||
| Cnidiummonnieri | |||||||||||||||
| (L.) Cusson | HeLa, SiHa, | C-33A, CaSki |
40, 80, 120, 160, 200, 240 µM; 24, 48 h | Induction of apoptosis Inhibition of proliferation |
↑Bax, c-caspase-3, -9 proteins, E-cadherin, H2AX ↓Bcl-2, MMP-2, -9, β-catenin, vimentin, N-cadherin, IKKα, p-IKKα, p65, p-p65, p50, NF-κB |
[39] | |||||||||
| Plant | Physcion | ||||||||||||||
| Rhamnus sphaerosperma | |||||||||||||||
| var. | |||||||||||||||
| pubescens | |||||||||||||||
| SiHa, C33A | 43.8, 87.5, 175 μg/mL; | 6, 12, 24 h |
Induction of apoptosis | ↓HOCl/OCl-, p-Akt | [29] | ||||||||||
| Plant | Phyto-synthesis of silver nanoparticles | Garlic, Green tea, Turmeric | HeLa | 2, 5, 10, 25, 50, 100 μg/mL; 48 h | Induction of apoptosis | ↓free radical | [40] | ||||||||
| Plant | Piperine | ||||||||||||||
| Piper nigrum | |||||||||||||||
| L. | HeLa, PTX | 50 µM; 6, 24, 72 h with paclitaxel |
Induction of apoptosis | ↑Bax, Bcl-2, c-PARP, caspase-3 ↓p-Akt, Mcl-1 |
[41] | ||||||||||
| Plant | Prenylflavonoids C1 | ||||||||||||||
| Mallotus conspurcatus | |||||||||||||||
| HeLa | 30 μM; 24 h | Induction of apoptosis | ↑EGFP, ROS, Bcl-2, cytochrome c, Apaf-1, caspase-3, -9 ↓c-Myc, hTERT |
[42] | |||||||||||
| Prenylflavonoids C5 | 10 μM; 24 h | ||||||||||||||
| Plant | Protodioscin | ||||||||||||||
| Dioscoreae rhizome | |||||||||||||||
| HeLa, C33A | 4 μM; 24, 48 h | Induction of apoptosis and mitochondrial dysfunction | ↑JNK, p38, PERK, ATF4, Bax, caspase-3, -8, -9, PARP ↓Bcl-2 |
[43] | |||||||||||
| Etc. | Pterostilbene | HPV E6, TC1, C57Bl/6 |
In vitro: 30 µM; 48 h In vivo: 1 mM; 5 days |
Induction of cell cycle arrest | ↑caspase-3 ↓PCNA, VEGF |
[44] | |||||||||
| Resveratrol | |||||||||||||||
| Plant | Tf-CT-ME | ||||||||||||||
| Tripterygium wilfordii | |||||||||||||||
| HeLa | 0.5, 1, 2 µg/mL; 24 h | Induction of cell cycle arrest and apoptosis Inhibition of proliferation |
↑c-caspase-3 ↓Bcl-2/Bax |
[45] | |||||||||||
| Seed | Thymoquinone | ||||||||||||||
| Nigella sativa | |||||||||||||||
| SiHa, CaSki | 10, 20, 40 μM; | 24, 36, 48 h |
Inhibition of migration and invasion | ↑Bax, E-cadherin ↓Bcl-2, Twist1, vimentin |
[46] | ||||||||||
| Plant | Triphala | ||||||||||||||
| Terminalia chebula | |||||||||||||||
| Retz., | |||||||||||||||
| Terminalia bellerica | |||||||||||||||
| (Gaertn) Roxb., | |||||||||||||||
| Phyllanthus emblica | |||||||||||||||
| Linn. | HeLa | 25-150 μg/mL; 48 h | Induction of apoptosis | ↑ERK, p53 ↓c-Myc, cyclin D1, p-Akt, p-NF-κB, p56, p-p44/42, MAPK |
[47] | ||||||||||
| Plant | 1′S-1′-acetoxychavicol acetate | ||||||||||||||
| Alpinia conchigera | |||||||||||||||
| CaSki, SiHa | 20, 30 μM; 6, 12, 48 h | Induction of apoptosis | ↑RSU1, GAPDH | [48] | |||||||||||
| Plant | 2D of oleanolic acid and glycyrrhetinic acid | ||||||||||||||
| Ligustri Lucidi Fructus, Glycyrrhiza uralensis | |||||||||||||||
| HeLa | 2, 4 µM; 24, 48 h | Induction of apoptosis Inhibition of proliferation |
↑ROS | [49] | |||||||||||
| 3O of oleanolic acid and glycyrrhetinic acid | 1, 2 μM; 48 h | ||||||||||||||
| Etc. | 3,5,4′-trimethoxystilbene | HeLa | 10 µM; 48 h | Induction of apoptosis | [50] | ||||||||||
| 5,6,7-trimethoxyflavone | |||||||||||||||
| Plant | 5′- | ||||||||||||||
| epi | |||||||||||||||
| -SPA-6952A | |||||||||||||||
| Streptomyces diastatochromogenes | |||||||||||||||
| HeLa | 2, 4, 8, 16 µg/mL; 24 h | Induction of apoptosis and cell cycle arrest Inhibition of proliferation | ↑Bax/Bcl-2, cytochrome c, caspase-3, -9, c-PARP, p53 ↓MMP |
[51] |
