Pancreatic fluid collection (PFC) is one of the local complications that occurs after acute pancreatitis. Recently, the gold standard for management of pancreatic fluid collection has changed from aggressive debridement to a more conservative approach. Endoscopic drainage and necrosectomy are now accepted treatment approaches for patients with symptomatic walled-off pancreatic necrosis (WON). The current recommendations advocate step-up approaches for the treatment of symptomatic WON. Previous recommendations stipulated that endoscopic intervention should be delayed until more than four weeks after the onset. Recent data on early drainage have been increasing and this option might be considered in well-encapsulated cases, but the percutaneous route is preferred if the drainage is performed within two weeks after onset or in nonencapsulated cases.
- Pancreatic Fluid Collection
- endoscopic drainage
- necrosectomy
- walled-off pancreatic necrosis
1. Introduction
Recently, the gold standard for management of pancreatic fluid collection has changed from aggressive debridement to a more conservative approach. Endoscopic treatment has been accepted as the standard treatment for this condition. However, the timing of endoscopic treatment was adopted from data collected using other approaches. With increasing data regarding the endoscopic treatment, the optimal timing for the procedure has been reconsidered. This review summarizes the data emphasizing the timing of endoscopic and other approaches for pancreatic walled-off necrosis drainage as well as endoscopic necrosectomy. To achieve this, a search was made of English-language human studies listed in the PubMed database, EMBASE, and others that were published between 2007 and November 2020. The following keywords were used alone or in combination with pancreatic walled-off necrosis: necrotizing pancreatitis, timing, early drainage, percutaneous drainage, surgical drainage, endoscopic drainage, necrosectomy, step-up approach, stents, lumen-apposing stents, and multigateway. The references of identified articles were also searched for potentially relevant studies. Systematic reviews, meta-analyses, and case reports of special techniques were included. Duplicated data or data published as abstracts in academic meetings were excluded.
2. Evolution of Pancreatic Fluid Collection
Based on the pathophysiology, acute pancreatitis can be divided into two types: interstitial edematous pancreatitis and necrotizing pancreatitis[1]. The edematous inflammations consist of pancreatic fluid leakage that then forms a peripancreatic fluid collection and develops into a pancreatic pseudocyst, while the necrotic collection forms into acute necrosis and later becomes a walled-off necrosis[1] (
). Most patients with interstitial pancreatitis have mild symptoms that resolve within one week[2]. On the other hand, 20% of patients will develop necrotizing pancreatitis, which later will turn into walled-off necrosis [3]. These patient usully have a more severe condition associated with higher rates of organ failure, ICU stay, and mortality [2].

The evolution of the pancreatic necrosis and well-encapsulated walled-off necrosis. (
) The encapsulation was not completed 10 days after onset. (
,
) The peripancreatic fluid collection formed an encapsulation (arrow) within two weeks of the onset. (
) This patient developed infected walled-off necrosis with a cavity containing air bubbles (arrowhead) that needed drainage at day 68 after the onset of pancreatitis.
3. Endoscopic Drainage
Endoscopic treatment for peripancreatic fluid collection has been used since 1975 for direct transluminal puncture and aspiration[4]. The procedure has shifted from endoscopically guided simple aspiration or fistulotomy to endoscopic ultrasound-guided drainage [5]. By placing a stent over the newly created tract, the necrotic fluid and debris can be drained into the luminal cavity and vice versa. For safe drainage without free peritoneal perforation, effective encapsulation of the collection is warranted. While a cutoff point of four weeks was estimated for the walled-off formation, full encapsulation could be seen in up to 43.3% of patients[6]. The timing of endoscopic drainage was adopted from the data using other interventions—that is, more than four weeks after the onset of acute pancreatitis[7]. However, in many cases, the indication for drainage occurs earlier and percutaneous intervention is generally recommended in such situations[8]. On the other hand, in cases where a lesion is located in the central area of the retroperitoneal region, it is much easier to approach by endoscopy, so endoscopic drainage might be performed after the encapsulation is confirmed[9].
There have been a few retrospective studies of early endoscopic drainage in walled-off necrosis. In one study, in a series of direct endoscopic necrosectomies using metallic stents, no procedure-related complications were reported. Another two comparative studies between early (<4 weeks) and delayed conventional drainage also showed no increase in morbidity or mortality if the procedure was performed in an encapsulated cavity[6]. The median time for early drainage in these retrospective studies was 19 to 23 days after the onset of acute pancreatitis[10]. Complications such as perforation or bleeding did not significantly increase in patients who received early drainage[9].
3.1. SEMS as an Adjunctive Strategy to Improve Endoscopic Drainage
The benefits of endoscopic drainage include lower invasiveness and good proximity to the retroperitoneal region. However, the access portal size is still the main limitation. In the case of walled-off necrosis, the tissue debris cannot be drained through multiple pigtail stents so additional procedures are usually needed (
). Before the development of dedicated stents for pancreatic fluid collection drainage, fully covered self-expandable metallic stents (FCSEMS), either biliary or esophageal, were used to aid the endoscopic removal of tissue debris[10][11][12]. In reports using esophageal FCSEMS with a diameter of 18 to 20 mm, total necrosectomy could be achieved within three sessions of endoscopic necrosectomy[11][12]. However, major complications such as migration and occlusion occurred[12]. To solve the migration problem, double pigtail stents were deployed within the SEMS and more dedicated FCSEMS with a flare-type, biflanged design (NAGI
, Taewoo-Medical, Ilsan, Korea) were developed[13]. Additional lumen-apposing properties were added in these fully covered short metal stents, which creates more apposition forces than just at the flared end[14]. These so-called lumen-apposing metal stents (LAMS) could not only provide a portal for necrotic tissue drainage but could be applied for entero-enteric or entero-biliary anastomosis creation[15]. By the improvement of stent visibility on endoscopic ultrasound (EUS), LAMS insertion could be performed without fluoroscopy[16]. These stents are available in many sizes, ranging from 8 to 20 mm in diameter and 10 to 30 mm in length[17][18]. With the development of an electrocautery-enhanced delivery system, the EUS-guided drainage procedure could be performed in a single step, which eliminates the need for other devices and reduces the procedure time[19][20].
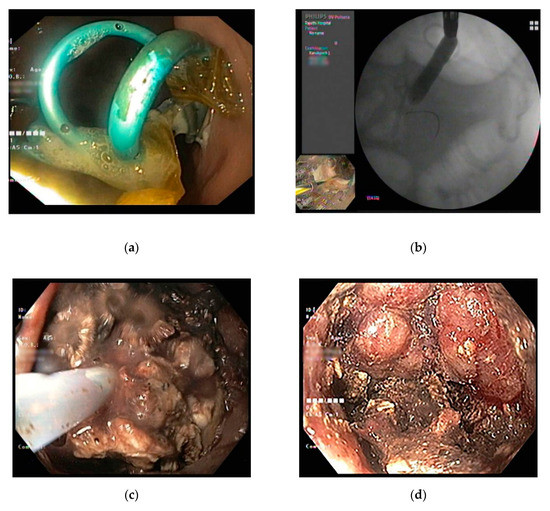
(
) Endoscopic necrosectomy after EUS-guided placement of multiple plastic stents. (
) After stent removal, the puncture site is dilated using a balloon and the scope is inserted into the cavity. (
) The debris is removed by irrigation and mechanical removal until (
) pink granulation tissue is seen.
The benefits of LAMS in WON are aiding in the drainage of the debris and easing the endoscopic necrosectomy procedure[8]. There have been many studies directly comparing the efficacy and safety of LAMS and conventional plastic stents (
1). Complications after LAMS placement included delayed bleeding and buried LAMS syndrome[21][22]. Data from randomized studies and meta-analyses did not show a significant difference in the overall clinical outcome and adverse events when compared with multiple plastic stents[23][24]. On the contrary, data from multicenter studies showed that the use of LAMS results in higher clinical success after initial drainage and a decreased need for endoscopic necrosectomy[25][26]. Recent data on LAMS as a multigateway approach are promising as it appears to improve the clinical outcome of patients with a large or complex cavity. Due to the high risk of complications in long-term LAMS, the stent should be removed within three weeks of placement if the WON has been resolved[23]. To prevent LAMS occlusion by necrotic debris and distal impaction to the WON cavity, some place another double pigtail stent inside the LAMS, either as primary [22][27] or secondary prophylaxis[28] for LAMS occlusion. In addition, due to the short length, caution should be employed if the distance between the EUS probe and the WON cavity is larger than 1 cm [25].
Comparative studies of each type of stents for the treatment of walled-off pancreatic necrosis (WON).
