Cattle are susceptible to heat stress, especially those kept on high levels of nutrition for the purpose of maximising growth rates, which leads to a significant heat increment in their bodies. Consequences include compromised health and productivity and mortalities during extreme events, as well as serious economic loss.
- body temperature
- heat stress
- hyperthermia
- infrared thermography
Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
1. Introduction
Welfare and productivity of cattle can be severely compromised in hot environments, especially for those kept in subtropical and tropical regions where animals are regularly exposed to high ambient temperature and/or relative humidity for prolonged periods [1]. In the literature, either “heat load” or “heat stress” are used to describe the animal’s suffering under hot conditions [2,3][2][3]. Cattle exposed to prolonged heat stress reduce their feed intake, milk production [4–6][4][5][6], growth and welfare [7], reproductive performance [8], health, and immunity. Consequences include serious economic loss and mortalities during extreme events [9,10][9][10]. Almost 20 years ago, In the US beef industry heat stress resulted in more than 300 million dollars of loss to the national economy [11]. In Australia, a 16.5-million-dollar loss to feedlot industry has been estimated over the summer period in 2006 [12] and more recently during 2019–2020, high temperatures cause not only heat stress but also bushfires affecting livestock industry, exact estimate awaited. Thermal acclimation to a heat stress event helps to maintain productivity but it is variable for different beef breeds, for example it has been reported as 9 and 14 days for Angus and Charolais, respectively [13].
The body temperature of animals increases in hot environmental conditions, including high humidity and/or high solar radiation, particularly when ambient temperature exceeds the upper critical temperature [14]. Animals suffering with these conditions attempt to cope by dissipating heat through different physiological means, that are coordinated by the hypothalamus [14,15][14][15]. These centre on heat dissipation from the surface of the skin through increased peripheral circulation, vasodilatation, and sweating [16].
The endocrine response to heat stress is mainly reflected in elevated glucocorticoids (cortisol), aldosterone, antidiuretic hormone, thyroxine, prolactin, and growth hormone (GH) [17,18][17][18]. The benefits of using faeces that has been discharged from the body as a suitable biological material for estimation of cortisol metabolites is that it provides a longer-term non-invasive approach, compared with conventional blood sampling, which is a short-term assessment and an invasive procedure, requiring handling of animals [19–21][19][20][21]. Higher plasma cortisol concentrations in cows were reported in earlier studies during acute heat stress conditions [22[22][23],23], but increased concentrations of cortisol metabolites in faeces have been shown in animals exposed to acute heat stress conditions [18].
Cattle core body temperature ranges from 38.0 to 39.3 °C during thermoneutral conditions [16,24][24]. Different methods are used for estimating core body temperature, by approaching through the rectum [24[24][25],25], vagina [26–28][26][27][28], tympanic membrane of the ear [29], rumen [3[30],30], or using infrared thermography of external body surfaces [31,32][31][32]. These all differ in their speed of response to heat stress, accuracy, and degree of invasiveness [33], and are discussed individually in this review.
Climate change means that cattle will be increasingly exposed to heat stress [34]. Economic losses, resulting from poor production performance of heat stressed animals, could be reduced by predicting the exact time for the implementation of corrective actions to manage the risk of heat stress. This could be accomplished through better understanding of the cattle responses during heat stress conditions. The measurement of faecal and hair cortisol hormone metabolites and infrared thermography of external body surfaces are among the most recent approaches, with the added advantage that they are non-invasive. We hypothesized that heat stress detection in cattle is supported by a growing literature that will eventually enable farmers to control this source of stress.
2. Stress Response to Hot Environment
Selye [35,36][35][36] demonstrated a common stress response, driven by the adrenal gland, to different stressors and defined stress as the biological responses upon exposure to an adverse environmental condition. The term “stressors” was used to define adverse environmental conditions. Stress does not always result in overall detrimental effects for animals [37], as this could be an indication for adaptation to a new environment [35].
Extreme environmental circumstances, hot or cold, are potential stressors [35]. In particular, high temperatures often lead to low productivity, lowered immune responses, high morbidity, and mortality. The exposure of animals with hot environmental conditions are described using one of the two terms either “heat load” and or “heat stress” [2,38][38]. Heat load would appear to be an indication of the levels of factors that are supplying the environmental impact on the animal and the heat stress is how the animal responds.
Heat stress affects cattle bioenergetics [17[39][40],39,40], and particularly afflicts rapidly growing beef cattle and high lactating cows because of their requirement for a high plane of nutrition and the significant heat increment arising from rumen fermentation [1]. Cattle respond to heat stress through increased heat dissipation [17] by physiological and behavioural thermoregulatory mechanisms [16,38,39][38][39]. These mechanisms involve increased heat dissipation through sweating and peripheral circulation, increased respiration, panting, and also reducing feed intake to lower metabolic heat [16].
