Melanocytes and melanin play a wide range of roles such as adsorption of metals, thermoregulation, and protection from foreign enemies by camouflage.
- melanosome,melanogenesis,toll-like receptor,innate immunity,microphthalmia-associated transcription factor
Thanks so much for your check. We sincerely hope you may create this entry based on your published paper. You can click the “submit” button to upload it and revise it. We will help you layout after you submit it. Moreover, we will link your article at the entry, and more scholars and students can look through it.
1. Introduction
Melanocytes play unique roles in the production of melanin, a fundamental molecule of skin pigment. Melanocytes synthesize melanin pigments by receiving various external stimuli such as ultraviolet rays and peptide hormones melanocortins. Melanocytes deliver melanin to the adjacent epidermal keratinocytes, and then the transported melanin functions as a UV filter in the keratinocytes. Thus, melanocytes and melanin play roles in the biological defense of human epidermis. Melanocytes are also involved in innate immunity, which conducts the initial responses in the elimination of microorganisms and viruses. Melanocytes augment melanogenesis and melanin transport by innate immune stimuli through toll-like receptors (TLRs). These findings suggest that melanin synthesis and melanin transport have connections with the immune systems. In this review, we provide an overview of the skin functions in innate immune systems and review the melanocyte functions in immune responses and pigment formation induced by innate immunity in order to discuss the significance of innate immunity in melanogenesis and skin pigmentation.
2. Melanocyte Functions in Immune System and Inflammation
2.1. Melanocyte Functions in Acquired Immunity
In epidermal cells, melanocytes and Langerhans cells are morphologically classified as dendritic cells. As professional antigen presenting cells (APCs), Langerhans cells as well as dermal dendritic cells have particularly strong antigen presenting ability. APCs take up a foreign antigen into cells by endocytosis and phagocytosis and present the antigen peptides. APCs express a glycoprotein called MHC class II and present the antigen to T cells by forming a complex with foreign antigen peptides. Although melanocytes are not recognized as a professional APCs, melanocytes express MHC class II by IFNγ stimulation[1][2][3] [15,16,17]. The ability for phagocytosis is also observed in melanocytes. Le Poole et al. documented that melanocytes had phagocytosis ability, which was examined using 1 µm latex beads in the presence of keratinocytes[4] [18]. Le Poole et al. also demonstrated that melanocytes can function as target cells for T cells by processing and presenting the phagocytosed antigen[1] [15]. Melanocytes express intercellular adhesion factor ICAM-1, which is responsible for the cell-cell interactions of the immune system, and CD40, which is mainly observed in mature dendritic cells and plays roles in adaptive immune systems[3][5][6][7] [17,19,20,21]. In a manner similar to that of the professional APCs, melanocytes also produce cytokines that modulate immune responses, such as IL-1α, IL-1β, IL-8, and TGF-β1[8][9][10] [22,23,24]. These finding suggest the possible roles of melanocytes as antigen-presenting cells.
2.2. Melanocytes and Immunodeficiency Disorders
Albinisms, hypopigmentary disorders, are caused by a genetic alteration of the melanin synthetases and melanosome-related molecules. Some albinisms are complicated by disorders of immune functions, suggesting genetic relationships between melanin production and immune function.
Griscelli syndromes (GS) are autosomal recessive disorders characterized by immunodeficiency and partial depigmentation due to aberrant melanosome distribution[11] [25]. Rab27A is one of the genes responsible for causing GS [12][26]. Because Rab27A is a regulator of intracellular membrane trafficking and is essential for melanosome transport to the dendrite tip in human melanocytes, a defect of the Rab27A function causes the depigmentation of skin observed in GS[13] [27] (Figure 1). Rab27A is also involved in immune functions. Individuals with the Rab27A mutation develop lymphocyte and macrophage activation syndrome. Rab27A-deficient T cells have reduced cytolytic granule exocytosis, which is caused by a defect of intracellular membrane trafficking[12] [26]. These findings suggest that Rab27A is an important effector of the cytotoxic granule transport, which is an essential pathway for immune homeostasis.
