Single-atom catalysts (SACs), as atomically dispersed metal active sites anchored or coordinated on suitable supports, demonstrate large potential for use in therapeutic applications. SACs have structural features similar to those of natural enzyme, while exhibiting remarkable catalytic activity, desirable stability, and excellent selectivity.
- single-atom catalysts
- enzyme-like
- biotherapy application
Note:All the information in this draft can be edited by authors. And the entry will be online only after authors edit and submit it.
1. Introduction
Single-atom catalysts (SACs), a novel class of catalysts in which all of the isolated, catalytically active metal atoms are stabilized by a support, were first proposed by Zhang and coworkers in 2011 [1]. Subsequently, the state-of-the-art SACs have been proven to exhibit superb performance in various chemical reactions on the basis of the optimized use of metal atoms with well-defined active centers [2–4][2][3][4]. Taking advantage of their properties, including but not limited to their extraordinary catalytic activity, outstanding selectivity, desirable stability, and 100% atom utilization, SACs offer the merits of both heterogeneous and homogeneous catalysts, and thus have attracted extensive attention in regard to chemical catalysis in the petrochemical industry, environmental protection, energy conversion, chemical transformation, and biomedical applications [5–9][5][6][7][8][9]. A considerable amount of evidence has demonstrated that SACs are powerful and effective in many typical heterogeneous catalytic reactions, such as electrochemical reactions, water-gas shift reactions, and hydrogenation reactions [10–12][10][11][12]. Moreover, SACs are low cost, abundant, and environmentally friendly resources [13,14][13][14].
In 2018, SACs were introduced into the field of biotherapy [15]. Despite the sizeable amount of evidence in industrial, energy, and ecological applications, the potential of SACs in biotherapy is much less understood. To the best of the authors’ knowledge, there is no systematic review on SACs in biotherapy applications that has been published. In a mini review published by Jiao and coworkers in 2020 [16], they summarized the recent progress in the synthesis, characterization, and application of SACs, which showed distinct advantages in environmental protection and biomedical applications. Nevertheless, they did not systematically analyze the therapeutic efficacy of SACs in biotherapy applications. In addition, it may have only captured a part of the available evidence without a systematic search of relevant databases.
2. Systematic Search Outcomes
The search of the three databases and the bibliographies of papers yielded 900 papers after excluding duplicate papers. Through screening by titles and abstracts, 25 studies were potentially included. Finally, 12 studies were included in this systematic review. The inter-reviewer reliability was high for both the title/abstract screening and full-text assessment processes (kappa =0.84 and kappa =0.87, respectively).
3. Characteristics of the Chosen Studies
The key characteristics of the included literature are detailed in Tables 1–3. The included studies were published in 2019 and 2020. The most common biotherapy applications were as an anticancer treatment (five studies), followed by an anti-infection therapy (four studies), an oxidative stress cytoprotection application (two studies), and a brain trauma therapy (one study).
4. Fabrication and Characterization of the SAC
The fabrication techniques and key characterization in the included studies have been used to synthesize and confirm the SACs and are summarized in Table 1. “Isolation-pyrolysis” is the most widely used path to fabricate SACs to disperse metal atoms on a 2D support. For instance, FeN5 single-atom nanozymes with carbon nanoframe-confined (SA/CNF), zinc-based single atom nanozyme (PMCS SA), single iron atoms anchored in nitrogen-doped amorphous carbon (SAF NCs), Co/PMCS, PEGylated single-atom Fe-containing nanocatalysts (PSAF NCs) Fe-N/C SACs, Fe-SAs/NC were developed through the use of pyrolysis [15, 17–22][15][17][18][19][20][21][22]. The deposition/coprecipitation strategy is also a common approach for SAC preparation, which was used to prepare carbon dot-supported atomically dispersed gold (MitoCAT-g), porphyrin-like single-atom Fe(III) metal-organic framework (P-MOF), and Pt/CeO2 [23–25][23][24][25]. Apart from these two methods, SACs can be prepared via self-assembly, exemplified by OxgeMCC-r SAE, and multistep reactions, exemplified by N-doped carbon sphere doped with a single-atom copper species (Cu-HNCS) [26,27][26][27].
