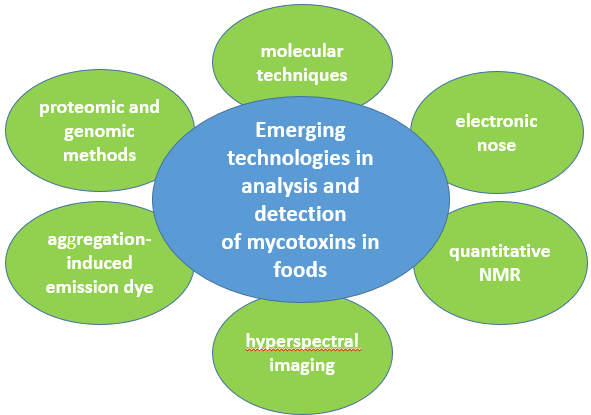
Mycotoxins are secondary metabolites produced by filamentous fungi that cause harmful effects on human and animal health as well as significant economic losses. As mycotoxins are responsible for food contamination and certain permissible limits have already been established, developing sensitive and reliable methods to detect them is a top priority. Proteomic and genomic methods, molecular techniques, electronic nose, aggregation-induced emission dye, quantitative NMR, and hyperspectral imaging are some innovative techniques which are applied in the analysis and determination of important mycotoxins in foods and are used alternatively in chromatographic techniques. Some of them have proven to be particularly effective in not only the detection of mycotoxins, but also in detecting mycotoxin-producing fungi. As mycotoxin-contaminated foods can appear anywhere in the world through international trade, their detection and identification are considered essential to the protection of human health by providing safe foods free of major food contaminants such as mycotoxins.
Mycotoxins are secondary metabolites produced by filamentous fungi that cause harmful effects on human and animal health as well as significant economic losses. As mycotoxins are responsible for food contamination and certain permissible limits have already been established, developing sensitive and reliable methods to detect them is a top priority. Proteomic and genomic methods, molecular techniques, electronic nose, aggregation-induced emission dye, quantitative NMR, and hyperspectral imaging are some innovative techniques which are applied in the analysis and determination of important mycotoxins in foods and are used alternatively in chromatographic techniques. Some of them have proven to be particularly effective in not only the detection of mycotoxins, but also in detecting mycotoxin-producing fungi. As mycotoxin-contaminated foods can appear anywhere in the world through international trade, their detection and identification are considered essential to the protection of human health by providing safe foods free of major food contaminants such as mycotoxins.
Keywords
mycotoxins; analysis; determination; emerging technologies; proteomic and genomic methods; molecular techniques; electronic nose; hyperspectral imaging; aggregation-induced emission dye; quantitative NMR
- mycotoxins
- analysis
- determination
- emerging technologies
- proteomic and genomic methods
- molecular techniques
- electronic nose
- hyperspectral imaging
- aggregation-induced emission dye
- quantitative NMR
1. Proteomic and Genomic Methods
Proteomic methods include initial mould peptide/protein extraction from food and further analysis by matrix-assisted laser desorption or ionization time-of-flight mass spectrometry (MALDI-TOF MS). The MALDI–TOF MS technique quickly detects fungal isolates and mycotoxins and identifies them with high precision, and is used alternatively in chromatographic techniques[1][2] [28][150]. This analysis is performed by detecting proteins in a mass range of 2–20 kDa after calculating the m/z values. The identification of the micro-organism is achieved through the fingerprint MS created for each one. The MALDI-TOF MS technique is a very hopeful approach as it identifies closely related species of filamentous fungi. However, the need to create a public database in which all in-house entries are accessible has been reported. Additionally, the food industry currently considers this technique as expensive[3] [151]. MALDI–TOF MS has been used to rapidly detect AFB1, CIT, DON, ZEA, T2, and griseofulvin[4] [152], and for rapid screening of Alternaria mycotoxins, alternariol (AOH), alternariol monomethyl ether (AME) and tentoxin (TEN)[5] [153].