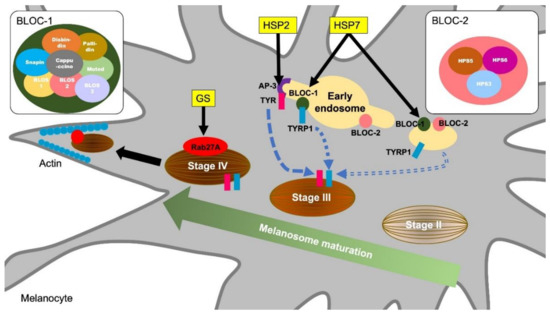
Figure 1. Molecular mechanisms of melanosome formation and maturation related to the hypopigmentary genetic diseases. Griscelli syndrome (GS) is an autosomal recessive disorder characterized by immunodeficiency and partial bleaching due to abnormal melanosome distribution. One of the causes of GS is a mutation in the Rab27A gene. Rab27A transports melanosome to the cell periphery, and Rab27A gene mutations result in a melanosome transport disturbance in melanocytes. Hermansky–Pudlak syndromes (HPS) are also rare autosomal recessive disorders and are associated with skin depigmentation and immune impairment. HPS2 patients have a deficiency in the β3A subunit of AP-3. AP-3 classifies the melanin synthesis-related enzyme tyrosinase (TYR) from endosomes to melanosomes. Patients with HSP7 do not express the dysbindin protein, one of the components of BLOC-1 (biogenesis of lysosome-related organelles complex 1). BLOC-1 regulates the transport and biosynthesis of lysosomal organelles. BLOC-1 interacts with AP-3 to promote the transport of tyrosinase-related protein 1 (TYRP1). BLOC-1 also interacts with BLOC-2 and promotes TYRP1 transport by a mechanism different from that of AP-3.
Hermansky-Pudlak syndromes (HPS) are autosomal recessive disorders known to be associated with depigmentation and immune disorders[14][15] [28,29]. Defects in cytoplasmic organelles such as melanosomes, platelet granules, and lysosomes cause ocular cutaneous albinism, bleeding tendency, and ceroid lipofuscin lysosomal storage disease[15] [29]. Pigment abnormalities in HSPs are caused by a decrease in the number of melanosomes and increase in immature melanosomes in melanocytes[16] [30]. Some types of HPS result from mutations in genes encoding endoplasmic reticulum transport proteins, which are involved in the biosynthesis of organelles containing melanosomes [17][31]. HPS2 is caused by a deficiency in the β3A subunit of the adaptor protein-3 (AP-3) complex[18] [32]. The AP-3 complex controls sorting of the protein cargo into specialized organelles, including endosomes, lysosomes, and melanosomes[19] [33]. Microbial antigens phagocytosed by APCs undergo antigen binding to MHC and CD1 molecules after being delivered to the lysosomal compartment. However, in AP-3 deficient cells derived from HPS2 patients, CD1b does not have efficient access to lysosomes, resulting in defective antigen presentation[20] [34]. In melanocytes, AP-3 functions to deliver the melanin synthesis-related enzyme tyrosinase to melanosomes from endosomes[21] [35] (Figure 1). Thus, HPS2 patients are characterized by partial albinism and signs of immunodeficiency because the AP-3 functions in both the lysosomal compartment in APCs and melanosome in melanocytes.