Cyan fluorescent protein (CFP); yellow fluorescent protein (YFP); Bcl-2-like1 (Bcl2-L1); Bcl-2 associated X protein (Bax); B-cell lymphoma 2 (Bcl-2); matrix metalloproteinase (MMP); transcription factor (TCF); cellular myelocytomatosis oncogene (c-Myc); cytochrome complex (cytochrome c); poly (ADP-ribose) polymerase (PARP); nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB); phospho-Akt (p-Akt); B-cell lymphoma-extra large (Bcl-xL); reactive oxygen species (ROS); x-linked inhibitor of apoptosis protein (XIAP); cleaved caspase (c-caspase); epidermal growth factor receptor (EGFR); 3-phosphate-dehydrogenase (3-p-dehydrogenase); phospho-phosphatase and tensin homolog (p-PTEN); epithelial cadherin (E-cadherin); H2A histone family member X (H2AX); neural cadherin (N-cadherin); inhibitor of NF-κB kinase α (IKKα); phospho- IKKα (p-IKKα); phospho-p65 (p-p65); cleaved PARP (c-PARP); myeloid cell leukemia sequence 1 (Mcl-1); enhanced green fluorescent protein (EGFP); apoptotic protease activating factor 1 (Apaf-1); human telomerase reverse transcriptase (hTERT); c-Jun N-terminal kinases (JNK); protein kinase RNA-like endoplasmic reticulum kinase (PERK); activating transcription factor 4 (ATF4); proliferating cell nuclear antigen (PCNA); vascular endothelial growth factor (VEGF); transferrin-modified microemulsion carrying coix seed oil and tripterine (Tf-CT-ME); extracellular signal-regulated kinases (ERK); phospho- NF-κB (p-NF-κB); phospho-p44 (p-p44); mitogen-activated protein kinase (MAPK); Ras suppressor protein 1 (RSU1); glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
2.1.2. Extracts
The 19 natural extracts have been found to induce cell death in cervical cancer (
). Swanepoel et al. showed that aqueous extracts of
Anemone nemorosa caused a delay in the early mitosis phase of the cell cycle [52]. Apoptosis was confirmed through fluorescent staining with annexin V-FITC. The treatment with IC
caused a delay in the early mitosis phase of the cell cycle [55]. Apoptosis was confirmed through fluorescent staining with annexin V-FITC. The treatment with IC
50
concentration of 20.33 ± 2.480 µg/mL elevated the expression level of PS translocation, c-caspase-3, -8, and ROS in 24 h. In 48 h, however, the level of MMP and ROS were suppressed. The result of this study indicated that induction of apoptosis and autophagy and inhibition of proliferation were induced in the pathway. Aril extracts isolated from
Strelitzia nicolai induced apoptosis and inhibited oxidant in HeLa cells at a concentration of 250 μg/mL for an incubation time of 24 h, 48 h, and 72 h [53]. The aril extracts decreased cell viability by 52% and induced apoptosis in HeLa cells. Additionally, aril extracts produced a higher radical scavenging activity than the bilirubin standard. Ethanol extracts from
induced apoptosis and inhibited oxidant in HeLa cells at a concentration of 250 μg/mL for an incubation time of 24 h, 48 h, and 72 h [56]. The aril extracts decreased cell viability by 52% and induced apoptosis in HeLa cells. Additionally, aril extracts produced a higher radical scavenging activity than the bilirubin standard. Ethanol extracts from
Astragalus membranaceus
,
Angelica gigas
, and
Trichosanthes kirilowii upregulated c-caspase-3, -8, and PARP-1 and downregulated Bax, cyclin D, CDK2, CDK4, CDK6, and p27 in HeLa cells at doses of 100 μg/mL, 200 μg/mL, and 400 μg/mL for 24 h and 48 h [54]. This result suggested that induction of apoptosis, cell cycle arrest, and reduction of cell viability are involved in the pathway. However, ethanol extracts did not affect the intrinsic mitochondria-mediated apoptosis pathway in HeLa cells. Expression of c-caspase-3, -8, RIP, and TNF-R1 were increased and MMP-2 and-9 were decreased in HeLa cells by treatment of ethanol extracts from