Figure. 1. Emerging technologies in analysis and detection of mycotoxins
Genomic methods include initial mould DNA or RNA extraction from foods followed by detection using polymerase chain reaction (PCR), quantitative PCR (qPCR), loop-mediated isothermal amplification (LAMP), and reverse transcription PCR (RT-PCR). Through these methods, fungal isolates are detected, but it is not possible to quantify them. It is also not possible to detect specific species, but only to detect strains with the ability to produce mycotoxins[1][6] [28,154]. Through the PCR technique, amplification of DNA sequences in vitro is achieved. Selective and repetitive amplification requires the existence of the primers and Taq DNA polymerase. The real-time combination of the sensitivity of conventional PCR with a specific fluorescent signal allows qPCR to quantify specific DNA targets. LAMP uses a DNA polymerase and four primers in order to amplify nucleic acids, and it is used alternatively to PCR for the detection of spoilage moulds. OTA-producing fungi have been detected by PCR-based methods[7] [155], Fusarium, Penicillium and Aspergillus species have also been detected by the same method, as well as analyzed as associated with the expression of genes involved in the biosynthetic pathway of several mycotoxins[1] [28].
2. Molecular Techniques
Among others, molecular techniques include PCR, fluorescent in situ hybridization (FISH) and DNA barcoding. Techniques that are varied with PCR such as qPCR, RT-PCR, PCR denaturing gradient gel electrophoresis (PCR–DGGE), and PCR–ELISA, can be used to control fungi in food[8] [156]. Among them, PCR–DGGE has the advantage of terminal-restriction fragment length polymorphism analysis (T-RFLP), achieving satisfactory identifications for toxigenic fungi[9] [29]. PCR–ELISA when compared to simple PCR, proved to be more sensitive. DNA amplification through LAMP was reported by Luo et al.[109] [157] in order to detect aflatoxin-producing Aspergillus species.
3. Electronic Nose
The physicochemical properties of secondary fungal metabolites can be assessed by the electronic nose in a way that works partially like a GC system. Specifically, this analysis is based on the detection of volatile compounds released by a contaminated food through a solid state sensor[1110] [158]. The sensitivity of the sensors that are used in this analysis allows the creation of a unique fingerprint for each food, characteristic of its taste and aroma. The detection of the characteristic odor gives initial information about the category of the produced metabolites[11][12][13] [159,160]. Apples, oranges, strawberries and peaches are some fruits in which the application of this technique has been successfully implemented for the detection of fungi that produce mycotoxins[13][14][15][16][17] [161–164].
4. Aggregation-Induced Emission Dye
The aggregation-induced emission (AIE) exploits the enhancement of the fluorescence for a group of fluorescent dyes in the aggregation state. Intense fluorescence of these dyes may be the result of reduced intramolecular rotations observed in the aggregate state. The development of fluorescent biosensors is based on the fluorescence analysis of AIE dyes[17][18][19][20][21] [165–168]. AIE dyes that show high fluorescence emission in the aggregate states, are 9,10-distyrylanthracene (DSA), tetraphenylethene (TPE), and silacyclopentadiene (silole)[1918] [166].
Zhu et al.[2221] [169] developed an AIE dye-based aptasensor for the detection of ΟΤΑ. AIE dyes showed high affinity to aptamers and fluoresce through the process of dye aggregation. Only one aptamer sequence was used in this study, with the detection limit reaching 0.4 ng/mL. In addition, it had significant specificity for the recognition of OTA. Moreover, the application was successfully used to analyze OTA in wine and coffee. The on-site detection of food contaminations and the simple operation make the application of AIE dyes very effective.
5. Quantitative NMR
Nuclear magnetic resonance (NMR) spectroscopy is a basic technique for the identification of organic compounds, and one of the significant analytical technologies frequently utilized in metabolomics[2322] [170]. Metabolomics is defined as the simultaneous detection and statistical interpretation of multiple endogenous metabolites in a living system. NMR-based metabolomics gives rich structural information by providing a ‘holistic approach’ of metabolites. The most commonly used NMR methodologies are based on studying the physical properties of hydrogen nuclei (protons) in tissue water, followed by proton NMR on other endogenous metabolites, and less frequently, other nuclei such as 31P, 13C, 19F and 23Na. Low sensitivity, low spectral resolution and poor time resolution are some of the disadvantages of NMR technology. Limited commercial software on the market and limited quantification methods also characterize this technique[2423] [171]. NMR has also several advantages such as straightforward sample preparation, high sample throughput, stable chemical shifts, quantification without standards, and reliable identification of isolate metabolites[2524] [172]. NMR technologies have been successfully used to elucidate rearrangement mechanism of the Fusarium mycotoxin Fusarin C[2625] [173].