3. Thermoregulatory Mechanism
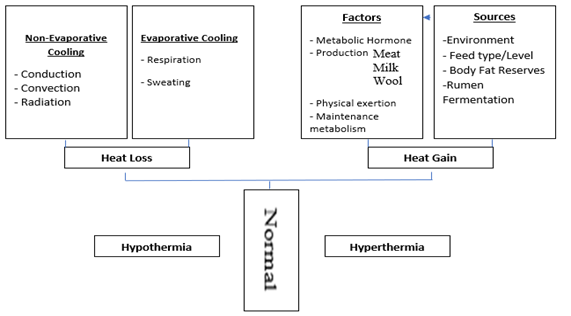
In homeotherms, initial thermoregulatory response is to maintain and restore constant core body temperature, allowing heat to dissipate from the body through insensible and sensible thermal heat exchange. Insensible heat exchange also known as evaporative heat loss uses evaporation, that involves a vapour gradient for thermal heat exchange. Sensible thermal heat exchange also known as non-evaporative heat loss that involves heat loss through a thermal gradient. Sensible thermal heat exchange includes convection, conduction, and radiation [7]. Thermoregulatory mechanisms have already been reviewed in literature [7[41],41], however, for a quick review, these mechanisms are detailed here in Figure 1.
Figure 1. Thermoregulatory mechanisms adapted from Tait [41] and Yousef [42]
4. Environmental Factors Related to Heat Stress in Cattle
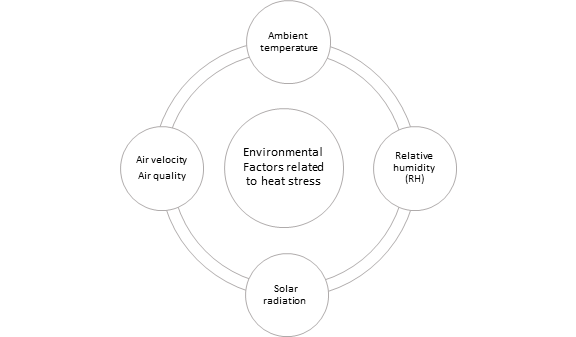
Environmental factors affecting cattle welfare due to heat stress include ambient temperature, relative humidity, and solar radiation, having direct or indirect impact on cattle welfare. Although brief exposure to hot environmental conditions may result an insignificant impact on production performance, during sustained exposure to hot conditions welfare problems can arise, especially for the high-producing dairy and feedlot cattle [17,43] [43]. The animal’s climatic environment is complex, especially in outdoor conditions. The impact of environmental factors related to heat stress in cattle has already been reviewed in literature [44], however for a quick review, these factors are outlined in Figure 2.
Figure 2. Environmental factors related to heat stress in cattle.
References
- Nienaber, J.A.; Hahn, G.L. Livestock production system management responses to thermal challenges. Int. J. Biometeorol. 2007, 52, 149–157, doi:10.1007/s00484-007-0103-x.
- Hansen, P.J. Physiological and cellular adaptations of zebu cattle to thermal stress. Anim. Reprod. Sci. 2004, 82, 349–360, doi:10.1016/j.anireprosci.2004.04.011.
- Lees, A.M. Biological Responses of Feedlot Cattle to Heat Load. Ph.D. Thesis, The University of Queensland, Gatton campus, Australia, 2016.
- West, J.W. Effects of heat-stress on production in dairy cattle. J. Dairy Sci. 2003. 86, 2131-2144, doi:10.3168/jds.S0022-0302(03)73803-X.
- Rhoads, M.L.; Rhoads, R.P.; VanBaale, M.J.; Collier, R.J.; Sanders, S.R.; Weber, W.J.; Crooker, B.A.; Baumgard, L.H. Effects of heat stress and plane of nutrition on lactating Holstein cows: I. Production, metabolism, and aspects of circulating somatotropin. J. Dairy Sci. 2009, 92, 1986–1997, doi:10.3168/jds.2008-1641.
- Renaudeau, D.; Collin, A.; Yahav, S.; De Basilio, V.; Gourdine, J.; Collier, R.J. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal 2012, 6, 707–728, doi:10.1017/S1751731111002448.
- Silanikove, N. Effects of heat stress on the welfare of extensively managed domestic ruminants. Livest. Prod. Sci. 2000, 67, 1–18, doi:10.1016/S0301-6226(00)00162-7.