HPS7 is caused by an alteration in DTNBP1, a gene for dysbindin protein[22] [36]. Sandy (sdy) is a mouse variant, which has a deletion of the Dtnbp1 gene and lack the dysbindin protein expression. Sdy mutant mice are used as an animal model for HSP because sdy mice show hypopigmentation due to melanosomes dysfunction as well as lysosome disfunction and platelet defects[23] [37]. Dtnbp1 is a component of the biogenesis of lysosome-related organelles complex 1 (BLOC-1) and regulates lysosomal organelle transport and biosynthesis. BLOC-1 is known to interact with AP-3 to promote the transport of tyrosinase-related protein 1 (TYRP1) in melanocytes (Figure 1). In addition, BLOC-1 also interacts with the biogenesis of lysosome-related organelles complex 2 (BLOC-2) and promotes melanin synthesis by promoting TYRP1 trafficking by a mechanism different from that of AP-3 [24] [38] (Figure 1). Although there is insufficient evidence that BLOC-1 deficiency is a direct cause of immunodeficiency, AP-3 and BLOC-1 are required for cytokine signaling from plasma membranes and endosomes[25] [39]. In endosomal TLR signaling in plasmacytoid dendritic cells (pDCs), AP-3, BLOC-1, and BLOC-2 are essential for signaling transduction via TLR7 and TLR9, which sense viral nucleic acids and induce type I interferon production[26] [40]. Thus, a dysfunction of lysosome and melanosome-related molecules is responsible for hypopigmentation and immune cell dysfunctions observed in HPS.
2.3. Melanin and Inflammatory Responses
It has been reported that the melanin pigment itself may play a role in controlling the immune response. Compared to melanocytes that contain a large amount of melanin, melanocytes containing little melanin produce a higher number of cytokines such as IL-6 and IL-10[27][28] [41,42]. l-DOPA, the intermediate product of melanin synthesis, and its oxidation products function as potent immunosuppressive agents that inhibit lymphocyte proliferation and abrogate inflammatory cytokine production by activated lymphocytes[27][28] [41,42]. Mohagheghpour et al. have shown that non-toxic concentrations of synthetic melanin suppress the production of cytokines such as TNF, IL-1β, IL-6, and IL-10 by reducing the efficiency of mRNA translation[29] [43]. They suggested the possibility of treatment with melanin for pathological symptoms such as rheumatoid arthritis and sepsis syndrome caused by the overproduction of inflammatory cytokines[29] [43]. Individuals with human immunodeficiency virus (HIV) infection often develop oral pigmentation for an unknown reason[30] [44]. In vitro, non-toxic concentrations of melanin inhibit HIV virus-induced syncytia formation and cytopathic effects, with potential therapeutic utility in the treatment of acquired immunodeficiency syndrome (AIDS)[31] [45]. These reports suggest that melanocytes have a role in controlling the immune response and that the presence of melanin pigments may cause fluctuation of the immune response levels.
3. Future Prospect for Studies of Melanogenesis in Immune Systems
Studies on innate immune systems have revealed that the TLR-mediated intracellular mechanism has multiple functions in melanogenesis. Since the melanin pigment itself has a role in the immune response, it is reasonable to consider that innate immune stimulation controls pigment formation. Exploring the role of melanin pigments in the immune response may lead to understanding how melanocytes and melanogenesis evolved to acquire their regulatory mechanisms and functions. Studies of the TLRs involvement in melanogenesis have only just been launched.
At present, little is known about how the TLRs affects the dynamics of melanosome and melanin in keratinocytes. Keratinocytes express functional TLRs. The link between TLRs and lysosome are extensively studied in the field of acquired and innate immunity. Therefore, TLRs might affect the melanosome degradation through lysosome and autophagy activation in keratinocytes. In the future, the involvement of immune signals in pigment formation should be explored to understand the cycle of melanin synthesis, transport, uptake, and degradation in keratinocytes, which are essential molecular actions controlling the skin color. The development of such studies will promote the understanding of the biological defense involving pigmentation control in the living body and elucidate the mechanisms of immune dysfunction in skin diseases with pigment abnormality.
Reference (we'll rearrange the references after you submitted it)
- Lee, S.H.; Jeong, S.K.; Ahn, S.K. An Update of the Defensive Barrier Function of Skin. Yonsei Med. J. 2006, 47, 293–306.
- Baroni, A.; Buommino, E.; De Gregorio, V.; Ruocco, E.; Ruocco, V.; Wolf, R. Structure and function of the epidermis related to barrier properties. Clin. Dermatol. 2012, 30, 257–262.
- Ermertcan, A.; Ozturk, F.; Gunduz, K. Toll-like receptors and skin. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 997–1006.