6. Hyperspectral Imaging
Hyperspectral imaging (HSI) is a technique that can be applied for fungal and mycotoxin assessment with significant advantages. Analyses by this technique are inexpensive, are performed without destroying the sample, are quick, and operate by obtaining spectral data for each pixel location. This technique is considered to be capable of replacing expensive and time-consuming techniques for mycotoxins analysis in cereals, contributing significantly to the screening of contaminated cereal grains. The review by Femenias et al.[2726] [31] describes the principle of HSI technique and the use of HSI for Fusarium pathogen and DON risk management in cereals. Also, HSI prediction algorithms are important innovative data for DON determination. HSI is an important grain-sorting tool with the ability to classify individual contaminated grains. An HSI system consists of a camera for spectral and spatial detection, a spectrograph for the production of a spectrum for each pixel, an objective lens which focuses the beam of light in the direction of the detector, an illumination device that produces and sends light to the sample[287][2928] [174,175], a moving unit with a translation stage and motor which are responsible for the movement of the sample, and a data acquisition instrument[3029] [176].
Tekle et al.[3130] [177] studied a short wavelength infrared camera with a mercury telluride detector for the monitoring of Fusarium and DON contaminated cereal kernels. The working wavelengths of the device were 1000–2500 nm. The HSI presented by Barbedo et al.[3231] [178] was a XENICS camera coupled to a VIS/NIR spectrometer with working wavelengths of 528–1785 nm. They investigated the use of HSI for DON screening in wheat kernels through the use of a new algorithm. The results showed that the algorithm designed exclusively for this study did not provide sufficient evidence for the correlation between DON infection and the presence of Fusarium. A new algorithm was designed to categorize the samples into batches. The new algorithm that emerged could be a valuable tool for detecting initial batches of wheat followed by DON analysis.
Recently, Ropelewska and Zapotoczny[3332] [179] developed images for the separation of healthy and infected cereals, using a charge coupled device camera, a VIS/NIR spectrometer with working wavelengths of 400–1100 nm and a fiber optic illuminator coupled with an infrared lamp. In a recent study, Liang et al.[3433] [30] used a charge coupled device camera and a spectrometer with working wavelengths of 400–1000 nm in order to identify and visualize the different DON levels in bulk wheat kernels.
References
- Alicia Rodríguez; Mar Rodríguez; María J. Andrade; Juan J. Córdoba; Detection of filamentous fungi in foods. Current Opinion in Food Science 2015, 5, 36-42, 10.1016/j.cofs.2015.07.007.
- Patricia Morales; Cledir Santos; Armando Venâncio; Nelson Lima; Species identification of Aspergillus section Flavi isolates from Portuguese almonds using phenotypic, including MALDI-TOF ICMS, and molecular approaches. Journal of Applied Microbiology 2011, 111, 877-892, 10.1111/j.1365-2672.2011.05116.x.
- Ashutosh Panda; Anup K. Ghosh; Bijay R Mirdha; Immaculata Xess; Saikat Paul; Jyotish C Samantaray; Alagiri Srinivasan; Shehla Khalil; Neha Rastogi; Yubhisha Dabas; et al. MALDI-TOF mass spectrometry for rapid identification of clinical fungal isolates based on ribosomal protein biomarkers. Journal of Microbiological Methods 2015, 109, 93-105, 10.1016/j.mimet.2014.12.014.
- Lukas Hleba; Miroslava Císarová; Mohammad Ali Shariati; Dana Tančinová; DETECTION OF MYCOTOXINS USING MALDI-TOF MASS SPECTROMETRY. Journal of Microbiology, Biotechnology and Food Sciences 2017, 7, 181-185, 10.15414/jmbfs.2017.7.2.181-185.