The type of SACs included single-atom nanozymes with carbon nanoframe-confined FeN5, single iron atom catalyst (Fe SAEs), zinc-based single-atom nanozyme (PMCS), single iron atoms anchored in nitrogen-doped amorphous carbon (SAF NCs), atomically dispersed Fe-N4 immobilized on a carbon substrate (Fe-N/C SACs), nitrogen-doped carbon-supported atomically dispersed Co-porphyrin centers (Co/PMCS), PEGylated single-atom Fe-containing nanocatalysts (PSAF NCs), carbon-dot-supported gold (Au/CDs), porphyrin-like single-atom Fe(III), hollow N-doped carbon sphere doped with a single-atom copper species (Cu-HNCS), single-atom Ru using Mn3[Co(CN)6]2 metal-organic framework (MOF) as the support material, and single-atom Pt/CeO2.
Aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) and scanning tunneling microscopy (STM) are the most commonly used characterization techniques to assess SACs because these techniques can directly observe the single-atom metals on supports. The detailed structural information (coordination environment, binding mode, and oxidation states) of single-metal sites in SACs was further investigated by synchrotron radiation-based X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) spectra.
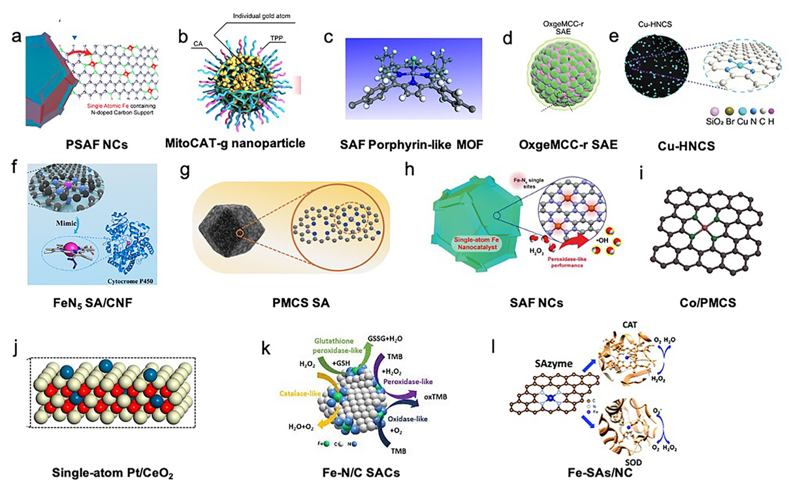
The active metal atoms of SACs included Fe, Zn, Co, Cu, Pt, Au, and Ru [15, 17–20,22–27][15][17][18][19][20][22][23][24][25][26][27]. The selected support materials of the SACs included carbon materials, such as carbon nanoframes, carbon nanospheres, nitrogen-doped carbon, hollow nitrogen-doped carbon spheres, carbon dots, MOFs, Mn3[Co(CN6)6]2, and CeO2 [15, 17–21,23–27][15][17][18][19][20][21][23][24][25][26][27]. The structures of the included SACs are presented in Figure 1.