|
Authors (Year) |
Stents |
Type of Study |
Number of Patients |
Outcome |
Remarks |
|
Mukai (2015) [29] |
DPS versus LAMS (Axios® 15 mm, Nagi® 16 mm, Spaxus® 12 mm) |
Retrospective |
70 |
No difference in success but a shorter procedure time with LAMS |
Nasocystic irrigation in all cases |
|
Ang (2016) [30] |
DPS versus Nagi® 16 mm) |
Retrospective |
49 |
DPS associated with higher need for secondary drainage |
Both pancreatic pseudocyst and WON included |
|
Bapaye (2017) [31] |
DPS versus FCSEMS (Nagi®, 16 mm) |
Retrospective |
133 |
FCSEMS superior to DPS in terms of clinical success, number of necrosectomies, salvage surgeries, and length of hospital stay |
Nasocystic irrigation in all cases |
|
Siddiqui (2017) [32] |
DPS versus FCSEMS (10 mm) versus LAMS (Axios® 10,15 mm) |
Retrospective |
313 |
FCSEMS and LAMs superior to DPS in efficacy. Fewer procedures are required in LAMS |
More acute adverse events in LAMS but fewer stent occlusions or migrations |
|
Abu Dayyeh (2018) [33] |
DPS versus FCSEMS (Axios®, Nagi®, 15, 18, 20 mm) |
Retrospective |
94 |
FCSEMS decreases the need for repeated necrosectomy and procedure-related hemorrhage |
|
|
Law (2018) [34] |
FCSEMS (10 mm) versus LAMS (Axios® 10, 15 mm) |
Retrospective |
68 |
Comparable efficacy and safety, but more revisions needed in LAMS |
|
|
Lang (2018) [27] |
DPS versus LAMS (Axios® 10, 15 mm) |
Retrospective |
103 |
Increased complications (bleeding, occlusion) in LAMS |
Both pancreatic pseudocyst and WON included |
|
Mohan (2019) [24] |
DPS versus LAMS |
Meta-analysis |
9 studies (737 patients) of LAMS, 7 studies (527 patients) of DPS |
Equal clinical outcomes and adverse events in DPS and LAMS |
|
|
Bang (2019) [23] |
DPS versus LAMS (Axios® 15 mm) |
RCT |
60 |
No significant differences in treatment outcome |
|
|
Chen (2019) [25] |
DPS versus LAMS |
Retrospective |
189 |
Higher clinical success, shorter procedure time, lower need for surgery, and lower rate of recurrence in LAMS |
|
|
Cho (2019) [35] |
DPS versus LAMS (HANARO® 10 mm) |
Retrospective |
28 |
No difference in clinical success rate and complications |
Pilot study. Included both pseudocyst and WON. New stent with antireflux and antimigration property |
|
Kayal (2020) [26] |
DPS versus FCSEMS tubular versus Axios® |
Historical cohort |
58 |
Higher clinical success in LAMS than FCSEMS and DPS (96.3% vs. 81.8% vs. 77.8%) |
Both pancreatic pseudocyst and WON included |
|
Zhu (2020) [36] |
DPS versus LAMS (Microtech, 16 mm) |
Retrospective |
84 |
Better outcome using LAMS in cases with debris <20% |
|
|
Rana (2020) [28] |
DPS versus LAMS (Nagi®, Plumber®, 14, 16 mm) |
Retrospective |
166 |
Similar technical success rate, complications, and resolution but shorter time to resolution in LAMS |
|
|
Ge (2020) [22] |
DPS versus LAMS (Axios® 10, 15 mm) |
Retrospective |
112 |
LAMS associated with faster resolution, lower recurrence, and decreased requirement for surgery but higher adverse event rates (bleeding, perforation) |
Additional DPS inserted through LAMS |
|
Parsa (2020) [37] |
LAMS (Axios®) 15 mm versus 20 mm |
Retrospective |
306 |
Comparable clinical success and safety but with fewer necrosectomies in larger LAMS |
|
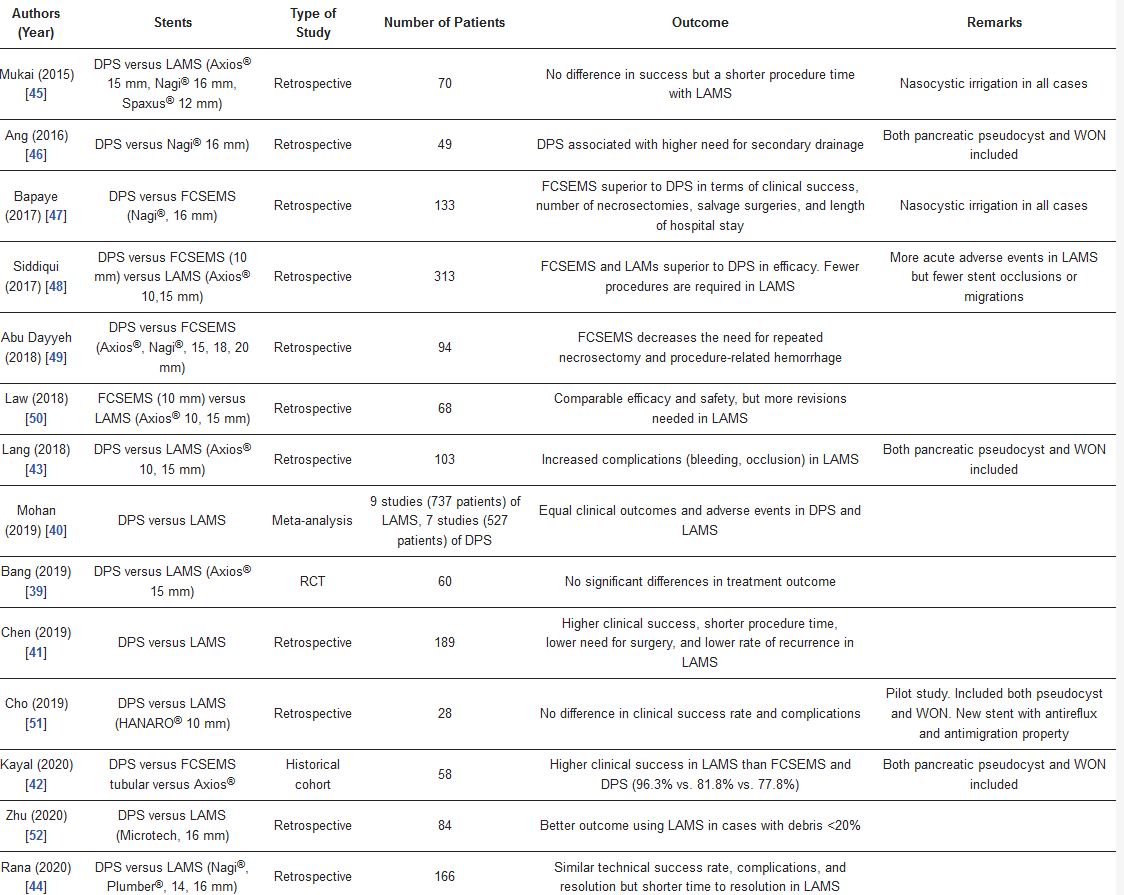
DPS = double pigtail stent, FCSEMS = fully covered self-expandable tubular stent, LAMS = lumen-apposing metal stent.
3.2. Endoscopic Necrosectomy
Endoscopic necrosectomy aims to remove the tissue debris and infected material, and open up multiple dead spaces that contain infected material. The procedure could be performed immediately after the initial endoscopic drainage (direct necrosectomy)[38][39] or after a failed clinical response after drainage as a step-up approach[40]. The optimal timing to start endoscopic necrosectomy after the initial procedure ranges from immediately to 48–72 h afterward[23][41] [
,
]. Generally, endoscopic necrosectomy is recommended only when there is no improvement in clinical response after initial drainage due to a high rate of procedure-related complications[8].
3.2.1. Technical Aspects of Endoscopic Necrosectomy
The technique of endoscopic necrosectomy includes mechanical removal and irrigation until pink granulation tissue is seen[42] (
C,D). The procedure could be performed via the transluminal tract or the percutaneous tract[43]. To aid the necrosectomy, fully covered metallic stents are usually placed after the initial puncture. In the case of transluminal drainage, fully covered esophageal stents or, preferably, lumen-apposing stents are placed[12]; a fully covered esophageal stent can only be used in the transcutaneous approach[44] (
).
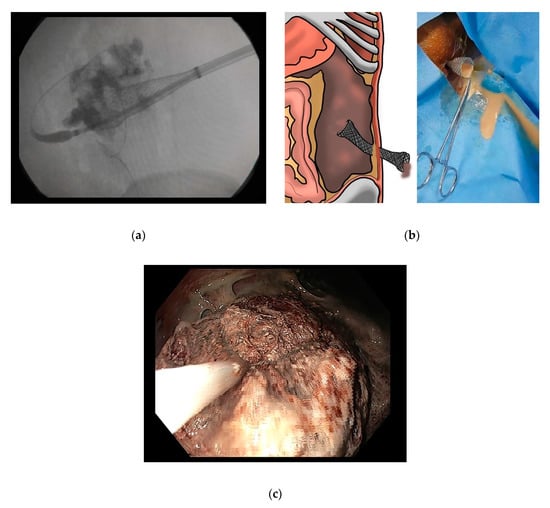
Percutaneous necrosectomy. (
,
) After percutaneous catheter insertion, a fully covered esophageal stent is placed in the cavity under fluoroscopic guidance. (
) Endoscopic necrosectomy can be performed using a small caliber endoscope.
The transluminal procedure is performed by using a flexible gastroscope with a water irrigation system and CO
insufflation, inserted through the fistula tract. Percutaneous necrosectomy can be performed by tract dilation until it is large enough for endoscopic insertion via an overtube or esophageal stent[45]. Tissue debris is mechanically fragmented and removed using a snare, basket, Roth net retriever, tripod/pentapod retriever, or large forceps[13][38][46][41][42].
4.2.2. Timing of Endoscopic Necrosectomy
In case of early drainage within four weeks after onset, endoscopic debridement can be performed without increasing local complications, regardless of the route of necrosectomy[6]. Interestingly, in comparative studies, perforation after necrosectomy seems to be higher in the late- (>4 weeks) intervention group[6][9]. This indicates that the four-week timing might not be a good general rule of safety for endoscopic procedures and that decisions should be made based on the individual case. However, due to poor encapsulation in the early stage of pancreatitis, endoscopic debridement should be avoided within two weeks of necrosis[5].
The interval between initial stent placement and first necrosectomy is still controversial. Although many endoscopists prefer to delay the first endoscopic necrosectomy until at least a week after the initial stent placement, some prefer to perform direct endoscopic necrosectomy in the first session for early mobilization of the necrotic debris. Concerns over safety and the benefits of early direct endoscopic necrosectomy (DEN) have been reported in a large multicenter study, which showed a decrease in the number of interventions if the endoscopic necrosectomy is performed immediately at the time of LAMS placement [47].
4.2.3. Adjunctive Techniques for Endoscopic Necrosectomy
There are reports of adjunctive techniques that can improve the efficacy of endoscopic necrosectomy. Many studies use a nasocystic tube with irrigation using normal saline[29][31], irrigation during necrosectomy using diluted bacitracin[38], or irrigation with hydrogen peroxide solution[41] and avoidance of acid-suppressing therapy to allow acid digestion of the necrotic debris [48]. Despite their widespread use, the benefits of these techniques are not very clear[49]. In cases where initial endoscopic necrosectomy is not effective, additional necrosectomy for the subcavity using the same entry site, so-called “single transluminal gateway transcystic multiple drainages” could be performed [50]. If these methods fail to achieve a clinical response, proceeding to laparoscopic debridement or surgical necrosectomy might be considered[8][51].
The proposed algorithm for timely endoscopic drainage and necrosectomy for walled-off necrosis is shown in
.
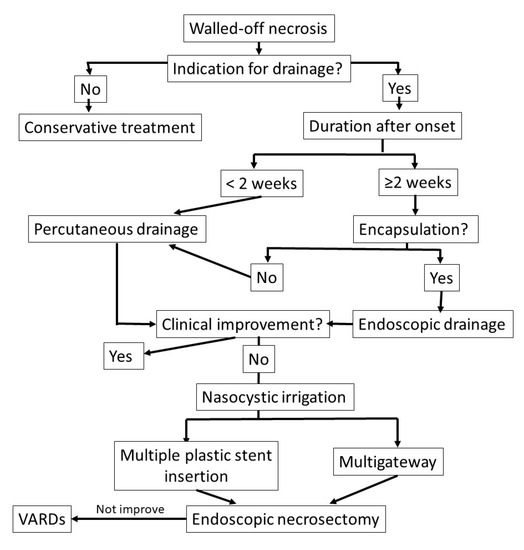
Proposed algorithm for the timely management of walled-off necrosis drainage and necrosectomy.