upregulated c-caspase-3, -8, and PARP-1 and downregulated Bax, cyclin D, CDK2, CDK4, CDK6, and p27 in HeLa cells at doses of 100 μg/mL, 200 μg/mL, and 400 μg/mL for 24 h and 48 h [57]. This result suggested that induction of apoptosis, cell cycle arrest, and reduction of cell viability are involved in the pathway. However, ethanol extracts did not affect the intrinsic mitochondria-mediated apoptosis pathway in HeLa cells. Expression of c-caspase-3, -8, RIP, and TNF-R1 were increased and MMP-2 and-9 were decreased in HeLa cells by treatment of ethanol extracts from
Bauhinia variegate candida at dose of 15 μg/mL for an incubation time of 24 h [55]. Controlling mechanisms, it could reduce cell viability and inhibit migration and induct apoptosis. In conclusion, it contained components with potential tumor-selective cytotoxic action. Ethanol extracts isolated from
at dose of 15 μg/mL for an incubation time of 24 h [58]. Controlling mechanisms, it could reduce cell viability and inhibit migration and induct apoptosis. In conclusion, it contained components with potential tumor-selective cytotoxic action. Ethanol extracts isolated from
Botryidiopsidaceae species induced apoptosis and inhibited oxidant, proliferation, migration, and invasion in HeLa cells [56]. It upregulated p53 and c-caspase-3 and downregulated Bcl-2 at doses of 6.25 μg/mL, 12.5 μg/mL, 25 μg/mL, and 50 μg/mL for an incubation time of 24 h. In conclusion, inhibitory effects of ethanol extracts on migration and invasion might occur via the modulation of genes related to the processes of cellular invasion and migration. Ethanol extracts from
species induced apoptosis and inhibited oxidant, proliferation, migration, and invasion in HeLa cells [59]. It upregulated p53 and c-caspase-3 and downregulated Bcl-2 at doses of 6.25 μg/mL, 12.5 μg/mL, 25 μg/mL, and 50 μg/mL for an incubation time of 24 h. In conclusion, inhibitory effects of ethanol extracts on migration and invasion might occur via the modulation of genes related to the processes of cellular invasion and migration. Ethanol extracts from
Chloromonas species upregulated c-caspase-3 and p53 and downregulated Bcl-2 in HeLa cells at dose of 12.5 μg/mL and 25 μg/mL for an incubation time of 24 h and 72 h [57]. The result meant that ethanol extracts dealt with cancer cells by increasing the pro-apoptotic protein and reducing the anti-apoptotic protein. It suggested that induction of apoptosis through the modulation of apoptosis associated genes and inhibition of proliferation and oxidant were involved in the pathway. Ethanol extracts from
species upregulated c-caspase-3 and p53 and downregulated Bcl-2 in HeLa cells at dose of 12.5 μg/mL and 25 μg/mL for an incubation time of 24 h and 72 h [60]. The result meant that ethanol extracts dealt with cancer cells by increasing the pro-apoptotic protein and reducing the anti-apoptotic protein. It suggested that induction of apoptosis through the modulation of apoptosis associated genes and inhibition of proliferation and oxidant were involved in the pathway. Ethanol extracts from
Dendrobium chrysanthum upregulated Bax, and p53 and downregulated Bcl-2 in HeLa cells at the density of 450 μg/mL for an incubation time of 24 h [58]. This suggested that induction of apoptosis, DNA fragmentation, and ROS-altered cell morphology were involved in the pathway. In addition, the anticancer potential of the
upregulated Bax, and p53 and downregulated Bcl-2 in HeLa cells at the density of 450 μg/mL for an incubation time of 24 h [61]. This suggested that induction of apoptosis, DNA fragmentation, and ROS-altered cell morphology were involved in the pathway. In addition, the anticancer potential of the
Dendrobium chrysanthum
was mediated through p53-dependent apoptosis. In vivo, antitumor activity exhibited a significant increase in the life span of Dalton’s lymphoma-bearing mice with significant decrease in abdominal size along with reduced tumor ascites. Ethanol extracts from
Rhamnus sphaerosperma
var.