- Kumaran Sivagnanam; Emy Komatsu; Christoph Rampitsch; Hélène Perreault; Tom Gräfenhan; Rapid screening of Alternaria mycotoxins using MALDI-TOF mass spectrometry. Journal of the Science of Food and Agriculture 2016, 97, 357-361, 10.1002/jsfa.7703.
- Anong Bintvihok; Supitchaya Treebonmuang; Kitiya Srisakwattana; Wisut Nuanchun; Koranis Patthanachai; Sungworn Usawang; A Rapid and Sensitive Detection of Aflatoxin-producing Fungus Using an Optimized Polymerase Chain Reaction (PCR). Toxicological Research 2016, 32, 81-87, 10.5487/TR.2016.32.1.081.
- Akhtar Hayat; Nathalie Paniel; Amina Rhouati; Jean-Louis Marty; Lise Barthelmebs; Recent advances in ochratoxin A-producing fungi detection based on PCR methods and ochratoxin A analysis in food matrices. Food Control 2012, 26, 401-415, 10.1016/j.foodcont.2012.01.060.
- Midorikawa, G.E.O.; Miller, R.N.G.; Bittencourt, D.M.d.C. Molecular Techniques in Food Biology: Safety, Biotechnology, Authenticity and Traceability, 1st ed.; El Sheikha, A.F., Levin, R., Xu, J., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; Volume 3, pp. 385–407.
- Aly F. El Sheikha; New Strategies for Tracing Foodstuffs: Biological Barcodes Utilising PCR-DGGE. AdvaJie Luo; Rudi F. Vogel; Ludwig Niessen; Development and application of a loop-mediated isothermal amplification assay for rapid identification of aflatoxigenic molds and their detection in food samples. Inctes in Food Technology and Nutrition Sciences – Open Journarnational Journal of Food Microbiol ogy 2015, 2, 1, S1-S7, 10.17140/aftnsoj-se-1-101.59, 214-224, 10.1016/j.ijfoodmicro.2012.08.018.
- Jie Luo; Rudi F. Vogel; Ludwig Niessen; Development and application of a loop-mediated isothermal amplification assay for rapid identification of aflatoxigenic molds and their detection in food samples. Matteo Falasconi; I. Concina; E. Gobbi; V. Sberveglieri; A. Pulvirenti; G. Sberveglieri; Electronic Nose for Microbiological Quality Control of Food Products. International Journal of Food MiElecrobiologtrochemistry 2012, , 20159, 214-224, 10.1016/j.ijfoodmicro.2012.08.018.2, 1-12, 10.1155/2012/715763.
- Matteo Falasconi; I. Concina; E. Gobbi; V. Sberveglieri; A. Pulvirenti; G. Sberveglieri; Electronic Nose for Microbiological Quality Control of Food Products. IntIda A. Casalinuovo; Nato Di Pierro; Massimiliano Coletta; Paolo Di Francesco; Application of Electronic Noses for Disease Diagnosis and Food Spoilage Detection. Sernational Journal of Electrochemisorstry 20012, 2012, 1-12, 10.1155/2012/715763.6, 6, 1428-1439, 10.3390/s6111428.
- Ida A. Casalinuovo; Nato Di Pierro; Massimiliano Coletta; Paolo Di Francesco; Application of Electronic Noses for Disease Diagnosis and Food Spoilage Detection. SAlireza Sanaeifar; Hassan Zakidizaji; Abdolabbas Jafari; Miguel De La Guardia; Early detection of contamination and defect in foodstuffs by electronic nose: A review. TrAC Trendsors in Analytical Chemistry 2006, 6, 1428-1439, 10.3390/s6111428.17, 97, 257-271, 10.1016/j.trac.2017.09.014.
- Alireza Sanaeifar; Hassan Zakidizaji; Abdolabbas Jafari; Miguel De La Guardia; Early detection of contamination and defect in foodstuffs by electronic nose: A review. TrAC TrWenshen Jia; Gang Liang; Hui Tian; Jing Sun; Cihui Wan; Electronic Nose-Based Technique for Rapid Detection and Recognition of Moldy Apples.. Sends in Analytical Chemiorstry 2017, 9, 197, 257-271, 10.1016/j.trac.2017.09.014., 1526, 10.3390/s19071526.