- Mitsui, H.; Watanabe, T.; Saeki, H.; Mori, K.; Fujita, H.; Tada, Y.; Asahina, A.; Nakamura, K.; Tamaki, K. Differential Expression and Function of Toll-like Receptors in Langerhans Cells: Comparison with Splenic Dendritic Cells. J. Investig. Dermatol. 2004, 122, 95–102.
- Baker, B.; Ovigne, J.-M.; Powles, A.; Corcoran, S.; Fry, L. Normal keratinocytes express Toll-like receptors (TLRs) 1, 2 and 5: Modulation of TLR expression in chronic plaque psoriasis. Br. J. Dermatol. 2003, 148, 670–679.
- Lebre, M.C.; Van Der Aar, A.M.G.; Van Baarsen, L.; Van Capel, T.M.M.; Schuitemaker, J.H.N.; Kapsenberg, M.L.; De Jong, E.C. Human Keratinocytes Express Functional Toll-Like Receptor 3, 4, 5, and 9. J. Investig. Dermatol. 2007, 127, 331–341.
- Yamasaki, K.; Gallo, R.L.; Kenshi, Y.; Richard, L.G. Antimicrobial peptides in human skin disease. Eur. J. Dermatol. 2007, 18, 11–21.
- Gilchrest, B.A.; Park, H.-Y.; Eller, M.S.; Yaar, M. Mechanisms of Ultraviolet Light-Induced Pigmentation. Photochem. Photobiol. 1996, 63, 1–10.
- Wang, Z.; Mascarenhas, N.; Eckman, L.; Miyamoto, Y.; Sun, X.; Kawakami, T.; Di Nardo, A. Skin microbiome promotes mast cell maturation by triggering stem cell factor (SCF) production in keratinocytes. J. Allergy Clin. Immunol. 2017, 139, 1205–1216.
- D’Mello, S.A.N.; Finlay, G.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144.
- Kobayashi, N.; Nakagawa, A.; Muramatsu, T.; Yamashina, Y.; Shirai, T.; Hashimoto, M.W.; Ishigaki, Y.; Ohnishi, T.; Mori, T. Supranuclear Melanin Caps Reduce Ultraviolet Induced DNA Photoproducts in Human Epidermis. J. Investig. Dermatol. 1998, 110, 806–810.
- Montagna, W.; Carlisle, K. The architecture of black and white facial skin. J. Am. Acad. Dermatol. 1991, 24, 929–937.
- Kollias, N.; Sayre, R.M.; Zeise, L.; Chedekel, M.R. Photoprotection by melanin. J. Photochem. Photobiol. B 1991, 9, 135–160.
- Hill, H.Z. The function of melanin or six blind people examine an elephant. BioEssays 1992, 14, 49–56.
- Le Poole, I.C.; Mutis, T.; Wijngaard, R.M.V.D.; Westerhof, W.; Ottenhoff, T.; De Vries, R.R.; Das, P.K. A novel, antigen-presenting function of melanocytes and its possible relationship to hypopigmentary disorders. J. Immunol. 1993, 151, 7284–7292.
- Tsujisaki, M.; Igarashi, M.; Sakaguchi, K.; Eisinger, M.; Herlyn, M.; Ferrone, S. Immunochemical and functional analysis of HLA class II antigens induced by recombinant immune interferon on normal epidermal melanocytes. J. Immunol. 1987, 138, 1310–1316.
- Hedley, S.J.; Metcalfe, R.; Gawkrodger, D.J.; Weetman, A.P.; Mac Neil, S. Vitiligo melanocytes in long-term culture show normal constitutive and cytokine-induced expression of intercellular adhesion molecule-1 and major histocompatibility complex class I and class II molecules. Br. J. Dermatol. 1998, 139, 965–973.
- Le Poole, I.; Wijngaard, R.V.D.; Westerhof, W.; Verkruisen, R.; Dutrieux, R.; Dingemans, K.; Das, P. Phagocytosis by Normal Human Melanocytes in Vitro. Exp. Cell Res. 1993, 205, 388–395.