pubescens (EERs) reduced activation of HOCl/OCl- and p-Akt at 25 μg/mL, 50 μg/mL, and 100 μg/mL for 6 h, 12 h, and 24 h for the treatment of SiHa and C33A cells [29]. The result showed that EERs were related to the induction of cell cytotoxicity, apoptosis, oxidative stress, and DNA damage. Ma et al. reported that EAEG, ethyl acetate extracts of
(EERs) reduced activation of HOCl/OCl- and p-Akt at 25 μg/mL, 50 μg/mL, and 100 μg/mL for 6 h, 12 h, and 24 h for the treatment of SiHa and C33A cells [29]. The result showed that EERs were related to the induction of cell cytotoxicity, apoptosis, oxidative stress, and DNA damage. Ma et al. reported that EAEG, ethyl acetate extracts of
Gynura formosana Kitam. leaves, exhibited antioxidant, anti-inflammatory, and autophagy-mediated inhibition of cell proliferation activity on HeLa cells [59]. The increased levels of LC3-II/LC3-I and decreased levels of P62/GAPDH and MCM7/GAPDH were observed at a concentration of 30 µg/mL for 72 h. Kuriakose et al. reported that ethyl acetate extracts of
Kitam. leaves, exhibited antioxidant, anti-inflammatory, and autophagy-mediated inhibition of cell proliferation activity on HeLa cells [62]. The increased levels of LC3-II/LC3-I and decreased levels of P62/GAPDH and MCM7/GAPDH were observed at a concentration of 30 µg/mL for 72 h. Kuriakose et al. reported that ethyl acetate extracts of
Penicillium sclerotiorum
, isolated from
Cassia fistula L., had cytotoxic activity [60]. At doses of 5 μg/mL, 25 μg/mL, and 50 μg/mL for 24 h, it arrested cells at S and G2/M phase of the cell cycle in a dose-dependent manner. Annexin V/propidium iodide double staining showed apoptosis more than necrosis. Moreover, the decreased Bcl-2 and increased Bax, p53, and Apaf-1 support apoptotic cell death. Ethyl acetate extracts from
L., had cytotoxic activity [63]. At doses of 5 μg/mL, 25 μg/mL, and 50 μg/mL for 24 h, it arrested cells at S and G2/M phase of the cell cycle in a dose-dependent manner. Annexin V/propidium iodide double staining showed apoptosis more than necrosis. Moreover, the decreased Bcl-2 and increased Bax, p53, and Apaf-1 support apoptotic cell death. Ethyl acetate extracts from
Streptomyces species upregulated caspase-3, -9, Bax, and LC3-II and downregulated PARP, LC3-I, Beclin1, and p62 in SiHa cells at concentrations of 20 μg/mL, 40 μg/mL, and 60 μg/mL for 24 h [61]. Half of the SiHa cells underwent death at a concentration of 20 μg/mL. This induced altered cell morphology and apoptosis, autophagy, and inhibited proliferation in the pathway. Consequently, ethyl acetate extracts-induced Bax-dependent mitochondrial permeabilization plays the role of an initiator of intrinsic pathway of apoptosis. Expression of p53 was increased and cyclin D, E, p21, and survivin were decreased in SiHa cells by treatment of blueberry extracts at the density of 50 mg/mL for an incubation time of 24 h with 4 Gy radiotherapy [62]. Through the mechanisms, it enhanced radiotherapy. Lipid-soluble extracts from
species upregulated caspase-3, -9, Bax, and LC3-II and downregulated PARP, LC3-I, Beclin1, and p62 in SiHa cells at concentrations of 20 μg/mL, 40 μg/mL, and 60 μg/mL for 24 h [64]. Half of the SiHa cells underwent death at a concentration of 20 μg/mL. This induced altered cell morphology and apoptosis, autophagy, and inhibited proliferation in the pathway. Consequently, ethyl acetate extracts-induced Bax-dependent mitochondrial permeabilization plays the role of an initiator of intrinsic pathway of apoptosis. Expression of p53 was increased and cyclin D, E, p21, and survivin were decreased in SiHa cells by treatment of blueberry extracts at the density of 50 mg/mL for an incubation time of 24 h with 4 Gy radiotherapy [65]. Through the mechanisms, it enhanced radiotherapy. Lipid-soluble extracts from
Pinellia pedatisecta Schott. upregulated β-catenin, c-Myc, cyclin D1, PPAR1, and downregulated Th2 and Th17 in HPV and TC-1 cells at a dose of 500 μg/mL dealing with 72 h [63]. This result suggested that induction of apoptosis and cell cycle arrest were involved in the pathway. The subset proportion of Th1 cells increased significantly and both Th2 cells and Th17 cells decreased profoundly. In vivo, T lymphocyte infiltration in tumor-burdened mice was enhanced with treatment. Methanol extracts from
Schott. upregulated β-catenin, c-Myc, cyclin D1, PPAR1, and downregulated Th2 and Th17 in HPV and TC-1 cells at a dose of 500 μg/mL dealing with 72 h [66]. This result suggested that induction of apoptosis and cell cycle arrest were involved in the pathway. The subset proportion of Th1 cells increased significantly and both Th2 cells and Th17 cells decreased profoundly. In vivo, T lymphocyte infiltration in tumor-burdened mice was enhanced with treatment. Methanol extracts from