- Bernabucci, U.; Lacetera, N.; Baumgard, L.H.; Rhoads, R.P.; Ronchi, B.; Nardone, A. Metabolic and hormonal acclimation to heat stress in domesticated ruminants. Animal 2010, 4, 1167, doi:10.1017/S175173111000090X.
- Lacetera, N.; Bernabucci, U.; Scalia, D.; Basiricò, L.; Morera, P.; Nardone, A. Heat stress elicits different responses in peripheral blood mononuclear cells from Brown Swiss and Holstein cows. J. Dairy Sci. 2006, 89, 4606–4612, doi:10.3168/jds.S0022-0302(06)72510-3.
- Lacerda, T.; Loureiro, B. Selecting thermotolerant animals as a strategy to improve fertility in Holstein cows. Glob. J. Anim. Sci. Res. 2015, 3, 119–127.
- St-Pierre, N.R.; Cobanov, B.; Schnitkey, G. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 2003, 86, E52–E77, doi:10.3168/jds. S0022-0302(03)74040-5.
- Sackett, D.; Holmes, P.; Abbot, K.; Jephcott, S.; Barber, M. Assessing the Economic Cost of Endemic Disease on the Profitability of Australian Beef Cattle and Sheep Producers; MLA Final Report AHW.087; Meat and Livestock Australia: Sydney, Australia, 2006.
- Senft, R.; Rittenhouse, L. A model of thermal acclimation in cattle. J. Anim. Sci. 1985, 61, 297–306, doi:10.2527/jas1985.612297x.
- Vermunt, J.J.; Tranter, B.P. Heat stress in dairy cattle—A review, and some of the potential risks associated with the nutritional management of this condition. In Proceedings of the Annual Conference of the Australian Veterinary Association—Queensland Division, Townsville, Australia, 25–27 March 2011; pp. 212–221.
- Veissier, I.; Laer, E.V.; Palme, R.; Moons, C.P.H.; Ampe, B.; Sonck, B.; Andanson, S.; Tuyttens, F.A. Heat stress in cows at pasture and benefit of shade in a temperate climate region. Int. J. Biometeorol. 2018, 62, 585–595, doi:10.1007/s00484-017-1468-0.
- Farooq, U.; Samad, H.; Shehzad, F.; Qayyum, A. Physiological responses of cattle to heat stress. World Appl. Sci. J. 2010, 8, 38–43.
- Scharf, B.A. Comparison of Thermoregulatory Mechanisms in Heat Sensitive and Tolerant Breeds of Bos taurus Cattle. Master’s Thesis, Science-University of Missouri-Columbia, MO 65211, United States, 2008, doi:10.32469/10355/5689.
- Rees, A.; Fischer-Tenhagen, C.; Heuwieser, W. Effect of Heat Stress on Concentrations of Faecal Cortisol Metabolites in Dairy Cows. Reprod. Domest. Anim. 2016, 51, 392–399, doi:10.1111/rda.12691.
- Palme, R.; Robia, C.; Messmann, S.; Hofer, J.; Mostl, E. Measurement of faecal cortisol metabolites in ruminants: A non-invasive parameter of adrenocortical function. Wien. Tierarztl. Monat. 1999, 86, 237–241.
- Möstl, E.; Palme, R. Hormones as indicators of stress. Domest. Anim. Endocrinol. 2002, 23, 67–74, doi:10.1016/S0739-7240(02)00146-7.
- Palme, R. Monitoring stress hormone metabolites as a useful, non-invasive tool for welfare assessment in farm animals. Anim. Welf. 2012, 21, 331, doi:10.7120/09627286.21.3.331.
- Christison, G.; Johnson, H. Cortisol turnover in heat-stressed cows 1. J. Anim. Sci. 1972, 35, 1005–1010.
- Beede, D.K.; Collier, R.J.; Mallonee, P.G.; Wilcox, C.J.; Buffington, D.E. Heat stress and dietary potassium effects on circadian profiles of blood prolactin and aldosterone in lactating cows. In American Society of Agricultural Engineers; ASAE publication: St. Joseph, MO, USA, 1982.
- Gaughan, J.B. Respiration Rate and Rectal Temperature Responses of Feedlot Cattle in Dynamic, Thermally Challenging Environments. Ph.D. Thesis, The University of Queensland, Gatton Campus, Australia, 2002.
- Reuter, R.R.; Carroll, J.A.; Hulbert, L.E.; Dailey, J.W.; Galyean, M.L. Development of a self-contained, indwelling rectal temperature probe for cattle research. J. Anim. Sci. 2010, 88, 3291–3295, doi:10.2527/jas.2010-3093.