- Al Badri, A.M.T.; Foulis, A.K.; Todd, P.M.; Garioch, J.J.; Gudgeon, J.E.; Stewart, D.G.; Gracie, J.A.; Goudie, R.B. Abnormal expression of MHC class II and ICAM-1 by melanocytes in vitiligo. J. Pathol. 1993, 169, 203–206.
- Smit, N.; Le Poole, I.; Wijngaard, R.V.D.; Tigges, A.; Westerhof, W.; Das, P. Expression of different immunological markers by cultured human melanocytes. Arch. Dermatol. Res. 1993, 285, 356–365.
- Lu, Y.; Zhu, W.-Y.; Tan, C.; Yu, G.-H.; Gu, J.-X. Melanocytes are potential immunocompetent cells: Evidence from recognition of immunological characteristics of cultured human melanocytes. Pigment Cell Res. 2002, 15, 454–460.
- Swope, V.B.; Sauder, D.N.; McKenzie, R.C.; Sramkoski, R.M.; A Krug, K.; Babcock, G.F.; Nordlund, J.J.; A Abdel-Malek, Z. Synthesis of interleukin-1 alpha and beta by normal human melanocytes. J. Investig. Dermatol. 1994, 102, 749–753.
- Zachariae, C.; Thestrup-Pedersen, K.; Matsushima, K. Expression and Secretion of Leukocyte Chemotactic Cytokines by Normal Human Melanocytes and Melanoma Cells. J. Investig. Dermatol. 1991, 97, 593–599.
- Le Poole, I.C.; Boyce, S.T. Keratinocytes suppress transforming growth factor-beta1 expression by fibroblasts in cultured skin substitutes. Br. J. Dermatol. 1999, 140, 409–416.
- Griscelli, C.; Prunieras, M. Pigment Dilution and Immunodeficiency: A New Syndrome. Int. J. Dermatol. 1978, 17, 788–791.
- Ménasché, G.; Pastural, E.; Feldmann, J.; Certain, S.; Ersoy, F.; Dupuis, S.; Wulffraat, N.; Bianchi, D.; Fischer, A.; Le Deist, F.; et al. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat. Genet. 2000, 25, 173–176.
- Bahadoran, P.; Aberdam, E.; Mantoux, F.; Busca, R.; Bille, K.; Yalman, N.; de Saint-Basile, G.; Casaroli-Marano, R.; Ortonne, J.P.; Ballotti, R. Rab27a: A key to melanosome transport in human melanocytes. J. Cell Biol. 2001, 152, 843–850.
- Hermansky, F.; Pudlak, P. Albinism Associated with Hemorrhagic Diathesis and Unusual Pigmented Reticular Cells in the Bone Marrow: Report of Two Cases with Histochemical Studies. Blood 1959, 14, 162–169.
- Spritz, R.A. Molecular genetics of the Hermansky-Pudlak and Chediak-Higashi syndromes. Platelets 1998, 9, 21–29.
- Schinella, R.A.; Greco, M.A.; Garay, S.M.; Lackner, H.; Wolman, S.R.; Fazzini, E.P. Hermansky-Pudlak syndrome: A clinicopathologic study. Hum. Pathol. 1985, 16, 366–376.
- Oh, J.; Liu, Z.-X.; Feng, G.H.; Raposo, G.; Spritz, R.A. The Hermansky-Pudlak syndrome (HPS) protein is part of a high molecular weight complex involved in biogenesis of early melanosomes. Hum. Mol. Genet. 2000, 9, 375–385.
- Huizing, M.; Scher, C.D.; Strovel, E.; Fitzpatrick, D.L.; Hartnell, L.M.; Anikster, Y.; Gahl, W.A. Nonsense mutations in ADTB3A cause complete deficiency of the beta3A subunit of adaptor complex-3 and severe Hermansky-Pudlak syndrome type 2. Pediatr. Res. 2002, 51, 150–158.
- Raposo, G.; Fevrier, B.; Stoorvogel, W.; Marks, M.S. Lysosome-Related Organelles: A View from Immunity and Pigmentation. Cell Struct. Funct. 2002, 27, 443–456.