Figure 1. Structures of single-atom catalysts (SACs) in biotherapy applications. (a) PEGylated single-atom Fe-containing nanocatalysts (PSAF NCs). Adapted with permission from [21]. Copyright 2019, American Chemical Society. (b) Carbon-dot-supported atomically dispersed gold (MitoCAT-g). Adapted with permission from [23]. Copyright 2019, Nature Publishing Group. (c) Crystal structure of an Fe porphyrin center in P-MOF. Adapted with permission from [24]. Copyright 2019, American Chemical Society. (d) Catalytically active single-atom Ru sites anchored in Mn3[Co(CN)6]2 loading with Ce6 (MCC) with an outer PVP protection layer (OxgeMCC-r). Adapted with permission from [26]. Copyright 2020, Nature Publishing Group. (e) Structure of hollow nitrogen-doped carbon sphere doped with a single-atom copper species (Cu-HNCS). Adapted with permission from [27]. Copyright 2020, Wiley-VCH. (f) Carbon nanoframe-confined atomically dispersed Fe sites with axial five-N coordination to mimic the active center of cytochrome P450. Adapted with permission from [17]. Copyright 2019, American Association for the Advancement of Science. (g) Porphyrin-like structural model of zinc-based single-atom nanozyme (PMCS). Adapted with permission from [18]. Copyright 2019, Wiley-VCH. (h) Single-atom Fe nanocatalysts (SAF NCs). Adapted with permission from [18]. Copyright 2019, Wiley-VCH. (i) Model of Co/PMCS. Co (purple), N (green), and C (gray). Adapted with permission from [20]. Copyright 2019, Wiley-VCH. (j) Single-atom Pt/CeO2 nanozymes. Adapted with permission from [25]. Copyright 2019, American Chemical Society. (k) Schematic diagram of the intrinsic activity of Fe-N/C SACs mimicking peroxidase, oxidase, catalase, and glutathione peroxidase enzymes. Adapted with permission from [22]. Copyright 2019, The Royal Society of Chemistry. (l) Atomically dispersed Fe-N4 sites anchored on N-doped porous carbon materials (Fe-SAs/NC), which mimic two antioxidative enzymes: catalase (CAT) and superoxide dismutase (SOD). Adapted with permission from [15]. Copyright 2018, The Royal Society of Chemistry.
5. Biocompatibility of the SACs
The biocompatibility/biosafety of the synthesized SACs is one of the critical factors for their biotherapy application; thus, the biocompatibility of SACs was reported in all of the included studies. In general, the vast majority of the studies (11 out of 12) reported the favorable biocompatibility of SACs. Minimal cytotoxicity was reported in 1 remaining study. The cytocompatibility studies included a CCK8 assay [17,18,23,27][17][18][23][27] and/or Live/Dead assay [24] or an MTT assay [15,20,22,25][15][20][22][25] or a Cell Titer-Fluor assay [26], while studies conducted by Huo and coworkers [19,21][19][21] revealed the biocompatibilities of SAF NCs via the hematoxylin and eosin (HE) staining of major organs dissected from mice in different therapeutic groups. In regard to cytocompatibility, the single-atom catalyst of SAs/NC with bifunctional antioxidative enzymes for oxidative-stress cytoprotection exhibited the minimal cytotoxicity, which was reported by Ma and coworkers [15].
6. Application of SACs in Cancer Treatments
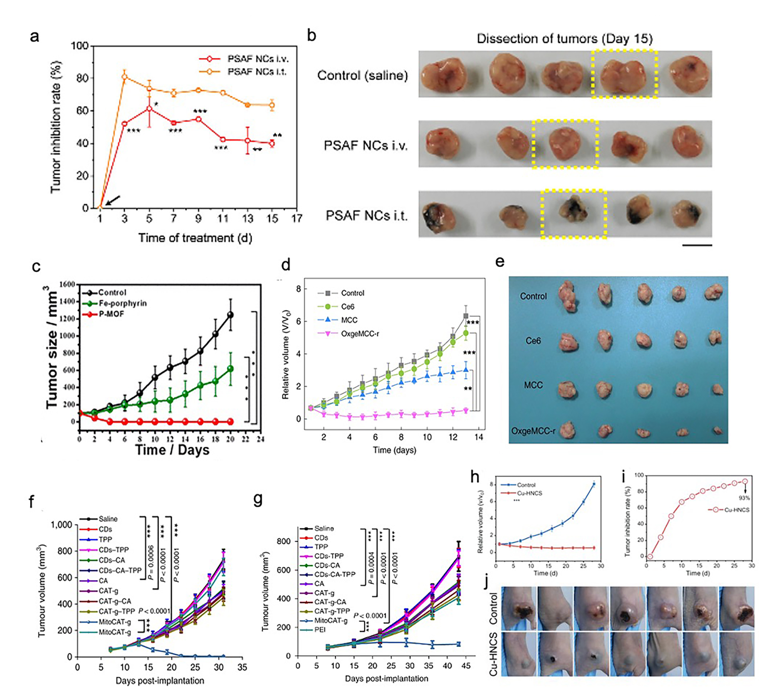