Allium atroviolaceum upregulated caspase-3, -5, and -9 and downregulated Bcl-2, CDK1, and p53 in HeLa cells at concentrations of 20 μg/mL, 40 μg/mL, 60 μg/mL, 80 μg/mL, and 100 μg/mL for 24 h, 48 h, and 72 h [64]. The efficacy was the best at 72 h. Controlling the mechanisms, it inhibited cell growth and proliferation, induced cell cycle arrest, and reduced cell viability. The level of caspase-3 was increased and the level of PARP-1 was decreased by methanol extracts from
upregulated caspase-3, -5, and -9 and downregulated Bcl-2, CDK1, and p53 in HeLa cells at concentrations of 20 μg/mL, 40 μg/mL, 60 μg/mL, 80 μg/mL, and 100 μg/mL for 24 h, 48 h, and 72 h [67]. The efficacy was the best at 72 h. Controlling the mechanisms, it inhibited cell growth and proliferation, induced cell cycle arrest, and reduced cell viability. The level of caspase-3 was increased and the level of PARP-1 was decreased by methanol extracts from
Corylus avellane in HeLa cells at dose of 250 μg/mL and 500 μg/mL for 24 h [65]. The expression of cleaved forms of caspase-3 and PARP-1 suggested that the extracts induced apoptosis through caspase-3 activation in HeLa cells. In conclusion, this result suggested that reduction of cell viability, induction of apoptosis, inhibition of oxidant and proliferation were involved in the pathway. Methanol extracts isolated from
in HeLa cells at dose of 250 μg/mL and 500 μg/mL for 24 h [68]. The expression of cleaved forms of caspase-3 and PARP-1 suggested that the extracts induced apoptosis through caspase-3 activation in HeLa cells. In conclusion, this result suggested that reduction of cell viability, induction of apoptosis, inhibition of oxidant and proliferation were involved in the pathway. Methanol extracts isolated from
Cyperus rotundus induced apoptosis and DNA fragmentation and inhibited migration in HeLa cells at doses of 25 μg/mL, 50 μg/mL, and 100 μg/mL for an incubation time of 24 h and 48 h [66]. Cytotoxic effects of methanol extracts on the tested cancer cell lines ranged from 4.52 ± 0.57 μg/mL
induced apoptosis and DNA fragmentation and inhibited migration in HeLa cells at doses of 25 μg/mL, 50 μg/mL, and 100 μg/mL for an incubation time of 24 h and 48 h [69]. Cytotoxic effects of methanol extracts on the tested cancer cell lines ranged from 4.52 ± 0.57 μg/mL
−1
to 9.85 ± 0.68 μg/mL
−1
. Moreover, methanol extracts showed anticancer and antimigration activity and it also induced nuclear fragmentation in HeLa cells. Expression of Bax, BAD, caspase-3, p21, and p53 were increased and Bcl-2 was decreased in HeLa cells by treatment of methanol extracts isolated from
Polyalthia longifolia at dose of 22 μg/mL for 6 h, 12 h, 24 h, 36 h [67]. Controlling the mechanisms, it altered cell morphology and induced apoptosis. Consequently, extracts produced distinctive porphological features of HeLa cell death that corresponds to apoptosis. Sul’ain et al. reported that the methanol extracts of
at dose of 22 μg/mL for 6 h, 12 h, 24 h, 36 h [70]. Controlling the mechanisms, it altered cell morphology and induced apoptosis. Consequently, extracts produced distinctive porphological features of HeLa cell death that corresponds to apoptosis. Sul’ain et al. reported that the methanol extracts of
Pyrrosia piloselloides showed antiproliferative effects on HeLa cells [68]. This efficacy was caused by treatment with an IC
showed antiproliferative effects on HeLa cells [71]. This efficacy was caused by treatment with an IC
50
of 16.25 μg/mL. Meanwhile,
Pyrrosia piloselloides
water extracts were without influence. Panicker et al. reported that methanol extracts from
Teucrium mascatense were shown to activate caspases and PARP on HeLa cells, following treatment with 25 µg/mL, 50 µg/mL, 125 µg/mL, and 250 µg/mL, for 72 h [69]. In addition, cell rounding, shrinkage, and detachment from other cells were shown by methanol extracts. This result suggested apoptosis and alteration of cell morphology were related to the pathway.
were shown to activate caspases and PARP on HeLa cells, following treatment with 25 µg/mL, 50 µg/mL, 125 µg/mL, and 250 µg/mL, for 72 h [72]. In addition, cell rounding, shrinkage, and detachment from other cells were shown by methanol extracts. This result suggested apoptosis and alteration of cell morphology were related to the pathway.