- Sugita, M.; Cao, X.; Watts, G.F.; Rogers, R.A.; Bonifacino, J.S.; Brenner, M.B. Failure of Trafficking and Antigen Presentation by CD1 in AP-3-Deficient Cells. Immunity 2002, 16, 697–706.
- Theos, A.C.; Tenza, D.; Martina, J.A.; Hurbain, I.; Peden, A.A.; Sviderskaya, E.V.; Stewart, A.; Robinson, M.S.; Bennett, D.C.; Cutler, D.F.; et al. Functions of Adaptor Protein (AP)-3 and AP-1 in Tyrosinase Sorting from Endosomes to Melanosomes. Mol. Biol. Cell 2005, 16, 5356–5372.
- Li, W.; Zhang, Q.; Oiso, N.; Novak, E.K.; Gautam, R.; O’Brien, E.P.; Tinsley, C.L.; Blake, D.J.; A Spritz, R.; Copeland, N.G.; et al. Hermansky-Pudlak syndrome type 7 (HPS-7) results from mutant dysbindin, a member of the biogenesis of lysosome-related organelles complex 1 (BLOC-1). Nat. Genet. 2003, 35, 84–89.
- Swank, R.T.; Sweet, H.O.; Davisson, M.T.; Reddington, M.; Novak, E.K. Sandy: A new mouse model for platelet storage pool deficiency. Genet. Res. 1991, 58, 51–62.
- Setty, S.R.G.; Tenza, D.; Truschel, S.T.; Chou, E.; Sviderskaya, E.V.; Theos, A.C.; Lamoreux, M.L.; Di Pietro, S.M.; Starcevic, M.; Bennett, D.C.; et al. BLOC-1 Is Required for Cargo-specific Sorting from Vacuolar Early Endosomes toward Lysosome-related Organelles. Mol. Biol. Cell 2007, 18, 768–780.
- Mantegazza, A.R.; Guttentag, S.H.; El-Benna, J.; Sasai, M.; Iwasaki, A.; Shen, H.; Laufer, T.M.; Marks, M.S. Adaptor Protein-3 in Dendritic Cells Facilitates Phagosomal Toll-like Receptor Signaling and Antigen Presentation to CD4+ T Cells. Immunity 2012, 36, 782–794.
- Blasius, A.L.; Arnold, C.N.; Georgel, P.; Rutschmann, S.; Xia, Y.; Lin, P.; Ross, C.; Li, X.; Smart, N.G.; Beutler, B.A. Slc15a4, AP-3, and Hermansky-Pudlak syndrome proteins are required for Toll-like receptor signaling in plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. USA 2010, 107, 19973–19978.
- Tam, I.; Dzierżęga-Lęcznar, A.; Stepień, K. Differential expression of inflammatory cytokines and chemokines in lipopolysaccharide-stimulated melanocytes from lightly and darkly pigmented skin. Exp. Dermatol. 2019, 28, 551–560.
- Slominski, A.; Zbytek, B.; Slominski, R. Inhibitors of melanogenesis increase toxicity of cyclophosphamide and lymphocytes against melanoma cells. Int. J. Cancer 2008, 124, 1470–1477.
- Mohagheghpour, N.; Waleh, N.; Garger, S.J.; Dousman, L.; Grill, L.K.; Tusé, D. Synthetic Melanin Suppresses Production of Proinflammatory Cytokines. Cell. Immunol. 2000, 199, 25–36.
- Feller, L.; Chandran, R.; Kramer, B.; Khammissa, R.A.; Altini, M.; Lemmer, J. Melanocyte Biology and Function with Reference to Oral Melanin Hyperpigmentation in HIV-Seropositive Subjects. AIDS Res. Hum. Retroviruses 2014, 30, 837–843.
- Montefiori, D.C.; Zhou, J. Selective antiviral activity of synthetic soluble l-tyrosine and l-dopa melanins against human immunodeficiency virus in vitro. Antivir. Res. 1991, 15, 11–25.