SACs were applied as a cancer therapy in five studies (Table 1) [21,23,24,26,27][21][23][24][26][27]. These studies targeted breast cancer (four studies) and liver cancer (one study). HeLa cells (two studies), 4T1 tumor cells (three studies), HEK 293 cells (one study), and HepG-2 cells (one study) were used in their in vitro assays. For studies targeting breast cancer, 4T1 tumor-bearing mice (three studies) and HeLa-bearing tumor mice (one study) were used. In addition, a mouse model based on xenografted HepG-2 tumors was used in a study focused on liver cancer. In general, all of the five studies reported that the synthesized SACs, which exhibited desirable biosafety, were highly efficient in both the in vitro and in vivo cancer treatments. PEGylated single-atom Fe-containing nanocatalysts (PSAF NCs), a metal-organic framework (MOF) rich in porphyrin-like single-atom Fe(III) (P-MOF), and single-atom Ru supported by a Mn3[Co(CN)6]2 MOF (OxgeMCC-r SAE) outperformed the saline or phosphate-Buffered Saline (PBS) control in inhibiting the growth profiles of breast tumors (Figure 2a–e) [21,24,26][21][24][26]. It is worth mentioning that both OxgeMCC-r SAE and P-MOF enhanced the therapeutic outcome of photodynamic therapy (PDT) in breast cancer via two different mechanisms. The OxgeMCC-r SAE exhibited a high loading capacity of Ce6 photosensitizer. On the other hand, the spin state of Fe(III) in P-MOF under NIR would facilitate the generation of singlet oxygen, which promoted PDT. Moreover, they also reported that P-MOF could serve as an agent for a photothermal therapy (PTT) cancer treatment, as well as photoacoustic imaging (PAI) of breast tumors. In addition, Gong et al. found that carbon dot-supported atomically dispersed gold (MitoCAT-g) significantly suppressed tumor growth in subcutaneous and orthotopic patient-derived xenograft hepatocellular carcinoma models without adverse effects or toxicity, when compared with the saline contro1 (Figure 2f,g) [23]. Recently, a study conducted by Lu and coworkers reported that the bioinspired Cu-HNCS could generate two types of reactive oxygen species (ROS) (O2•− and •OH) through two parallel reactions, which significantly enhanced the efficacy of the parallel catalytic of tumors in in vitro and in vivo (Figure 2h–j) [27].
Figure 2. Effect of SACs in cancer treatments. (a) Relative tumor inhibition rates of tumors treated with PEGylated single-atom Fe-containing nanocatalysts (PSAF NCs) intravenously and intratumorally, in contrast to the control group, (b) digital photographs of the dissected tumors of the control, PSAF NCs i.v. and PSAF NCs i.t. groups. (a) and (b) Adapted with permission from [21]. Copyright 2019, American Chemical Society. (c) Tumor growth curves with different treatments. Adapted with permission from [24]. Copyright 2019, American Chemical Society. (d) Relative tumor volumes in mice after various treatments (control, Ce6, MCC, and OxgeMCC-r), (e) Photographic images of tumors excised from different groups after various treatments indicated. (d) and (e) Adapted with permission from [26]. Copyright 2020, Nature Publishing Group. (f) Tumor growth in the MitoCAT-g-treated group compared with the saline control group in a subcutaneous tumor model. (g) Tumor volumes in the different treatment groups in the orthotopic hepatic patient-derived xenograft (PDX) tumor model in non-obese diabetic/severe combined immune-deficiency (NOD-SCID) mice. (f) and (g) Adapted with permission from [23]. Copyright 2019, Nature Publishing Group. (h) Tumor proliferation curves using parallel catalytic therapy in a subcutaneous tumor model. (i) Relative tumor inhibition rates. (j) Photographs of 4T1 tumors 28 days after treating bagg albino, laboratory-bred strain of the house mouse/c (BALB/c) nude mice with blank hydrogels or Cu-HNCS hydrogels. (h–j) Adapted with permission from [27]. Copyright 2020, Wiley-VCH.