Table 2.
Apoptosis inducing natural products-extracts.
| Classification | Extract | Source | Cell Line/ Animal Model |
Dose; Duration | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|---|---|
| Plant | Aqueous extract | Anemone nemorosa | HeLa | 20.33 ± 2.480 μg/mL; 24, 48 h |
Induction of apoptosis Inhibition of proliferation |
↑PS translocation, c-caspase-3, -8, ROS (24 h) ↓MMP, ROS (48 h) |
[52][55] |
| Plant | Aril extract | Strelitzia nicolai | HeLa | 250 μg/mL; 24, 48, 72 h | Inhibition of oxidative stress Induction of apoptosis |
[53][56] | |
| Plant | Ethanol extract | Astragalus membranaceus, Angelica gigas, Trichosanthes kirilowii Maximowicz. |
HeLa | 100, 200, 400 μg/mL; 24, 48 h |
Induction of apoptosis and cell cycle arrest Inhibition of cell viability |
↑c-caspase-3, -8, PARP-1 ↓Bax, cyclin D, CDK2, CDK4, CDK6, p27 |
[54][57] |
| Plant | Ethanol extract | Bauhinia variegate candida | HeLa | ||||
| Plant | |||||||
| Methanol extract | |||||||
| Allium atroviolaceum | |||||||
| HeLa | |||||||
| 20, 40, 60, 80, 100 μg/mL; | 24, 48, 72 h | Induction of cell cycle arrest | ↑caspase-3, -5, -9 | ↓Bcl-2, CDK1, p53 | [64][67] | ||
| Plant | Methanol extract | Corylus avellane L. | HeLa | 250, 500 μg/mL; 24 h | Inhibition of oxidative stress Induction of apoptosis |
↑caspase-3 ↓PARP-1 |
[65][68] |
| Plant | Methanol extract | Cyperus rotundus | HeLa | 25, 50, 100 μg/mL; 24, 48 h |
Induction of apoptosis | [66][69] | |
| Plant | Methanol extract | Polyalthia longifolia | HeLa | 22 μg/mL; 6, 12, 24, 36 h | Induction of apoptosis | ↑Bax, BAD, caspase-3, p21, p53 ↓Bcl-2 |
[67][70] |
| Plant | Methanol extract | Pyrrosia piloselloides | HeLa, | 16.25 μg/mL; 24, 48, 72 h | Inhibition of proliferation | [68][71] | |
| Plant | Methanol extract | Teucrium mascatense | HeLa | 25, 50, 125, 250 μg/mL; 72 h | Induction of apoptosis Inhibition of proliferation |
↑c-caspase-7, -8, -9, PARP | [69][72] |
Phosphatidylserine (PS); cyclin-dependant kinase (CDK); receptor-interacting protein (RIP); tumor necrosis factor receptor 1 (TNF-R1); light chain (LC); minichromosome maintenance protein complex (MCM); peroxisome proliferator-activated receptor 1 (PPAR1); T helper cell (Th); Bcl-2-associated death promoter (BAD).
2.2. Anti-angiogenesis
3.2. Anti-angiogenesis
Angiogenesis is a major cause in the development and metastasis of a variety of tumor types [70]. Local angiogenesis provides oxygen and essential nutrients to the growing tumor, supports tumor expansion and invasion into nearby normal tissue, and is essential for distant metastasis [71]. To be specific, in cervical cancer, angiogenesis plays a leading role in initiation, proliferation, and progression, and also relates to blocking p53 and stabilizing hypoxia-inducible factor-1α, which led to expression of VEGF [72]. So, preventing angiogenesis could be significant in treatment of the disease. Four natural products have this ability toward HeLa cells and CaSki cells (
Angiogenesis is a major cause in the development and metastasis of a variety of tumor types [74]. Local angiogenesis provides oxygen and essential nutrients to the growing tumor, supports tumor expansion and invasion into nearby normal tissue, and is essential for distant metastasis [75]. To be specific, in cervical cancer, angiogenesis plays a leading role in initiation, proliferation, and progression, and also relates to blocking p53 and stabilizing hypoxia-inducible factor-1α, which led to expression of VEGF [76]. So, preventing angiogenesis could be significant in treatment of the disease. Four natural products have this ability toward HeLa cells and CaSki cells (
). When HeLa cells were treated by