- Conclusions
5. Conclusions
Endoscopic drainage and necrosectomy in walled-off pancreatic necrosis should be performed in a step-up manner. The optimal duration of four weeks was established based on previous studies, but recent studies have pointed to more flexible timing, decided based on individual cases. Early interventions might be performed in the case of walled-off necrosis with the presence of encapsulation, but careful consideration should be given to endoscopic drainage in the very early stage (< 2 weeks) since there are limited safety data and encapsulation is not usually present. Several adjunctive methods have been proposed but the benefits are still unclear and the decision should be made on case-by-case basis.
References
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S.; Acute Pancreatitis Classification Working, G. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102-111, doi:10.1136/gutjnl-2012-302779.Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S.; Acute Pancreatitis Classification Working, G. Classification of acute pancreatitis-2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111, doi:10.1136/gutjnl-2012-302779.
- Singh, V.K.; Bollen, T.L.; Wu, B.U.; Repas, K.; Maurer, R.; Yu, S.; Mortele, K.J.; Conwell, D.L.; Banks, P.A. An assessment of the severity of interstitial pancreatitis. Clin Gastroenterol Hepatol 2011, 9, 1098-1103, doi:10.1016/j.cgh.2011.08.026.Singh, V.K.; Bollen, T.L.; Wu, B.U.; Repas, K.; Maurer, R.; Yu, S.; Mortele, K.J.; Conwell, D.L.; Banks, P.A. An assessment of the severity of interstitial pancreatitis. Clin. Gastroenterol. Hepatol. 2011, 9, 1098–1103, doi:10.1016/j.cgh.2011.08.026.
- van Brunschot, S.; Bakker, O.J.; Besselink, M.G.; Bollen, T.L.; Fockens, P.; Gooszen, H.G.; van Santvoort, H.C.; Dutch Pancreatitis Study, G. Treatment of necrotizing pancreatitis. Clin Gastroenterol Hepatol 2012, 10, 1190-1201, doi:10.1016/j.cgh.2012.05.005.van Brunschot, S.; Bakker, O.J.; Besselink, M.G.; Bollen, T.L.; Fockens, P.; Gooszen, H.G.; van Santvoort, H.C.; Dutch Pancreatitis Study, G. Treatment of necrotizing pancreatitis. Clin. Gastroenterol. Hepatol. 2012, 10, 1190–1201, doi:10.1016/j.cgh.2012.05.005.
- Arvanitakis, M.; Dumonceau, J.M.; Albert, J.; Badaoui, A.; Bali, M.A.; Barthet, M.; Besselink, M.; Deviere, J.; Oliveira Ferreira, A.; Gyokeres, T., et al. Endoscopic management of acute necrotizing pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) evidence-based multidisciplinary guidelines. Endoscopy 2018, 50, 524-546, doi:10.1055/a-0588-5365.Rogers, B.H.; Cicurel, N.J.; Seed, R.W. Transgastric needle aspiration of pancreatic pseudocyst through an endoscope. Gastrointest. Endosc. 1975, 21, 133–134, doi:10.1016/s0016-5107(75)73821-x.
- Baron, T.H.; DiMaio, C.J.; Wang, A.Y.; Morgan, K.A. American Gastroenterological Association Clinical Practice Update: Management of Pancreatic Necrosis. Gastroenterology 2020, 158, 67-75 e61, doi:10.1053/j.gastro.2019.07.064.Yip, H.C.; Teoh, A.Y.B. Endoscopic Management of Peri-Pancreatic Fluid Collections. Gut Liver 2017, 11, 604–611, doi:10.5009/gnl16178.
- Windsor, J.A. Infected pancreatic necrosis: drain first, but do it better. HPB (Oxford) 2011, 13, 367-368, doi:10.1111/j.1477-2574.2011.00313.x.Trikudanathan, G.; Tawfik, P.; Amateau, S.K.; Munigala, S.; Arain, M.; Attam, R.; Beilman, G.; Flanagan, S.; Freeman, M.L.; Mallery, S. Early (<4 Weeks) Versus Standard (>/= 4 Weeks) Endoscopically Centered Step-Up Interventions for Necrotizing Pancreatitis. Am. J. Gastroenterol. 2018, 113, 1550–1558, doi:10.1038/s41395-018-0232-3.
- Committee, A.S.o.P.; Muthusamy, V.R.; Chandrasekhara, V.; Acosta, R.D.; Bruining, D.H.; Chathadi, K.V.; Eloubeidi, M.A.; Faulx, A.L.; Fonkalsrud, L.; Gurudu, S.R., et al. The role of endoscopy in the diagnosis and treatment of inflammatory pancreatic fluid collections. Gastrointest Endosc 2016, 83, 481-488, doi:10.1016/j.gie.2015.11.027.van Santvoort, H.C.; Bakker, O.J.; Bollen, T.L.; Besselink, M.G.; Ahmed Ali, U.; Schrijver, A.M.; Boermeester, M.A.; van Goor, H.; Dejong, C.H.; van Eijck, C.H.; et al. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology 2011, 141, 1254–1263, doi:10.1053/j.gastro.2011.06.073.
- Mier, J.; Leon, E.L.; Castillo, A.; Robledo, F.; Blanco, R. Early versus late necrosectomy in severe necrotizing pancreatitis. Am J Surg 1997, 173, 71-75, doi:10.1016/S0002-9610(96)00425-4.Baron, T.H.; DiMaio, C.J.; Wang, A.Y.; Morgan, K.A. American Gastroenterological Association Clinical Practice Update: Management of Pancreatic Necrosis. Gastroenterology 2020, 158, 67–75, doi:10.1053/j.gastro.2019.07.064.
- van Grinsven, J.; van Santvoort, H.C.; Boermeester, M.A.; Dejong, C.H.; van Eijck, C.H.; Fockens, P.; Besselink, M.G.; Dutch Pancreatitis Study, G. Timing of catheter drainage in infected necrotizing pancreatitis. Nat Rev Gastroenterol Hepatol 2016, 13, 306-312, doi:10.1038/nrgastro.2016.23.Chantarojanasiri, T.; Yamamoto, N.; Nakai, Y.; Saito, T.; Saito, K.; Hakuta, R.; Ishigaki, K.; Takeda, T.; Uchino, R.; Takahara, N.; et al. Comparison of early and delayed EUS-guided drainage of pancreatic fluid collection. Endosc. Int. Open 2018, 6, E1398–E1405, doi:10.1055/a-0751-2698.
- van Santvoort, H.C.; Bakker, O.J.; Bollen, T.L.; Besselink, M.G.; Ahmed Ali, U.; Schrijver, A.M.; Boermeester, M.A.; van Goor, H.; Dejong, C.H.; van Eijck, C.H., et al. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology 2011, 141, 1254-1263, doi:10.1053/j.gastro.2011.06.073.Hritz, I.; Fejes, R.; Szekely, A.; Szekely, I.; Horvath, L.; Sarkany, A.; Altorjay, A.; Madacsy, L. Endoscopic transluminal pancreatic necrosectomy using a self-expanding metal stent and high-flow water-jet system. World J. Gastroenterol. 2013, 19, 3685–3692, doi:10.3748/wjg.v19.i23.3685.
- van Santvoort, H.C.; Besselink, M.G.; Bakker, O.J.; Hofker, H.S.; Boermeester, M.A.; Dejong, C.H.; van Goor, H.; Schaapherder, A.F.; van Eijck, C.H.; Bollen, T.L., et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010, 362, 1491-1502, doi:10.1056/NEJMoa0908821.Belle, S.; Collet, P.; Post, S.; Kaehler, G. Temporary cystogastrostomy with self-expanding metallic stents for pancreatic necrosis. Endoscopy 2010, 42, 493–495, doi:10.1055/s-0029-1244021.
- Isayama, H.; Nakai, Y.; Rerknimitr, R.; Khor, C.; Lau, J.; Wang, H.P.; Seo, D.W.; Ratanachu-Ek, T.; Lakhtakia, S.; Ang, T.L., et al. Asian consensus statements on endoscopic management of walled-off necrosis Part 1: Epidemiology, diagnosis, and treatment. J Gastroenterol Hepatol 2016, 31, 1546-1554, doi:10.1111/jgh.13394.Attam, R.; Trikudanathan, G.; Arain, M.; Nemoto, Y.; Glessing, B.; Mallery, S.; Freeman, M.L. Endoscopic transluminal drainage and necrosectomy by using a novel, through-the-scope, fully covered, large-bore esophageal metal stent: Preliminary experience in 10 patients. Gastrointest. Endosc. 2014, 80, 312–318, doi:10.1016/j.gie.2014.02.013.
- Baron, T.H.; Kozarek, R.A. Endotherapy for organized pancreatic necrosis: perspectives after 20 years. Clin Gastroenterol Hepatol 2012, 10, 1202-1207, doi:10.1016/j.cgh.2012.07.009.Tellez-Avila, F.I.; Villalobos-Garita, A.; Ramirez-Luna, M.A. Use of a novel covered self-expandable metal stent with an anti-migration system for endoscopic ultrasound-guided drainage of a pseudocyst. World J. Gastrointest. Endosc. 2013, 5, 297–299, doi:10.4253/wjge.v5.i6.297.
- Rana, S.S.; Gupta, R.; Kang, M.; Sharma, V.; Sharma, R.; Gorsi, U.; Bhasin, D.K. Percutaneous catheter drainage followed by endoscopic transluminal drainage/necrosectomy for treatment of infected pancreatic necrosis in early phase of illness. Endosc Ultrasound 2018, 7, 41-47, doi:10.4103/eus.eus_94_17.Teoh, A.Y.; Ng, E.K.; Chan, S.M.; Lai, M.; Moran, S.; Binmoeller, K.F.; Moon, J.H.; Ho, K.Y. Ex vivo comparison of the lumen-apposing properties of EUS-specific stents (with video). Gastrointest. Endosc. 2016, 84, 62–68, doi:10.1016/j.gie.2015.11.041.
- Varadarajulu, S.; Phadnis, M.A.; Christein, J.D.; Wilcox, C.M. Multiple transluminal gateway technique for EUS-guided drainage of symptomatic walled-off pancreatic necrosis. Gastrointest Endosc 2011, 74, 74-80, doi:10.1016/j.gie.2011.03.1122.Bank, J.; Adler, D. Lumen apposing metal stents: A review of current uses and outcomes. Gastrointest. Interv. 2017, 6, 9–14, doi:10.18528/gii160033.