7. Application of SACs in Anti-Infection Therapies
There were four studies on the application of SACs for anti-infection therapies (Table 2) [17–20][17][18][19][20]. Among them, three studies targeted wound disinfection applications [17–19][17][18][19], and one study examined sepsis management [20]. These four studies consistently showed that the synthesized SACs demonstrated superior antibacterial activity without, or at least very little, toxicity. In comparison to the control group, single-atom nanozymes with carbon nanoframe-confined FeN5 (FeN5 SA/CNF), zinc-based single atom nanozyme (PMCS SAzyme), and single iron atoms anchored in nitrogen-doped carbon (SAF NCs) exhibited very high statistically significant differences in inhibiting the growth of Pseudomonas aeruginosa (P. aeruginosa), Escherichia coli (E. coli)/Staphylococcus aureus (S. aureus) in vitro, and significantly promoted wound healing without toxicity in vivo. Cao and coworkers reported that the synthesized Co/PMCS could significantly reduce the number of bacteria in the liver, lung, kidney, intestines, and blood of E. coli-induced bacteremia in mice, as well as reducing the white blood cells (WBC), ROS, alanine transaminase (ALT), RNS, IL-6, TNF-α, Lym, Neu, and urea, in organs and blood, when compared with those of the PBS-treated control group. Additionally, they found that all the blood indexes of lipopolysaccharide (LPS)-induced sepsis mice in the Co/PMCS-treated group were reduced to normal. In other words, Co/PMCS could significantly eliminate the systematic production of reactive oxygen and nitrogen species (RONS) and lower proinflammatory cytokine levels compared with those of the PBS-treated group, thereby indicating that Co/PMCS could promote the recovery of multiple organ functions in sepsis mice (Figure 3) [20].
Figure 3. Effect of SACs in anti-infection therapies. (a) Photographs of the in vivo mice wound model. Photographs of the Escherichia coli infected wound treated with PBS buffer and carbon nanoframe-confined FeN5 (FeN5 CNF) solutions at the pre-treatment stage and after 1–3 days, and their corresponding histologic analyses. Related wound size of mice in each group after different treatments (diagram in the left corner). Adapted with permission from [17]. Copyright 2019, American Association for the Advancement of Science. (b) Photographs of bacterial colonies formed by Pseudomonas aeruginosa after exposure to (I) NaAc buffer; (II) NaAc buffer + H2O2; (III) PMCS, and (IV) PMCS + H2O2. (c) Photographs of P. aeruginosa-infected wound treated with (I)–(IV) after different lengths of time. (b) and (c) Adapted with permission from [18]. Copyright 2019, Wiley-VCH. (d) Digital photographs of remaining bacteria-inoculated agar plates. (e) Bacterial activity of treatment. (f) Relative wound area. (g) Digital photographs of mice in E. coli-infected group. (h) Relative wound area. i) Digital photographs of mice in Staphylococcus aureus-infected group. (d–i) Adapted with permission from [19]. Copyright 2019, Wiley-VCH. (j) Illustration of in vivo LPS-induced sepsis model. (k–l) Detection of TNF-α and IL-6 in vivo in different groups (UT: untreated, L: LPS, C: Co/PMCS). (m) Illustration of in vivo bacteremia model, (n) and (o) Detection of TNF-α and IL-6 in vivo in different groups (UT: untreated, B: bacteremia, C: Co/PMCS). (j–o) Adapted with permission from [20]. Copyright 2019, Wiley-VCH.
References
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Chem. 2011, 3, 634.
- Zhu, C.; Fu, S.; Shi, Q.; Du, D.; Lin, Y. Single‐atom electrocatalysts. Chem. Int. Ed. 2017, 56, 13944–13960.
- Zhang, H.; Liu, G.; Shi, L.; Ye, J. Single‐atom catalysts: Emerging multifunctional materials in heterogeneous catalysis. Energy Mater. 2018, 8, 1701343.
- Pelletier, J.D.; Basset, J.-M. Catalysis by design: Well-defined single-site heterogeneous catalysts. Chem. Res. 2016, 49, 664–677.