Praecitrullus fistulosus lectin protein (PfLP), which was grown and consumed in subtropical countries, MMP-2 and -9 became downregulated and it led to the induction of apoptosis and inhibition of angiogenesis [73]. This mechanism was efficient at a dose of 50 µg/mL with 24 h. In vivo, anticancer and anti-angiogenic properties of PfLP were observed at dose of 10 mg/kg on day 7, 9, and 11 after the tumor cell transplantation. Moreover, it did not show any side effects or secondary complications in the study. Purified Flaxseed hydrolysate (PFH), extracted from lignan, was shown to induce apoptosis and inhibit angiogenesis and metastasis on HeLa cells [15]. This process was caused by increasing caspase-3 and downregulating MMP-2 and VEGF. The efficient dose was 17.4 µg/mL, dealing with a timeframe of 48 h. These results suggest that PFH could be great treatment with its anticancer activity. Based on our findings, Kuriakose et al. reported that expression of Bax, p53, and Apaf-1 were increased and Bcl-2 was decreased in HeLa cells by treatment of ethyl acetate extracts of
lectin protein (PfLP), which was grown and consumed in subtropical countries, MMP-2 and -9 became downregulated and it led to the induction of apoptosis and inhibition of angiogenesis [77]. This mechanism was efficient at a dose of 50 µg/mL with 24 h. In vivo, anticancer and anti-angiogenic properties of PfLP were observed at dose of 10 mg/kg on day 7, 9, and 11 after the tumor cell transplantation. Moreover, it did not show any side effects or secondary complications in the study. Purified Flaxseed hydrolysate (PFH), extracted from lignan, was shown to induce apoptosis and inhibit angiogenesis and metastasis on HeLa cells [15]. This process was caused by increasing caspase-3 and downregulating MMP-2 and VEGF. The efficient dose was 17.4 µg/mL, dealing with a timeframe of 48 h. These results suggest that PFH could be great treatment with its anticancer activity. Based on our findings, Kuriakose et al. reported that expression of Bax, p53, and Apaf-1 were increased and Bcl-2 was decreased in HeLa cells by treatment of ethyl acetate extracts of
Penicillium sclerotiorum
from
Cassia fistula L. [60]. Hexadecanoic acid, oleic acid, and benzoic acid were the major active parts in this treatment. Through controlling mechanisms, it could induce cell cycle arrest and inhibit angiogenesis at a dose of 7.75 µg/mL. Seifaddinipour et al. reported that ethyl acetate extracts isolated from
L. [63]. Hexadecanoic acid, oleic acid, and benzoic acid were the major active parts in this treatment. Through controlling mechanisms, it could induce cell cycle arrest and inhibit angiogenesis at a dose of 7.75 µg/mL. Seifaddinipour et al. reported that ethyl acetate extracts isolated from
| Plant | |||||||
| Ethyl acetate extract | |||||||
| Pistacia vera | L. | CaSki | 81.17 ± 2.87 µg/mL; 72 h | Induction of apoptosis | Inhibition of angiogenesis |
↓ TNF, Bcl-2, IAP, TRAF | [74][78] |
Praecitrullus fistulosus lectin protein (PfLP); inhibitor of apoptosis protein (IAP); TNF receptor-associated factor (TRAF).
A total of four natural products exhibited anti-angiogenesis, and the mechanisms were very diverse. MMP performs a complex and important role in cancer growth and metastasis [75], and two substances inhibit it. Bcl-2 was inhibited by two substances, and in addition, it showed an anti-angiogenesis effect through a mechanism that inhibits factors such as VEGF, TNF, and IAP. In the study using
A total of four natural products exhibited anti-angiogenesis, and the mechanisms were very diverse. MMP performs a complex and important role in cancer growth and metastasis [
Phosphoinositide 3-kinase (PI3K); Zinc finger E-box-binding homeobox 1 (Zeb1).