- Binda, C.; Dabizzi, E.; Anderloni, A.; Cennamo, V.; Fiscaletti, M.; Fugazza, A.; Jovine, E.; Ercolani, G.; Gasbarrini, A.; Fabbri, C. Single-step endoscopic ultrasound-guided multiple gateway drainage of complex walled-off necrosis with lumen apposing metal stents. Eur J Gastroenterol Hepatol 2020, 32, 1401-1404, doi:10.1097/MEG.0000000000001793.Braden, B.; Koutsoumpas, A.; Silva, M.A.; Soonawalla, Z.; Dietrich, C.F. Endoscopic ultrasound-guided drainage of pancreatic walled-off necrosis using self-expanding metal stents without fluoroscopy. World J. Gastrointest. Endosc. 2018, 10, 93–98, doi:10.4253/wjge.v10.i5.93.
- Mukai, S.; Itoi, T.; Sofuni, A.; Itokawa, F.; Kurihara, T.; Tsuchiya, T.; Ishii, K.; Tsuji, S.; Ikeuchi, N.; Tanaka, R., et al. Expanding endoscopic interventions for pancreatic pseudocyst and walled-off necrosis. J Gastroenterol 2015, 50, 211-220, doi:10.1007/s00535-014-0957-8.Weilert, F.; Binmoeller, K.F. Specially designed stents for translumenal drainage. Gastrointest. Interv. 2015, 4, 40–45, doi:10.1016/j.gii.2015.03.003.
- Sugimoto, M.; Sonntag, D.P.; Flint, G.S.; Boyce, C.J.; Kirkham, J.C.; Harris, T.J.; Carr, S.M.; Nelson, B.D.; Bell, D.A.; Barton, J.G., et al. Better Outcomes if Percutaneous Drainage Is Used Early and Proactively in the Course of Necrotizing Pancreatitis. J Vasc Interv Radiol 2016, 27, 418-425, doi:10.1016/j.jvir.2015.11.054.Anderloni, A.; Fabbri, C.; Nieto, J.; Uwe, W.; Dollhopf, M.; Aparicio, J.R.; Perez-Miranda, M.; Tarantino, I.; Arlt, A.; Vleggaar, F.; et al. The safety and efficacy of a new 20 mm lumen apposing metal stent (lams) for the endoscopic treatment of pancreatic and peripancreatic fluid collections: A large international, multicenter study. Surg. Endosc. 2020, doi:10.1007/s00464-020-07567-8.
- van Baal, M.C.; van Santvoort, H.C.; Bollen, T.L.; Bakker, O.J.; Besselink, M.G.; Gooszen, H.G.; Dutch Pancreatitis Study, G. Systematic review of percutaneous catheter drainage as primary treatment for necrotizing pancreatitis. Br J Surg 2011, 98, 18-27, doi:10.1002/bjs.7304.Adler, D.G.; Taylor, L.J.; Hasan, R.; Siddiqui, A.A. A retrospective study evaluating endoscopic ultrasound-guided drainage of pancreatic fluid collections using a novel lumen-apposing metal stent on an electrocautery enhanced delivery system. Endosc. Ultrasound 2017, 6, 389–393, doi:10.4103/eus.eus_4_17.
- Mallick, B.; Dhaka, N.; Gupta, P.; Gulati, A.; Malik, S.; Sinha, S.K.; Yadav, T.D.; Gupta, V.; Kochhar, R. An audit of percutaneous drainage for acute necrotic collections and walled off necrosis in patients with acute pancreatitis. Pancreatology 2018, 18, 727-733, doi:10.1016/j.pan.2018.08.010.Weigand, K.; Mehrl, A.; Goessmann, H.; Mueller, M.; Kandulski, A. Endoscopic Necrosectomy of Walled-Off Necrosis following Severe Pancreatitis Using a Hot AxiosTM Stent-A Case Series. Dig. Dis. 2019, 1–4, doi:10.1159/000503991.
- Besselink, M.G.; Verwer, T.J.; Schoenmaeckers, E.J.; Buskens, E.; Ridwan, B.U.; Visser, M.R.; Nieuwenhuijs, V.B.; Gooszen, H.G. Timing of surgical intervention in necrotizing pancreatitis. Arch Surg 2007, 142, 1194-1201, doi:10.1001/archsurg.142.12.1194.Bang, J.Y.; Hasan, M.; Navaneethan, U.; Hawes, R.; Varadarajulu, S. Lumen-apposing metal stents (LAMS) for pancreatic fluid collection (PFC) drainage: May not be business as usual. Gut 2017, 66, 2054–2056, doi:10.1136/gutjnl-2016-312812.
- Rogers, B.H.; Cicurel, N.J.; Seed, R.W. Transgastric needle aspiration of pancreatic pseudocyst through an endoscope. Gastrointest Endosc 1975, 21, 133-134, doi:10.1016/s0016-5107(75)73821-x.Ge, P.S.; Young, J.Y.; Jirapinyo, P.; Dong, W.; Ryou, M.; Thompson, C.C. Comparative Study Evaluating Lumen Apposing Metal Stents Versus Double Pigtail Plastic Stents for Treatment of Walled-Off Necrosis. Pancreas 2020, 49, 236–241, doi:10.1097/MPA.0000000000001476.
- Yip, H.C.; Teoh, A.Y.B. Endoscopic Management of Peri-Pancreatic Fluid Collections. Gut Liver 2017, 11, 604-611, doi:10.5009/gnl16178.Bang, J.Y.; Navaneethan, U.; Hasan, M.K.; Sutton, B.; Hawes, R.; Varadarajulu, S. Non-superiority of lumen-apposing metal stents over plastic stents for drainage of walled-off necrosis in a randomised trial. Gut 2019, 68, 1200–1209, doi:10.1136/gutjnl-2017-315335.
- Trikudanathan, G.; Tawfik, P.; Amateau, S.K.; Munigala, S.; Arain, M.; Attam, R.; Beilman, G.; Flanagan, S.; Freeman, M.L.; Mallery, S. Early (<4 Weeks) Versus Standard (>/= 4 Weeks) Endoscopically Centered Step-Up Interventions for Necrotizing Pancreatitis. Am J Gastroenterol 2018, 113, 1550-1558, doi:10.1038/s41395-018-0232-3.Mohan, B.P.; Jayaraj, M.; Asokkumar, R.; Shakhatreh, M.; Pahal, P.; Ponnada, S.; Navaneethan, U.; Adler, D.G. Lumen apposing metal stents in drainage of pancreatic walled-off necrosis, are they any better than plastic stents? A systematic review and meta-analysis of studies published since the revised Atlanta classification of pancreatic fluid collections. Endosc. Ultrasound 2019, 8, 82–90, doi:10.4103/eus.eus_7_19.
- Chantarojanasiri, T.; Yamamoto, N.; Nakai, Y.; Saito, T.; Saito, K.; Hakuta, R.; Ishigaki, K.; Takeda, T.; Uchino, R.; Takahara, N., et al. Comparison of early and delayed EUS-guided drainage of pancreatic fluid collection. Endosc Int Open 2018, 6, E1398-E1405, doi:10.1055/a-0751-2698.Chen, Y.I.; Yang, J.; Friedland, S.; Holmes, I.; Law, R.; Hosmer, A.; Stevens, T.; Franco, M.C.; Jang, S.; Pawa, R.; et al. Lumen apposing metal stents are superior to plastic stents in pancreatic walled-off necrosis: A large international multicenter study. Endosc. Int. Open 2019, 7, E347–E354, doi:10.1055/a-0828-7630.
- Hritz, I.; Fejes, R.; Szekely, A.; Szekely, I.; Horvath, L.; Sarkany, A.; Altorjay, A.; Madacsy, L. Endoscopic transluminal pancreatic necrosectomy using a self-expanding metal stent and high-flow water-jet system. World J Gastroenterol 2013, 19, 3685-3692, doi:10.3748/wjg.v19.i23.3685.Kayal, A.; Taghizadeh, N.; Ishikawa, T.; Gonzalez-Moreno, E.; Bass, S.; Cole, M.J.; Heitman, S.J.; Mohamed, R.; Turbide, C.; Chen, Y.I.; et al. Endosonography-guided transmural drainage of pancreatic fluid collections: Comparative outcomes by stent type. Surg. Endosc. 2020, doi:10.1007/s00464-020-07699-x.
- Belle, S.; Collet, P.; Post, S.; Kaehler, G. Temporary cystogastrostomy with self-expanding metallic stents for pancreatic necrosis. Endoscopy 2010, 42, 493-495, doi:10.1055/s-0029-1244021.Lang, G.D.; Fritz, C.; Bhat, T.; Das, K.K.; Murad, F.M.; Early, D.S.; Edmundowicz, S.A.; Kushnir, V.M.; Mullady, D.K. EUS-guided drainage of peripancreatic fluid collections with lumen-apposing metal stents and plastic double-pigtail stents: Comparison of efficacy and adverse event rates. Gastrointest. Endosc. 2018, 87, 150–157, doi:10.1016/j.gie.2017.06.029.
- Attam, R.; Trikudanathan, G.; Arain, M.; Nemoto, Y.; Glessing, B.; Mallery, S.; Freeman, M.L. Endoscopic transluminal drainage and necrosectomy by using a novel, through-the-scope, fully covered, large-bore esophageal metal stent: preliminary experience in 10 patients. Gastrointest Endosc 2014, 80, 312-318, doi:10.1016/j.gie.2014.02.013.Rana, S.S.; Sharma, R.; Dhalaria, L.; Gupta, R. Efficacy and safety of plastic versus lumen-apposing metal stents for transmural drainage of walled-off necrosis: A retrospective single-center study. Ann. Gastroenterol. 2020, 33, 426–432, doi:10.20524/aog.2020.0499.
- Tellez-Avila, F.I.; Villalobos-Garita, A.; Ramirez-Luna, M.A. Use of a novel covered self-expandable metal stent with an anti-migration system for endoscopic ultrasound-guided drainage of a pseudocyst. World J Gastrointest Endosc 2013, 5, 297-299, doi:10.4253/wjge.v5.i6.297.Mukai, S.; Itoi, T.; Baron, T.H.; Sofuni, A.; Itokawa, F.; Kurihara, T.; Tsuchiya, T.; Ishii, K.; Tsuji, S.; Ikeuchi, N.; et al. Endoscopic ultrasound-guided placement of plastic vs. biflanged metal stents for therapy of walled-off necrosis: A retrospective single-center series. Endoscopy 2015, 47, 47–55, doi:10.1055/s-0034-1377966.