- Peng, Y.; Lu, B.; Chen, S. Carbon‐supported single atom catalysts for electrochemical energy conversion and storage. Mater. 2018, 30, 1801995.
- Jiao, L.; Xu, W.; Yan, H.; Wu, Y.; Liu, C.; Du, D.; Lin, Y.; Zhu, C. Fe-N-C single-atom nanozymes for the intracellular hydrogen peroxide detection. Chem. 2019, 91, 11994–11999.
- Yang, S.; Kim, J.; Tak, Y.J.; Soon, A.; Lee, H. Single‐atom catalyst of platinum supported on titanium nitride for selective electrochemical reactions. Chem. Int. Ed. 2016, 55, 2058–2062.
- An, S.; Zhang, G.; Wang, T.; Zhang, W.; Li, K.; Song, C.; Miller, J.T.; Miao, S.; Wang, J.; Guo, X. High-density ultra-small clusters and single-atom Fe sites embedded in graphitic carbon nitride (g-C3N4) for highly efficient catalytic advanced oxidation processes. ACS Nano 2018, 12, 9441–9450, doi:10.1021/acsnano.8b04693.
- Wang, J.; Li, Z.; Wu, Y.; Li, Y. Fabrication of single‐atom catalysts with precise structure and high metal loading. Mater. 2018, 30, 1801649.
- Chen, Y.; Ji, S.; Chen, C.; Peng, Q.; Wang, D.; Li, Y. Single-atom catalysts: Synthetic strategies and electrochemical applications. Joule 2018, 2, 1242–1264, doi:10.1016/j.joule.2018.06.019.
- Lin, J.; Wang, A.; Qiao, B.; Liu, X.; Yang, X.; Wang, X.; Liang, J.; Li, J.; Liu, J.; Zhang, T. Remarkable performance of Ir1/FeOx single-atom catalyst in water gas shift reaction. Am. Chem. Soc. 2013, 135, 15314–15317.
- Zhang, Z.; Zhu, Y.; Asakura, H.; Zhang, B.; Zhang, J.; Zhou, M.; Han, Y.; Tanaka, T.; Wang, A.; Zhang, T. Thermally stable single atom Pt/m-Al2O3 for selective hydrogenation and CO oxidation. Commun. 2017, 8, 1–10.
- Yi, L.; Lan, F.; Li, J.; Zhao, C. Efficient noble-metal-free Co-NG/TiO2 photocatalyst for H2 evolution: Synergistic effect between single-atom Co and N-doped graphene for enhanced photocatalytic activity. ACS Sustain. Chem. Eng. 2018, 6, 12766–12775, doi:10.1021/acssuschemeng.8b02001.
- Pu, Z.; Amiinu, I.S.; Cheng, R.; Wang, P.; Zhang, C.; Mu, S.; Zhao, W.; Su, F.; Zhang, G.; Liao, S. Single-atom catalysts for electrochemical hydrogen evolution reaction: Recent advances and future perspectives. Nano-Micro Lett. 2020, 12, 1–29.
- Ma, W.; Mao, J.; Yang, X.; Pan, C.; Chen, W.; Wang, M.; Yu, P.; Mao, L.; Li, Y. A single-atom Fe-N4 catalytic site mimicking bifunctional antioxidative enzymes for oxidative stress cytoprotection. Commun. 2018, 55, 159–162, doi:10.1039/c8cc08116f.
- Jiao, L.; Yan, H.; Wu, Y.; Gu, W.; Zhu, C.; Du, D.; Lin, Y. When nanozymes meet single-atom catalysis. Chem. Int. Ed. 2020, 59, 2565–2576, doi:10.1002/anie.201905645.
- Huang, L.; Chen, J.; Gan, L.; Wang, J.; Dong, S. Single-atom nanozymes. Adv. 2019, 5, eaav5490, doi:10.1126/sciadv.aav5490.
- Xu, B.; Wang, H.; Wang, W.; Gao, L.; Li, S.; Pan, X.; Wang, H.; Yang, H.; Meng, X.; Wu, Q.; et al. A single-atom nanozyme for wound disinfection applications. Chem. Int. Ed. 2019, 58, 4911–4916, doi:10.1002/anie.201813994.