Expression of E-cadherin was increased and p38 and PI3K were decreased in SiHa cells by treatment of astragaloside IV from Radix Astragali [78]. Additionally, astragaloside IV demonstrated inhibition of cell metastasis on SiHa cell lines. The efficient dose was 200 µg/mL, dealing with a timeframe of 24 h. Expression of MMP-2, -9, and VEGF was decreased in HeLa cells by treatment of epigallocatechingallate (EGCG) from green tea [79]. Thus, it induced apoptosis at a dose of 50 µg/mL for 48 h. In addition, it inhibited cell metastasis and proliferation in cervical cancer cells by reducing VEGF, CDK2, and ERK1/2. Praeruptorin-B isolated from
Expression of E-cadherin was increased and p38 and PI3K were decreased in SiHa cells by treatment of astragaloside IV from Radix Astragali [82]. Additionally, astragaloside IV demonstrated inhibition of cell metastasis on SiHa cell lines. The efficient dose was 200 µg/mL, dealing with a timeframe of 24 h. Expression of MMP-2, -9, and VEGF was decreased in HeLa cells by treatment of epigallocatechingallate (EGCG) from green tea [83]. Thus, it induced apoptosis at a dose of 50 µg/mL for 48 h. In addition, it inhibited cell metastasis and proliferation in cervical cancer cells by reducing VEGF, CDK2, and ERK1/2. Praeruptorin-B isolated from
Peucedanum praeruptorum Dunn. downregulated NF-κB, MMP-2, and -9 in HeLa and SiHa cells [80]. Inhibition of cell metastasis could be observed following the treatment of praeruptorin-B at doses of 40 µg and 60 µg over 24 h. Moreover, it blocked Akt phosphorylation without affecting the MAPK pathway. These results suggested that praeruptorin-B could be good at anticancer activity, especially in cervical cancer cells. Thymoquinone from
Dunn. downregulated NF-κB, MMP-2, and -9 in HeLa and SiHa cells [84]. Inhibition of cell metastasis could be observed following the treatment of praeruptorin-B at doses of 40 µg and 60 µg over 24 h. Moreover, it blocked Akt phosphorylation without affecting the MAPK pathway. These results suggested that praeruptorin-B could be good at anticancer activity, especially in cervical cancer cells. Thymoquinone from
Nigella sativa was shown to induce apoptosis, migration, and invasion on CaSki and HeLa cells [46]. It was efficient when CaSki cells and HeLa cells were treated at a concentration of 5 µM for 24 h. Furthermore, it included the mechanism of increasing E-cadherin level and decreasing the level of Twist1 and Zeb1. Ethanol extracts from
was shown to induce apoptosis, migration, and invasion on CaSki and HeLa cells [46]. It was efficient when CaSki cells and HeLa cells were treated at a concentration of 5 µM for 24 h. Furthermore, it included the mechanism of increasing E-cadherin level and decreasing the level of Twist1 and Zeb1. Ethanol extracts from
Bauhinia variegata candida decreased the level of MMP-2 and -9 on HeLa cells [55]. This mechanism was caused when HeLa cells were treated at a concentration of 25 µg/mL for 24 h. Subsequently, it reduced cell viability, migration, and invasion. Lee et al. reported that the decline of MMP-9 and ERK1/2 was observed after the exposure of
decreased the level of MMP-2 and -9 on HeLa cells [58]. This mechanism was caused when HeLa cells were treated at a concentration of 25 µg/mL for 24 h. Subsequently, it reduced cell viability, migration, and invasion. Lee et al. reported that the decline of MMP-9 and ERK1/2 was observed after the exposure of
Terminalia catappa ethanol extracts on HeLa and SiHa cells [81]. This process was efficient when the dose was 25 µg/mL, 50 µg/mL, and 75 µg/mL, dealing with a timeframe of 24 h and it led to inhibition of cell metastasis. In conclusion, ethanol extracts blocked the MMP-9 through ERK1/2 pathway by anti-metastatic effects.
ethanol extracts on HeLa and SiHa cells [85]. This process was efficient when the dose was 25 µg/mL, 50 µg/mL, and 75 µg/mL, dealing with a timeframe of 24 h and it led to inhibition of cell metastasis. In conclusion, ethanol extracts blocked the MMP-9 through ERK1/2 pathway by anti-metastatic effects.
A total of six substances derived from natural products inhibited the metastasis of cervical cancer, and MMP control was the most important mechanism. Four substances regulated MMP, MMP-2 was inhibited in three substances, and MMP-9 was inhibited in four substances. E-cadherin is a protein remarkably related to tumor invasion, metastatic transmission, and poor patient prognosis [82]. It was derived from two substances, and in addition, it showed anti-metastasis action through inhibitory mechanisms such as p38, VEGF, and Twist1. In particular, ethanol extracts from
A total of six substances derived from natural products inhibited the metastasis of cervical cancer, and MMP control was the most important mechanism. Four substances regulated MMP, MMP-2 was inhibited in three substances, and MMP-9 was inhibited in four substances. E-cadherin is a protein remarkably related to tumor invasion, metastatic transmission, and poor patient prognosis [86]. It was derived from two substances, and in addition, it showed anti-metastasis action through inhibitory mechanisms such as p38, VEGF, and Twist1. In particular, ethanol extracts from
Bauhinia variegate candida exhibited multi-effect, inhibited migration and invasion at 25 µg/mL, and also induced apoptosis at 15 µg/mL [55]. However, the concentration of the natural product was so high (200 µg/mL) that there was a study involving cytotoxicity concerns [78].
exhibited multi-effect, inhibited migration and invasion at 25 µg/mL, and also induced apoptosis at 15 µg/mL [58]. However, the concentration of the natural product was so high (200 µg/mL) that there was a study involving cytotoxicity concerns [82].