- Teoh, A.Y.; Ng, E.K.; Chan, S.M.; Lai, M.; Moran, S.; Binmoeller, K.F.; Moon, J.H.; Ho, K.Y. Ex vivo comparison of the lumen-apposing properties of EUS-specific stents (with video). Gastrointest Endosc 2016, 84, 62-68, doi:10.1016/j.gie.2015.11.041.Ang, T.L.; Kongkam, P.; Kwek, A.B.; Orkoonsawat, P.; Rerknimitr, R.; Fock, K.M. A two-center comparative study of plastic and lumen-apposing large diameter self-expandable metallic stents in endoscopic ultrasound-guided drainage of pancreatic fluid collections. Endosc. Ultrasound 2016, 5, 320–327, doi:10.4103/2303-9027.191659.
- Bank, J.; Adler, D. Lumen apposing metal stents: A review of current uses and outcomes. Gastrointestinal Intervention 2017, 6, 9-14, doi:10.18528/gii160033.Bapaye, A.; Dubale, N.A.; Sheth, K.A.; Bapaye, J.; Ramesh, J.; Gadhikar, H.; Mahajani, S.; Date, S.; Pujari, R.; Gaadhe, R. Endoscopic ultrasonography-guided transmural drainage of walled-off pancreatic necrosis: Comparison between a specially designed fully covered bi-flanged metal stent and multiple plastic stents. Dig. Endosc. 2017, 29, 104–110, doi:10.1111/den.12704.
- Braden, B.; Koutsoumpas, A.; Silva, M.A.; Soonawalla, Z.; Dietrich, C.F. Endoscopic ultrasound-guided drainage of pancreatic walled-off necrosis using self-expanding metal stents without fluoroscopy. World J Gastrointest Endosc 2018, 10, 93-98, doi:10.4253/wjge.v10.i5.93.Siddiqui, A.A.; Kowalski, T.E.; Loren, D.E.; Khalid, A.; Soomro, A.; Mazhar, S.M.; Isby, L.; Kahaleh, M.; Karia, K.; Yoo, J.; et al. Fully covered self-expanding metal stents versus lumen-apposing fully covered self-expanding metal stent versus plastic stents for endoscopic drainage of pancreatic walled-off necrosis: Clinical outcomes and success. Gastrointest. Endosc. 2017, 85, 758–765, doi:10.1016/j.gie.2016.08.014.
- Weilert, F.; Binmoeller, K.F. Specially designed stents for translumenal drainage. Gastrointestinal Intervention 2015, 4, 40-45, doi:10.1016/j.gii.2015.03.003.Abu Dayyeh, B.K.; Mukewar, S.; Majumder, S.; Zaghlol, R.; Vargas Valls, E.J.; Bazerbachi, F.; Levy, M.J.; Baron, T.H.; Gostout, C.J.; Petersen, B.T.; et al. Large-caliber metal stents versus plastic stents for the management of pancreatic walled-off necrosis. Gastrointest. Endosc. 2018, 87, 141–149, doi:10.1016/j.gie.2017.04.032.
- Anderloni, A.; Fabbri, C.; Nieto, J.; Uwe, W.; Dollhopf, M.; Aparicio, J.R.; Perez-Miranda, M.; Tarantino, I.; Arlt, A.; Vleggaar, F., et al. The safety and efficacy of a new 20-mm lumen apposing metal stent (lams) for the endoscopic treatment of pancreatic and peripancreatic fluid collections: a large international, multicenter study. Surgical Endoscopy 2020, 10.1007/s00464-020-07567-8, doi:10.1007/s00464-020-07567-8.Law, S.T.; De La Serna Higuera, C.; Simon, P.G.; Castillo, M.P.-M. Comparison of clinical efficacies and safeties of lumen-apposing metal stent and conventional-type metal stent-assisted EUS-guided pancreatic wall-off necrosis drainage: A real-life experience in a tertiary hospital. Surg. Endosc. 2018, 32, 2448–2453, doi:10.1007/s00464-017-5946-6.
- Adler, D.G.; Taylor, L.J.; Hasan, R.; Siddiqui, A.A. A retrospective study evaluating endoscopic ultrasound-guided drainage of pancreatic fluid collections using a novel lumen-apposing metal stent on an electrocautery enhanced delivery system. Endosc Ultrasound 2017, 6, 389-393, doi:10.4103/eus.eus_4_17.Cho, I.R.; Chung, M.J.; Jo, J.H.; Lee, H.S.; Park, J.Y.; Bang, S.; Park, S.W.; Song, S.Y. A novel lumen-apposing metal stent with an anti-reflux valve for endoscopic ultrasound-guided drainage of pseudocysts and walled-off necrosis: A pilot study. PLoS ONE 2019, 14, e0221812, doi:10.1371/journal.pone.0221812.
- Weigand, K.; Mehrl, A.; Goessmann, H.; Mueller, M.; Kandulski, A. Endoscopic Necrosectomy of Walled-Off Necrosis following Severe Pancreatitis Using a Hot AxiosTM Stent - A Case Series. Dig Dis 2019, 10.1159/000503991, 1-4, doi:10.1159/000503991.Zhu, H.; Xie, P.; Wang, Y.; Jin, Z.; Li, Z.; Du, Y. The role of solid debris in endoscopic ultrasound-guided drainage of walled-off necrosis: A large cohort study. J. Gastroenterol. Hepatol. 2020, doi:10.1111/jgh.15086.
- Bang, J.Y.; Hasan, M.; Navaneethan, U.; Hawes, R.; Varadarajulu, S. Lumen-apposing metal stents (LAMS) for pancreatic fluid collection (PFC) drainage: may not be business as usual. Gut 2017, 66, 2054-2056, doi:10.1136/gutjnl-2016-312812.Parsa, N.; Nieto, J.M.; Powers, P.; Mitsuhashi, S.; Abdelqader, A.; Hadzinakos, G.; Anderloni, A.A.; Fugazza, A.; James, T.W.; Arlt, A.; et al. Endoscopic ultrasound-guided drainage of pancreatic walled-off necrosis using 20 mm versus 15 mm lumen-apposing metal stents: An international, multicenter, case-matched study. Endoscopy 2020, 52, 211–219, doi:10.1055/a-1096-3299.
- Ge, P.S.; Young, J.Y.; Jirapinyo, P.; Dong, W.; Ryou, M.; Thompson, C.C. Comparative Study Evaluating Lumen Apposing Metal Stents Versus Double Pigtail Plastic Stents for Treatment of Walled-Off Necrosis. Pancreas 2020, 49, 236-241, doi:10.1097/MPA.0000000000001476.Thompson, C.C.; Kumar, N.; Slattery, J.; Clancy, T.E.; Ryan, M.B.; Ryou, M.; Swanson, R.S.; Banks, P.A.; Conwell, D.L. A standardized method for endoscopic necrosectomy improves complication and mortality rates. Pancreatology 2016, 16, 66–72, doi:10.1016/j.pan.2015.12.001.
- Bang, J.Y.; Navaneethan, U.; Hasan, M.K.; Sutton, B.; Hawes, R.; Varadarajulu, S. Non-superiority of lumen-apposing metal stents over plastic stents for drainage of walled-off necrosis in a randomised trial. Gut 2019, 68, 1200-1209, doi:10.1136/gutjnl-2017-315335.Gardner, T.B.; Chahal, P.; Papachristou, G.I.; Vege, S.S.; Petersen, B.T.; Gostout, C.J.; Topazian, M.D.; Takahashi, N.; Sarr, M.G.; Baron, T.H. A comparison of direct endoscopic necrosectomy with transmural endoscopic drainage for the treatment of walled-off pancreatic necrosis. Gastrointest. Endosc. 2009, 69, 1085–1094, doi:10.1016/j.gie.2008.06.061.
- Mohan, B.P.; Jayaraj, M.; Asokkumar, R.; Shakhatreh, M.; Pahal, P.; Ponnada, S.; Navaneethan, U.; Adler, D.G. Lumen apposing metal stents in drainage of pancreatic walled-off necrosis, are they any better than plastic stents? A systematic review and meta-analysis of studies published since the revised Atlanta classification of pancreatic fluid collections. Endosc Ultrasound 2019, 8, 82-90, doi:10.4103/eus.eus_7_19.Isayama, H.; Nakai, Y.; Rerknimitr, R.; Khor, C.; Lau, J.; Wang, H.P.; Seo, D.W.; Ratanachu-Ek, T.; Lakhtakia, S.; Ang, T.L.; et al. Asian consensus statements on endoscopic management of walled-off necrosis Part 1: Epidemiology, diagnosis, and treatment. J. Gastroenterol. Hepatol. 2016, 31, 1546–1554, doi:10.1111/jgh.13394.
- Chen, Y.I.; Yang, J.; Friedland, S.; Holmes, I.; Law, R.; Hosmer, A.; Stevens, T.; Franco, M.C.; Jang, S.; Pawa, R., et al. Lumen apposing metal stents are superior to plastic stents in pancreatic walled-off necrosis: a large international multicenter study. Endosc Int Open 2019, 7, E347-E354, doi:10.1055/a-0828-7630.Bansal, R.; Puri, R.; Choudhary, N.; Bhatia, S.; Patel, N.; Patle, S.; Patil, G.; Agarwal, A.; Prabha, C.; Sud, R. Endoscopic pancreatic necrosectomy: Why scuff when you can flush the muck-Make it an easy row to hoe. Endosc. Int. Open 2017, 5, E847–E853, doi:10.1055/s-0043-112854.
- Kayal, A.; Taghizadeh, N.; Ishikawa, T.; Gonzalez-Moreno, E.; Bass, S.; Cole, M.J.; Heitman, S.J.; Mohamed, R.; Turbide, C.; Chen, Y.I., et al. Endosonography-guided transmural drainage of pancreatic fluid collections: comparative outcomes by stent type. Surg Endosc 2020, 10.1007/s00464-020-07699-x, doi:10.1007/s00464-020-07699-x.Isayama, H.; Nakai, Y.; Rerknimitr, R.; Khor, C.; Lau, J.; Wang, H.P.; Seo, D.W.; Ratanachu-Ek, T.; Lakhtakia, S.; Ang, T.L.; et al. Asian consensus statements on endoscopic management of walled-off necrosis. Part 2: Endoscopic management. J. Gastroenterol. Hepatol. 2016, 31, 1555–1565, doi:10.1111/jgh.13398.