- Huo, M.; Wang, L.; Zhang, H.; Zhang, L.; Chen, Y.; Shi, J. Construction of single-iron-atom nanocatalysts for highly efficient catalytic antibiotics. Small 2019, 15, e1901834, doi:10.1002/smll.201901834.
- Cao, F.; Zhang, L.; You, Y.; Zheng, L.; Ren, J.; Qu, X. An enzyme-mimicking single-atom catalyst as an efficient multiple reactive oxygen and nitrogen species scavenger for sepsis management. Chem. Int. Ed. 2020, doi:10.1002/anie.201912182.
References
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Chem. 2011, 3, 634.
- Zhu, C.; Fu, S.; Shi, Q.; Du, D.; Lin, Y. Single‐atom electrocatalysts. Chem. Int. Ed. 2017, 56, 13944–13960.
- Zhang, H.; Liu, G.; Shi, L.; Ye, J. Single‐atom catalysts: Emerging multifunctional materials in heterogeneous catalysis. Energy Mater. 2018, 8, 1701343.
- Pelletier, J.D.; Basset, J.-M. Catalysis by design: Well-defined single-site heterogeneous catalysts. Chem. Res. 2016, 49, 664–677.
- Peng, Y.; Lu, B.; Chen, S. Carbon‐supported single atom catalysts for electrochemical energy conversion and storage. Mater. 2018, 30, 1801995.
- Jiao, L.; Xu, W.; Yan, H.; Wu, Y.; Liu, C.; Du, D.; Lin, Y.; Zhu, C. Fe-N-C single-atom nanozymes for the intracellular hydrogen peroxide detection. Chem. 2019, 91, 11994–11999.
- Yang, S.; Kim, J.; Tak, Y.J.; Soon, A.; Lee, H. Single‐atom catalyst of platinum supported on titanium nitride for selective electrochemical reactions. Chem. Int. Ed. 2016, 55, 2058–2062.
- An, S.; Zhang, G.; Wang, T.; Zhang, W.; Li, K.; Song, C.; Miller, J.T.; Miao, S.; Wang, J.; Guo, X. High-density ultra-small clusters and single-atom Fe sites embedded in graphitic carbon nitride (g-C3N4) for highly efficient catalytic advanced oxidation processes. ACS Nano 2018, 12, 9441–9450, doi:10.1021/acsnano.8b04693.
- Wang, J.; Li, Z.; Wu, Y.; Li, Y. Fabrication of single‐atom catalysts with precise structure and high metal loading. Mater. 2018, 30, 1801649.
- Chen, Y.; Ji, S.; Chen, C.; Peng, Q.; Wang, D.; Li, Y. Single-atom catalysts: Synthetic strategies and electrochemical applications. Joule 2018, 2, 1242–1264, doi:10.1016/j.joule.2018.06.019.
- Lin, J.; Wang, A.; Qiao, B.; Liu, X.; Yang, X.; Wang, X.; Liang, J.; Li, J.; Liu, J.; Zhang, T. Remarkable performance of Ir1/FeOx single-atom catalyst in water gas shift reaction. Am. Chem. Soc. 2013, 135, 15314–15317.
- Zhang, Z.; Zhu, Y.; Asakura, H.; Zhang, B.; Zhang, J.; Zhou, M.; Han, Y.; Tanaka, T.; Wang, A.; Zhang, T. Thermally stable single atom Pt/m-Al2O3 for selective hydrogenation and CO oxidation. Commun. 2017, 8, 1–10.
- Yi, L.; Lan, F.; Li, J.; Zhao, C. Efficient noble-metal-free Co-NG/TiO2 photocatalyst for H2 evolution: Synergistic effect between single-atom Co and N-doped graphene for enhanced photocatalytic activity. ACS Sustain. Chem. Eng. 2018, 6, 12766–12775, doi:10.1021/acssuschemeng.8b02001.
- Pu, Z.; Amiinu, I.S.; Cheng, R.; Wang, P.; Zhang, C.; Mu, S.; Zhao, W.; Su, F.; Zhang, G.; Liao, S. Single-atom catalysts for electrochemical hydrogen evolution reaction: Recent advances and future perspectives. Nano-Micro Lett. 2020, 12, 1–29.