- Hillman, P.; Willard, S.; Lee, C.; Kennedy, S. Efficacy of a Vaginal Temperature Logger to Record Body Temperatures of Dairy Cows; ASAE: Las Vegas, NV, USA, 2003.
- Hillman, P.; Lee, C.; Willard, S. Thermoregulatory responses associated with lying and standing in heat-stressed dairy cows. Trans. Am. Soc. Agric. Engr. 2005, 48, 795–801, doi:10.13031/2013.18322.
- Hillman, P.E.; Gebremedhin, K.G.; Willard, S.T.; Lee, C.N.; Kennedy, A.D. Continuous measurements of vaginal temperature of female cattle using a data logger encased in a plastic anchor. Appl. Eng. Agric. 2009, 25, 291–296, doi:10.13031/2013.26332.
- Brown-Brandl, T.; Eigenberg, R.; Hahn, G.; Nienaber, J. Measurements of bioenergetic responses in livestock. In American Society of Agricultural Engineers Special Meetings and Conferences Papers; ASAE: Las Vegas, NV, USA, 1999; p. 994210.
- Brown–Brandl, T.M.; Yanagi, T.; Xin, H.; Gates, R.S.; Bucklin, R.A.; Ross, G.S. A new telemetry system for measuring core body temperature in livestock and poultry. Appl. Eng. Agric. 2003, 19, 583, doi:10.13031/2013.15316.
- Uddin, J.; Phillips, C.J.C.; Goma, A.A.; McNeill, D.M. Relationships between infrared temperature and laterality. Appl. Anim. Behav. Sci. 2019, 220, 104855, doi:10.1016/j.applanim.2019.104855.
- Uddin, J.; McNeill, D.M.; Lisle, A.T.; Phillips, C.J.C. A sampling strategy for the determination of infrared temperature of relevant external body surfaces of dairy cows. Int. J. Biometeorol. 2020, 64, 1583–1592, doi:10.1007/s00484-020-01939-4.
- Lee, C.; Gebremedhin, K.; Parkhurst, A.; Hillman, P. Placement of temperature probe in bovine vagina for continuous measurement of core-body temperature. Int. J. Biometeorol. 2015, 59, 1201–1205, doi:10.1007/s00484-014-0931-4.
- Nardone, A.; Ronchi, B.; Lacetera, N.; Ranieri, M.S.; Bernabucci, U. Effects of climate changes on animal production and sustainability of livestock systems. Livest. Sci. 2010, 130, 57–69, doi:10.1016/j.livsci.2010.02.011.
- Selye, H. Stress and the General Adaptation Syndrome. Br. Med. J. 1950, 1, 1383–1392, doi:10.1136%2Fbmj.1.4667.1383.
- Selye, H. The Evolution of the Stress Concept: The originator of the concept traces its development from the discovery in 1936 of the alarm reaction to modern therapeutic applications of syntoxic and catatoxic hormones. Am. Sci. 1973, 61, 692-699.
- Moberg, G.P.; Mench, J.A. The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare; CABI Publishing: New York, NY, USA, 2000.
- Young, B.A. Implications of excessive heat load to the welfare of cattle in feedlots. In Recent advances in animal nutrition in Australia, Farrell, D.J., Ed.; University of New England Publishing Unit: Armidale: Australia, 1993; pp. 45–50.
- Young, B.A.; Hall, A.B. Heat load in cattle in the Australian environment. In Australian Beef; Coombs B., Ed.; Morescope Pty Ltd: Melbourne, Victoria, Australia 1993; pp. 143–148.
- Cartwright, T.C. Responses of beef cattle to high ambient temperatures. J. Anim. Sci. 1955, 14, 350–362, doi:10.2527/jas1955.142350x.
- Tait, L. Heat Load Alleviation in Beef Cattle: Water Application during Continuous High Temperature Exposure. Ph.D. Thesis, The University of Queensland, Gatton campus, Australia, 2015.
- Yousef, M.K. Stress Physiology in Livestock. Volume I. Basic Principles; CRC Press: Boca Raton, FL, USA, 1985; ISBN: 0849356679.
- Hahn, G. Dynamic responses of cattle to thermal heat loads. J. Anim. Sci. 1999, 77, 10–20, doi:10.2527/1997.77suppl_210x.
- Mader, T.L.; Davis, M.S.; Brown-Brandl, T. Environmental factors influencing heat stress in feedlot cattle. J. Anim. Sci. 2006, 84, 712–719, doi:10.2527/2006.843712x.