- Lang, G.D.; Fritz, C.; Bhat, T.; Das, K.K.; Murad, F.M.; Early, D.S.; Edmundowicz, S.A.; Kushnir, V.M.; Mullady, D.K. EUS-guided drainage of peripancreatic fluid collections with lumen-apposing metal stents and plastic double-pigtail stents: comparison of efficacy and adverse event rates. Gastrointest Endosc 2018, 87, 150-157, doi:10.1016/j.gie.2017.06.029.Yamamoto, N.; Isayama, H.; Takahara, N.; Sasahira, N.; Miyabayashi, K.; Mizuno, S.; Kawakubo, K.; Mohri, D.; Kogure, H.; Sasaki, T.; et al. Percutaneous direct-endoscopic necrosectomy for walled-off pancreatic necrosis. Endoscopy 2013, 45, E44–E45, doi:10.1055/s-0032-1309927.
- Rana, S.S.; Sharma, R.; Dhalaria, L.; Gupta, R. Efficacy and safety of plastic versus lumen-apposing metal stents for transmural drainage of walled-off necrosis: a retrospective single-center study. Ann Gastroenterol 2020, 33, 426-432, doi:10.20524/aog.2020.0499.Ke, L.; Mao, W.; Zhou, J.; Ye, B.; Li, G.; Zhang, J.; Wang, P.; Tong, Z.; Windsor, J.; Li, W. Stent-Assisted Percutaneous Endoscopic Necrosectomy for Infected Pancreatic Necrosis: Technical Report and a Pilot Study. World J. Surg. 2019, 43, 1121–1128, doi:10.1007/s00268-018-04878-9.
- Mukai, S.; Itoi, T.; Baron, T.H.; Sofuni, A.; Itokawa, F.; Kurihara, T.; Tsuchiya, T.; Ishii, K.; Tsuji, S.; Ikeuchi, N., et al. Endoscopic ultrasound-guided placement of plastic vs. biflanged metal stents for therapy of walled-off necrosis: a retrospective single-center series. Endoscopy 2015, 47, 47-55, doi:10.1055/s-0034-1377966.Rana, S.S.; Gupta, R.; Kang, M.; Sharma, V.; Sharma, R.; Gorsi, U.; Bhasin, D.K. Percutaneous catheter drainage followed by endoscopic transluminal drainage/necrosectomy for treatment of infected pancreatic necrosis in early phase of illness. Endosc. Ultrasound 2018, 7, 41–47, doi:10.4103/eus.eus_94_17.
- Ang, T.L.; Kongkam, P.; Kwek, A.B.; Orkoonsawat, P.; Rerknimitr, R.; Fock, K.M. A two-center comparative study of plastic and lumen-apposing large diameter self-expandable metallic stents in endoscopic ultrasound-guided drainage of pancreatic fluid collections. Endosc Ultrasound 2016, 5, 320-327, doi:10.4103/2303-9027.191659.Gardner, T.B.; Chahal, P.; Papachristou, G.I.; Vege, S.S.; Petersen,
- Bapaye, A.; Dubale, N.A.; Sheth, K.A.; Bapaye, J.; Ramesh, J.; Gadhikar, H.; Mahajani, S.; Date, S.; Pujari, R.; Gaadhe, R. Endoscopic ultrasonography-guided transmural drainage of walled-off pancreatic necrosis: Comparison between a specially designed fully covered bi-flanged metal stent and multiple plastic stents. Dig Endosc 2017, 29, 104-110, doi:10.1111/den.12704.Yan, L.; Dargan, A.; Nieto, J.; Shariaha, R.Z.; Binmoeller, K.F.; Adler, D.G.; DeSimone, M.; Berzin, T.; Swahney, M.; Draganov, P.V.; et al. Direct endoscopic necrosectomy at the time of transmural stent placement results in earlier resolution of complex walled-off pancreatic necrosis: Results from a large multicenter United States trial. Endosc. Ultrasound 2019, 8, 172–179, doi:10.4103/eus.eus_108_17.
- Siddiqui, A.A.; Kowalski, T.E.; Loren, D.E.; Khalid, A.; Soomro, A.; Mazhar, S.M.; Isby, L.; Kahaleh, M.; Karia, K.; Yoo, J., et al. Fully covered self-expanding metal stents versus lumen-apposing fully covered self-expanding metal stent versus plastic stents for endoscopic drainage of pancreatic walled-off necrosis: clinical outcomes and success. Gastrointest Endosc 2017, 85, 758-765, doi:10.1016/j.gie.2016.08.014.Powers, P.C.; Siddiqui, A.; Sharaiha, R.Z.; Yang, G.; Dawod, E.; Novikov, A.A.; Javia, A.; Edirisuriya, C.; Noor, A.; Mumtaz, T.; et al. Discontinuation of proton pump inhibitor use reduces the number of endoscopic procedures required for resolution of walled-off pancreatic necrosis. Endosc. Ultrasound 2019, 8, 194–198, doi:10.4103/eus.eus_59_18.
- Abu Dayyeh, B.K.; Mukewar, S.; Majumder, S.; Zaghlol, R.; Vargas Valls, E.J.; Bazerbachi, F.; Levy, M.J.; Baron, T.H.; Gostout, C.J.; Petersen, B.T., et al. Large-caliber metal stents versus plastic stents for the management of pancreatic walled-off necrosis. Gastrointestinal Endoscopy 2018, 87, 141-149, doi:10.1016/j.gie.2017.04.032.Arvanitakis, M.; Dumonceau, J.M.; Albert, J.; Badaoui, A.; Bali, M.A.; Barthet, M.; Besselink, M.; Deviere, J.; Oliveira Ferreira, A.; Gyokeres, T.; et al. Endoscopic management of acute necrotizing pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) evidence-based multidisciplinary guidelines. Endoscopy 2018, 50, 524–546, doi:10.1055/a-0588-5365.
- Law, S.T.; De La SernaHiguera, C.; Simon, P.G.; Perez-MirandaCastillo, M. Comparison of clinical efficacies and safeties of lumen-apposing metal stent and conventional-type metal stent-assisted EUS-guided pancreatic wall-off necrosis drainage: a real-life experience in a tertiary hospital. Surg Endosc 2018, 32, 2448-2453, doi:10.1007/s00464-017-5946-6.Mukai, S.; Itoi, T.; Sofuni, A.; Itokawa, F.; Kurihara, T.; Tsuchiya, T.; Ishii, K.; Tsuji, S.; Ikeuchi, N.; Tanaka, R.; et al. Novel single transluminal gateway transcystic multiple drainages after EUS-guided drainage for complicated multilocular walled-off necrosis (with videos). Gastrointest. Endosc. 2014, 79, 531–535, doi:10.1016/j.gie.2013.10.004.
- Cho, I.R.; Chung, M.J.; Jo, J.H.; Lee, H.S.; Park, J.Y.; Bang, S.; Park, S.W.; Song, S.Y. A novel lumen-apposing metal stent with an anti-reflux valve for endoscopic ultrasound-guided drainage of pseudocysts and walled-off necrosis: A pilot study. PLoS One 2019, 14, e0221812, doi:10.1371/journal.pone.0221812.Fagenholz, P.J.; Thabet, A.; Mueller, P.R.; Forcione, D.G. Combined endoscopic trangastric drainage and video assisted retroperitoneal pancreatic debridement–The best of both worlds for extensive pancreatic necrosis with enteric fistulae. Pancreatology 2016, 16, 788–790, doi:10.1016/j.pan.2016.06.009.
- Zhu, H.; Xie, P.; Wang, Y.; Jin, Z.; Li, Z.; Du, Y. The role of solid debris in endoscopic ultrasound-guided drainage of walled-off necrosis: A large cohort study. J Gastroenterol Hepatol 2020, 10.1111/jgh.15086, doi:10.1111/jgh.15086.
- Parsa, N.; Nieto, J.M.; Powers, P.; Mitsuhashi, S.; Abdelqader, A.; Hadzinakos, G.; Anderloni, A.A.; Fugazza, A.; James, T.W.; Arlt, A., et al. Endoscopic ultrasound-guided drainage of pancreatic walled-off necrosis using 20-mm versus 15-mm lumen-apposing metal stents: an international, multicenter, case-matched study. Endoscopy 2020, 52, 211-219, doi:10.1055/a-1096-3299.
- Thompson, C.C.; Kumar, N.; Slattery, J.; Clancy, T.E.; Ryan, M.B.; Ryou, M.; Swanson, R.S.; Banks, P.A.; Conwell, D.L. A standardized method for endoscopic necrosectomy improves complication and mortality rates. Pancreatology 2016, 16, 66-72, doi:10.1016/j.pan.2015.12.001.
- Gardner, T.B.; Chahal, P.; Papachristou, G.I.; Vege, S.S.; Petersen, B.T.; Gostout, C.J.; Topazian, M.D.; Takahashi, N.; Sarr, M.G.; Baron, T.H. A comparison of direct endoscopic necrosectomy with transmural endoscopic drainage for the treatment of walled-off pancreatic necrosis. Gastrointest Endosc 2009, 69, 1085-1094, doi:10.1016/j.gie.2008.06.061.
- Bansal, R.; Puri, R.; Choudhary, N.; Bhatia, S.; Patel, N.; Patle, S.; Patil, G.; Agarwal, A.; Prabha, C.; Sud, R. Endoscopic pancreatic necrosectomy: why scuff when you can flush the muck – make it an easy row to hoe. Endoscopy International Open 2017, 05, E847-E853, doi:10.1055/s-0043-112854.
- Isayama, H.; Nakai, Y.; Rerknimitr, R.; Khor, C.; Lau, J.; Wang, H.P.; Seo, D.W.; Ratanachu-Ek, T.; Lakhtakia, S.; Ang, T.L., et al. Asian consensus statements on endoscopic management of walled-off necrosis. Part 2: Endoscopic management. J Gastroenterol Hepatol 2016, 31, 1555-1565, doi:10.1111/jgh.13398.
- Yamamoto, N.; Isayama, H.; Takahara, N.; Sasahira, N.; Miyabayashi, K.; Mizuno, S.; Kawakubo, K.; Mohri, D.; Kogure, H.; Sasaki, T., et al. Percutaneous direct-endoscopic necrosectomy for walled-off pancreatic necrosis. Endoscopy 2013, 45 Suppl 2 UCTN, E44-45, doi:10.1055/s-0032-1309927.
- Ke, L.; Mao, W.; Zhou, J.; Ye, B.; Li, G.; Zhang, J.; Wang, P.; Tong, Z.; Windsor, J.; Li, W. Stent-Assisted Percutaneous Endoscopic Necrosectomy for Infected Pancreatic Necrosis: Technical Report and a Pilot Study. World J Surg 2019, 43, 1121-1128, doi:10.1007/s00268-018-04878-9.