- Ma, W.; Mao, J.; Yang, X.; Pan, C.; Chen, W.; Wang, M.; Yu, P.; Mao, L.; Li, Y. A single-atom Fe-N4 catalytic site mimicking bifunctional antioxidative enzymes for oxidative stress cytoprotection. Commun. 2018, 55, 159–162, doi:10.1039/c8cc08116f.
- Jiao, L.; Yan, H.; Wu, Y.; Gu, W.; Zhu, C.; Du, D.; Lin, Y. When nanozymes meet single-atom catalysis. Chem. Int. Ed. 2020, 59, 2565–2576, doi:10.1002/anie.201905645.
- Huang, L.; Chen, J.; Gan, L.; Wang, J.; Dong, S. Single-atom nanozymes. Adv. 2019, 5, eaav5490, doi:10.1126/sciadv.aav5490.
- Xu, B.; Wang, H.; Wang, W.; Gao, L.; Li, S.; Pan, X.; Wang, H.; Yang, H.; Meng, X.; Wu, Q.; et al. A single-atom nanozyme for wound disinfection applications. Chem. Int. Ed. 2019, 58, 4911–4916, doi:10.1002/anie.201813994.
- Huo, M.; Wang, L.; Zhang, H.; Zhang, L.; Chen, Y.; Shi, J. Construction of single-iron-atom nanocatalysts for highly efficient catalytic antibiotics. Small 2019, 15, e1901834, doi:10.1002/smll.201901834.
- Cao, F.; Zhang, L.; You, Y.; Zheng, L.; Ren, J.; Qu, X. An enzyme-mimicking single-atom catalyst as an efficient multiple reactive oxygen and nitrogen species scavenger for sepsis management. Chem. Int. Ed. 2020, doi:10.1002/anie.201912182.
- Huo, M.; Wang, L.; Wang, Y.; Chen, Y.; Shi, J. Nanocatalytic tumor therapy by single-atom catalysts. ACS Nano 2019, 13, 2643–2653.
- Lu, M.; Wang, C.; Ding, Y.; Peng, M.; Zhang, W.; Li, K.; Wei, W.; Lin, Y. Fe-N/C single-atom catalysts exhibiting multienzyme activity and ROS scavenging ability in cells. Chem. Commun. 2019, 55, 14534–14537.
- Gong, N.; Ma, X.; Ye, X.; Zhou, Q.; Chen, X.; Tan, X.; Yao, S.; Huo, S.; Zhang, T.; Chen, S.; et al. Carbon-dot-supported atomically dispersed gold as a mitochondrial oxidative stress amplifier for cancer treatment. Nat. Nanotechnol. 2019, 14, 379–387.
- Wang, L.; Qu, X.; Zhao, Y.; Weng, Y.; Waterhouse, G.I.; Yan, H.; Guan, S.; Zhou, S. Exploiting single atom iron centers in a porphyrin-like MOF for efficient cancer phototherapy. ACS Appl. Mater. Interfaces 2019, 11, 35228–35237.
- Yan, R.; Sun, S.; Yang, J.; Long, W.; Wang, J.; Mu, X.; Li, Q.; Hao, W.; Zhang, S.; Liu, H. Nanozyme-based bandage with single-atom catalysis for brain trauma. ACS Nano 2019, 13, 11552–11560.
- Wang, D.; Wu, H.; Phua, S.Z.F.; Yang, G.; Lim, W.Q.; Gu, L.; Qian, C.; Wang, H.; Guo, Z.; Chen, H. Self-assembled single-atom nanozyme for enhanced photodynamic therapy treatment of tumor. Nat. Commun. 2020, 11, 1–13.
- Lu, X.; Gao, S.; Lin, H.; Yu, L.; Han, Y.; Zhu, P.; Bao, W.; Yao, H.; Chen, Y.; Shi, J. Bioinspired Copper Single-Atom Catalysts for Tumor Parallel Catalytic Therapy. Adv. Mater. 2020, 32, 2002246.