- Yan, L.; Dargan, A.; Nieto, J.; Shariaha, R.Z.; Binmoeller, K.F.; Adler, D.G.; DeSimone, M.; Berzin, T.; Swahney, M.; Draganov, P.V., et al. Direct endoscopic necrosectomy at the time of transmural stent placement results in earlier resolution of complex walled-off pancreatic necrosis: Results from a large multicenter United States trial. Endosc Ultrasound 2019, 8, 172-179, doi:10.4103/eus.eus_108_17.
- Powers, P.C.; Siddiqui, A.; Sharaiha, R.Z.; Yang, G.; Dawod, E.; Novikov, A.A.; Javia, A.; Edirisuriya, C.; Noor, A.; Mumtaz, T., et al. Discontinuation of proton pump inhibitor use reduces the number of endoscopic procedures required for resolution of walled-off pancreatic necrosis. Endosc Ultrasound 2019, 8, 194-198, doi:10.4103/eus.eus_59_18.
- Mukai, S.; Itoi, T.; Sofuni, A.; Itokawa, F.; Kurihara, T.; Tsuchiya, T.; Ishii, K.; Tsuji, S.; Ikeuchi, N.; Tanaka, R., et al. Novel single transluminal gateway transcystic multiple drainages after EUS-guided drainage for complicated multilocular walled-off necrosis (with videos). Gastrointestinal Endoscopy 2014, 79, 531-535, doi:10.1016/j.gie.2013.10.004.
- Fagenholz, P.J.; Thabet, A.; Mueller, P.R.; Forcione, D.G. Combined endoscopic trangastric drainage and video assisted retroperitoneal pancreatic debridement – The best of both worlds for extensive pancreatic necrosis with enteric fistulae. Pancreatology 2016, 16, 788-790, doi:10.1016/j.pan.2016.06.009
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S.; Acute Pancreatitis Classification Working, G. Classification of acute pancreatitis-2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111, doi:10.1136/gutjnl-2012-302779.
- Singh, V.K.; Bollen, T.L.; Wu, B.U.; Repas, K.; Maurer, R.; Yu, S.; Mortele, K.J.; Conwell, D.L.; Banks, P.A. An assessment of the severity of interstitial pancreatitis. Clin. Gastroenterol. Hepatol. 2011, 9, 1098–1103, doi:10.1016/j.cgh.2011.08.026.
- van Brunschot, S.; Bakker, O.J.; Besselink, M.G.; Bollen, T.L.; Fockens, P.; Gooszen, H.G.; van Santvoort, H.C.; Dutch Pancreatitis Study, G. Treatment of necrotizing pancreatitis. Clin. Gastroenterol. Hepatol. 2012, 10, 1190–1201, doi:10.1016/j.cgh.2012.05.005.
- Rogers, B.H.; Cicurel, N.J.; Seed, R.W. Transgastric needle aspiration of pancreatic pseudocyst through an endoscope. Gastrointest. Endosc. 1975, 21, 133–134, doi:10.1016/s0016-5107(75)73821-x.
- Yip, H.C.; Teoh, A.Y.B. Endoscopic Management of Peri-Pancreatic Fluid Collections. Gut Liver 2017, 11, 604–611, doi:10.5009/gnl16178.
- Trikudanathan, G.; Tawfik, P.; Amateau, S.K.; Munigala, S.; Arain, M.; Attam, R.; Beilman, G.; Flanagan, S.; Freeman, M.L.; Mallery, S. Early (<4 Weeks) Versus Standard (>/= 4 Weeks) Endoscopically Centered Step-Up Interventions for Necrotizing Pancreatitis. Am. J. Gastroenterol. 2018, 113, 1550–1558, doi:10.1038/s41395-018-0232-3.
- Chantarojanasiri, T.; Yamamoto, N.; Nakai, Y.; Saito, T.; Saito, K.; Hakuta, R.; Ishigaki, K.; Takeda, T.; Uchino, R.; Takahara, N.; et al. Comparison of early and delayed EUS-guided drainage of pancreatic fluid collection. Endosc. Int. Open 2018, 6, E1398–E1405, doi:10.1055/a-0751-2698.
- Hritz, I.; Fejes, R.; Szekely, A.; Szekely, I.; Horvath, L.; Sarkany, A.; Altorjay, A.; Madacsy, L. Endoscopic transluminal pancreatic necrosectomy using a self-expanding metal stent and high-flow water-jet system. World J. Gastroenterol. 2013, 19, 3685–3692, doi:10.3748/wjg.v19.i23.3685.
- Baron, T.H.; DiMaio, C.J.; Wang, A.Y.; Morgan, K.A. American Gastroenterological Association Clinical Practice Update: Management of Pancreatic Necrosis. Gastroenterology 2020, 158, 67–75, doi:10.1053/j.gastro.2019.07.064.
- van Santvoort, H.C.; Bakker, O.J.; Bollen, T.L.; Besselink, M.G.; Ahmed Ali, U.; Schrijver, A.M.; Boermeester, M.A.; van Goor, H.; Dejong, C.H.; van Eijck, C.H.; et al. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology 2011, 141, 1254–1263, doi:10.1053/j.gastro.2011.06.073.
- Belle, S.; Collet, P.; Post, S.; Kaehler, G. Temporary cystogastrostomy with self-expanding metallic stents for pancreatic necrosis. Endoscopy 2010, 42, 493–495, doi:10.1055/s-0029-1244021.
- Attam, R.; Trikudanathan, G.; Arain, M.; Nemoto, Y.; Glessing, B.; Mallery, S.; Freeman, M.L. Endoscopic transluminal drainage and necrosectomy by using a novel, through-the-scope, fully covered, large-bore esophageal metal stent: Preliminary experience in 10 patients. Gastrointest. Endosc. 2014, 80, 312–318, doi:10.1016/j.gie.2014.02.013.
- Tellez-Avila, F.I.; Villalobos-Garita, A.; Ramirez-Luna, M.A. Use of a novel covered self-expandable metal stent with an anti-migration system for endoscopic ultrasound-guided drainage of a pseudocyst. World J. Gastrointest. Endosc. 2013, 5, 297–299, doi:10.4253/wjge.v5.i6.297.
- Teoh, A.Y.; Ng, E.K.; Chan, S.M.; Lai, M.; Moran, S.; Binmoeller, K.F.; Moon, J.H.; Ho, K.Y. Ex vivo comparison of the lumen-apposing properties of EUS-specific stents (with video). Gastrointest. Endosc. 2016, 84, 62–68, doi:10.1016/j.gie.2015.11.041.
- Bank, J.; Adler, D. Lumen apposing metal stents: A review of current uses and outcomes. Gastrointest. Interv. 2017, 6, 9–14, doi:10.18528/gii160033.
- Braden, B.; Koutsoumpas, A.; Silva, M.A.; Soonawalla, Z.; Dietrich, C.F. Endoscopic ultrasound-guided drainage of pancreatic walled-off necrosis using self-expanding metal stents without fluoroscopy. World J. Gastrointest. Endosc. 2018, 10, 93–98, doi:10.4253/wjge.v10.i5.93.
- Weilert, F.; Binmoeller, K.F. Specially designed stents for translumenal drainage. Gastrointest. Interv. 2015, 4, 40–45, doi:10.1016/j.gii.2015.03.003.
- Anderloni, A.; Fabbri, C.; Nieto, J.; Uwe, W.; Dollhopf, M.; Aparicio, J.R.; Perez-Miranda, M.; Tarantino, I.; Arlt, A.; Vleggaar, F.; et al. The safety and efficacy of a new 20 mm lumen apposing metal stent (lams) for the endoscopic treatment of pancreatic and peripancreatic fluid collections: A large international, multicenter study. Surg. Endosc. 2020, doi:10.1007/s00464-020-07567-8.
- Adler, D.G.; Taylor, L.J.; Hasan, R.; Siddiqui, A.A. A retrospective study evaluating endoscopic ultrasound-guided drainage of pancreatic fluid collections using a novel lumen-apposing metal stent on an electrocautery enhanced delivery system. Endosc. Ultrasound 2017, 6, 389–393, doi:10.4103/eus.eus_4_17.
- Weigand, K.; Mehrl, A.; Goessmann, H.; Mueller, M.; Kandulski, A. Endoscopic Necrosectomy of Walled-Off Necrosis following Severe Pancreatitis Using a Hot AxiosTM Stent-A Case Series. Dig. Dis. 2019, 1–4, doi:10.1159/000503991.
- Ge, P.S.; Young, J.Y.; Jirapinyo, P.; Dong, W.; Ryou, M.; Thompson, C.C. Comparative Study Evaluating Lumen Apposing Metal Stents Versus Double Pigtail Plastic Stents for Treatment of Walled-Off Necrosis. Pancreas 2020, 49, 236–241, doi:10.1097/MPA.0000000000001476.
- Bang, J.Y.; Navaneethan, U.; Hasan, M.K.; Sutton, B.; Hawes, R.; Varadarajulu, S. Non-superiority of lumen-apposing metal stents over plastic stents for drainage of walled-off necrosis in a randomised trial. Gut 2019, 68, 1200–1209, doi:10.1136/gutjnl-2017-315335.
- Mohan, B.P.; Jayaraj, M.; Asokkumar, R.; Shakhatreh, M.; Pahal, P.; Ponnada, S.; Navaneethan, U.; Adler, D.G. Lumen apposing metal stents in drainage of pancreatic walled-off necrosis, are they any better than plastic stents? A systematic review and meta-analysis of studies published since the revised Atlanta classification of pancreatic fluid collections. Endosc. Ultrasound 2019, 8, 82–90, doi:10.4103/eus.eus_7_19.
- Chen, Y.I.; Yang, J.; Friedland, S.; Holmes, I.; Law, R.; Hosmer, A.; Stevens, T.; Franco, M.C.; Jang, S.; Pawa, R.; et al. Lumen apposing metal stents are superior to plastic stents in pancreatic walled-off necrosis: A large international multicenter study. Endosc. Int. Open 2019, 7, E347–E354, doi:10.1055/a-0828-7630.
- Kayal, A.; Taghizadeh, N.; Ishikawa, T.; Gonzalez-Moreno, E.; Bass, S.; Cole, M.J.; Heitman, S.J.; Mohamed, R.; Turbide, C.; Chen, Y.I.; et al. Endosonography-guided transmural drainage of pancreatic fluid collections: Comparative outcomes by stent type. Surg. Endosc. 2020, doi:10.1007/s00464-020-07699-x.
- Lang, G.D.; Fritz, C.; Bhat, T.; Das, K.K.; Murad, F.M.; Early, D.S.; Edmundowicz, S.A.; Kushnir, V.M.; Mullady, D.K. EUS-guided drainage of peripancreatic fluid collections with lumen-apposing metal stents and plastic double-pigtail stents: Comparison of efficacy and adverse event rates. Gastrointest. Endosc. 2018, 87, 150–157, doi:10.1016/j.gie.2017.06.029.
- Rana, S.S.; Sharma, R.; Dhalaria, L.; Gupta, R. Efficacy and safety of plastic versus lumen-apposing metal stents for transmural drainage of walled-off necrosis: A retrospective single-center study. Ann. Gastroenterol. 2020, 33, 426–432, doi:10.20524/aog.2020.0499.
- Bang, J.Y.; Hasan, M.; Navaneethan, U.; Hawes, R.; Varadarajulu, S. Lumen-apposing metal stents (LAMS) for pancreatic fluid collection (PFC) drainage: May not be business as usual. Gut 2017, 66, 2054–2056, doi:10.1136/gutjnl-2016-312812.
- Mukai, S.; Itoi, T.; Baron, T.H.; Sofuni, A.; Itokawa, F.; Kurihara, T.; Tsuchiya, T.; Ishii, K.; Tsuji, S.; Ikeuchi, N.; et al. Endoscopic ultrasound-guided placement of plastic vs. biflanged metal stents for therapy of walled-off necrosis: A retrospective single-center series. Endoscopy 2015, 47, 47–55, doi:10.1055/s-0034-1377966.
- Ang, T.L.; Kongkam, P.; Kwek, A.B.; Orkoonsawat, P.; Rerknimitr, R.; Fock, K.M. A two-center comparative study of plastic and lumen-apposing large diameter self-expandable metallic stents in endoscopic ultrasound-guided drainage of pancreatic fluid collections. Endosc. Ultrasound 2016, 5, 320–327, doi:10.4103/2303-9027.191659.
- Bapaye, A.; Dubale, N.A.; Sheth, K.A.; Bapaye, J.; Ramesh, J.; Gadhikar, H.; Mahajani, S.; Date, S.; Pujari, R.; Gaadhe, R. Endoscopic ultrasonography-guided transmural drainage of walled-off pancreatic necrosis: Comparison between a specially designed fully covered bi-flanged metal stent and multiple plastic stents. Dig. Endosc. 2017, 29, 104–110, doi:10.1111/den.12704.
- Siddiqui, A.A.; Kowalski, T.E.; Loren, D.E.; Khalid, A.; Soomro, A.; Mazhar, S.M.; Isby, L.; Kahaleh, M.; Karia, K.; Yoo, J.; et al. Fully covered self-expanding metal stents versus lumen-apposing fully covered self-expanding metal stent versus plastic stents for endoscopic drainage of pancreatic walled-off necrosis: Clinical outcomes and success. Gastrointest. Endosc. 2017, 85, 758–765, doi:10.1016/j.gie.2016.08.014.
- Abu Dayyeh, B.K.; Mukewar, S.; Majumder, S.; Zaghlol, R.; Vargas Valls, E.J.; Bazerbachi, F.; Levy, M.J.; Baron, T.H.; Gostout, C.J.; Petersen, B.T.; et al. Large-caliber metal stents versus plastic stents for the management of pancreatic walled-off necrosis. Gastrointest. Endosc. 2018, 87, 141–149, doi:10.1016/j.gie.2017.04.032.
- Law, S.T.; De La Serna Higuera, C.; Simon, P.G.; Castillo, M.P.-M. Comparison of clinical efficacies and safeties of lumen-apposing metal stent and conventional-type metal stent-assisted EUS-guided pancreatic wall-off necrosis drainage: A real-life experience in a tertiary hospital. Surg. Endosc. 2018, 32, 2448–2453, doi:10.1007/s00464-017-5946-6.
- Parsa, N.; Nieto, J.M.; Powers, P.; Mitsuhashi, S.; Abdelqader, A.; Hadzinakos, G.; Anderloni, A.A.; Fugazza, A.; James, T.W.; Arlt, A.; et al. Endoscopic ultrasound-guided drainage of pancreatic walled-off necrosis using 20 mm versus 15 mm lumen-apposing metal stents: An international, multicenter, case-matched study. Endoscopy 2020, 52, 211–219, doi:10.1055/a-1096-3299.
- Thompson, C.C.; Kumar, N.; Slattery, J.; Clancy, T.E.; Ryan, M.B.; Ryou, M.; Swanson, R.S.; Banks, P.A.; Conwell, D.L. A standardized method for endoscopic necrosectomy improves complication and mortality rates. Pancreatology 2016, 16, 66–72, doi:10.1016/j.pan.2015.12.001.
- Gardner, T.B.; Chahal, P.; Papachristou, G.I.; Vege, S.S.; Petersen, B.T.; Gostout, C.J.; Topazian, M.D.; Takahashi, N.; Sarr, M.G.; Baron, T.H. A comparison of direct endoscopic necrosectomy with transmural endoscopic drainage for the treatment of walled-off pancreatic necrosis. Gastrointest. Endosc. 2009, 69, 1085–1094, doi:10.1016/j.gie.2008.06.061.
- Bansal, R.; Puri, R.; Choudhary, N.; Bhatia, S.; Patel, N.; Patle, S.; Patil, G.; Agarwal, A.; Prabha, C.; Sud, R. Endoscopic pancreatic necrosectomy: Why scuff when you can flush the muck-Make it an easy row to hoe. Endosc. Int. Open 2017, 5, E847–E853, doi:10.1055/s-0043-112854.
- Isayama, H.; Nakai, Y.; Rerknimitr, R.; Khor, C.; Lau, J.; Wang, H.P.; Seo, D.W.; Ratanachu-Ek, T.; Lakhtakia, S.; Ang, T.L.; et al. Asian consensus statements on endoscopic management of walled-off necrosis. Part 2: Endoscopic management. J. Gastroenterol. Hepatol. 2016, 31, 1555–1565, doi:10.1111/jgh.13398.
- Yamamoto, N.; Isayama, H.; Takahara, N.; Sasahira, N.; Miyabayashi, K.; Mizuno, S.; Kawakubo, K.; Mohri, D.; Kogure, H.; Sasaki, T.; et al. Percutaneous direct-endoscopic necrosectomy for walled-off pancreatic necrosis. Endoscopy 2013, 45, E44–E45, doi:10.1055/s-0032-1309927.
- Ke, L.; Mao, W.; Zhou, J.; Ye, B.; Li, G.; Zhang, J.; Wang, P.; Tong, Z.; Windsor, J.; Li, W. Stent-Assisted Percutaneous Endoscopic Necrosectomy for Infected Pancreatic Necrosis: Technical Report and a Pilot Study. World J. Surg. 2019, 43, 1121–1128, doi:10.1007/s00268-018-04878-9.
- Gardner, T.B.; Chahal, P.; Papachristou, G.I.; Vege, S.S.; Petersen,
- Rana, S.S.; Gupta, R.; Kang, M.; Sharma, V.; Sharma, R.; Gorsi, U.; Bhasin, D.K. Percutaneous catheter drainage followed by endoscopic transluminal drainage/necrosectomy for treatment of infected pancreatic necrosis in early phase of illness. Endosc. Ultrasound 2018, 7, 41–47, doi:10.4103/eus.eus_94_17.
- Yan, L.; Dargan, A.; Nieto, J.; Shariaha, R.Z.; Binmoeller, K.F.; Adler, D.G.; DeSimone, M.; Berzin, T.; Swahney, M.; Draganov, P.V.; et al. Direct endoscopic necrosectomy at the time of transmural stent placement results in earlier resolution of complex walled-off pancreatic necrosis: Results from a large multicenter United States trial. Endosc. Ultrasound 2019, 8, 172–179, doi:10.4103/eus.eus_108_17.
- Powers, P.C.; Siddiqui, A.; Sharaiha, R.Z.; Yang, G.; Dawod, E.; Novikov, A.A.; Javia, A.; Edirisuriya, C.; Noor, A.; Mumtaz, T.; et al. Discontinuation of proton pump inhibitor use reduces the number of endoscopic procedures required for resolution of walled-off pancreatic necrosis. Endosc. Ultrasound 2019, 8, 194–198, doi:10.4103/eus.eus_59_18.
- Mukai, S.; Itoi, T.; Sofuni, A.; Itokawa, F.; Kurihara, T.; Tsuchiya, T.; Ishii, K.; Tsuji, S.; Ikeuchi, N.; Tanaka, R.; et al. Novel single transluminal gateway transcystic multiple drainages after EUS-guided drainage for complicated multilocular walled-off necrosis (with videos). Gastrointest. Endosc. 2014, 79, 531–535, doi:10.1016/j.gie.2013.10.004.
- Fagenholz, P.J.; Thabet, A.; Mueller, P.R.; Forcione, D.G. Combined endoscopic trangastric drainage and video assisted retroperitoneal pancreatic debridement–The best of both worlds for extensive pancreatic necrosis with enteric fistulae. Pancreatology 2016, 16, 788–790, doi:10.1016/j.pan.2016.06.009.
- Arvanitakis, M.; Dumonceau, J.M.; Albert, J.; Badaoui, A.; Bali, M.A.; Barthet, M.; Besselink, M.; Deviere, J.; Oliveira Ferreira, A.; Gyokeres, T.; et al. Endoscopic management of acute necrotizing pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) evidence-based multidisciplinary guidelines. Endoscopy 2018, 50, 524–546, doi:10.1055/a-0588-5365.
- Cho, I.R.; Chung, M.J.; Jo, J.H.; Lee, H.S.; Park, J.Y.; Bang, S.; Park, S.W.; Song, S.Y. A novel lumen-apposing metal stent with an anti-reflux valve for endoscopic ultrasound-guided drainage of pseudocysts and walled-off necrosis: A pilot study. PLoS ONE 2019, 14, e0221812, doi:10.1371/journal.pone.0221812.
- Zhu, H.; Xie, P.; Wang, Y.; Jin, Z.; Li, Z.; Du, Y. The role of solid debris in endoscopic ultrasound-guided drainage of walled-off necrosis: A large cohort study. J. Gastroenterol. Hepatol. 2020, doi:10.1111/jgh.15086.
- Isayama, H.; Nakai, Y.; Rerknimitr, R.; Khor, C.; Lau, J.; Wang, H.P.; Seo, D.W.; Ratanachu-Ek, T.; Lakhtakia, S.; Ang, T.L.; et al. Asian consensus statements on endoscopic management of walled-off necrosis Part 1: Epidemiology, diagnosis, and treatment. J. Gastroenterol. Hepatol. 2016, 31, 1546–1554, doi:10.1111/jgh.13394.