- Wenshen Jia; Gang Liang; Hui Tian; Jing Sun; Cihui Wan; Electronic Nose-Based Technique for Rapid Detection and Recognition of Moldy Apples.. Jonas Gruber; Henry M. Nascimento; Elaine Y. Yamauchi; Rosamaria W.C. Li; Carlos H.A. Esteves; Gustavo Pamplona Rehder; Christine Gaylarde; Márcia A. Shirakawa; A conductive polymer based electronic nose for early detection of Penicillium digitatum in post-harvest oranges. Materials Sciensors ce and Engineering: C 2019, 19, 1526, 10.3390/s19071526.3, 33, 2766-2769, 10.1016/j.msec.2013.02.043.
- Jonas Gruber; Henry M. Nascimento; Elaine Y. Yamauchi; Rosamaria W.C. Li; Carlos H.A. Esteves; Gustavo Pamplona Rehder; Christine Gaylarde; Márcia A. Shirakawa; A conductive polymer based electronic nose for early detection of Penicillium digitatum in post-harvest oranges. MQiang Liu; Ke Sun; Nan Zhao; Jia Yang; Yuren Zhang; Chen Ma; Leiqing Pan; Kang Tu; Information fusion of hyperspectral imaging and electronic nose for evaluation of fungal contamination in strawberries during decay. Posthatrverials Science and Engineering: C st Biology and Technology 2013, 9, 1533, 2766-2769, 10.1016/j.msec.2013.02.043., 152-160, 10.1016/j.postharvbio.2019.03.017.
- Qiang Liu; Ke Sun; Nan Zhao; Jia Yang; Yuren Zhang; Chen Ma; Leiqing Pan; Kang Tu; Information fusion of hyperspectral imaging and electronic nose for evaluation of fungal contamination in strawberries during decay. PQiang Liu; Nan Zhao; Dandan Zhou; Ye Sun; Ke Sun; Leiqing Pan; Kang Tu; Discrimination and growth tracking of fungi contamination in peaches using electronic nose. Foostd Charvest Biologemistry and Technology 2019, 153, 152-160, 10.1016/j.postharvbio.2019.03.017.8, 262, 226-234, 10.1016/j.foodchem.2018.04.100.
- Qiang Liu; Nan Zhao; Dandan Zhou; Ye Sun; Ke Sun; Leiqing Pan; Kang Tu; Discrimination and growth tracking of fungi contamination in peaches using electronic nose. Food Yuning Hong; Jacky W. Y. Lam; Ben Zhong Tang; Aggregation-induced emission. Chemiscal Societry Reviews 20118, 262, 226-234, 10.1016/j.foodchem.2018.04.100., 40, 5361, 10.1039/c1cs15113d.
- Yuning Hong; Jacky W. Y. Lam; Ben Zhong Tang; Aggregation-induced emission. ChHaibo Wang; Gongyan Liu; Advances in luminescent materials with aggregation-induced emission (AIE) properties for biomedical applications.. Journal of Matemrical Society Reviews als Chemistry B 2011, 40, 5361, 10.1039/c1cs15113d.8, 6, 4029-4042, 10.1039/c8tb00674a.
- Haibo Wang; Gongyan Liu; Advances in luminescent materials with aggregation-induced emission (AIE) properties for biomedical applications.. JHaibo Wang; Gongyan Liu; Haiqi Gao; Yunbing Wang; A pH-responsive drug delivery system with an aggregation-induced emission feature for cell imaging and intracellular drug delivery. Pournal of Materialsymer Chemistry B 2018, 5, 6, 4029-4042, 10.1039/c8tb00674a., 4715-4718, 10.1039/C5PY00584A.
- Haibo Wang; Gongyan Liu; Haiqi Gao; Yunbing Wang; A pH-responsive drug delivery system with an aggregation-induced emission feature for cell imaging and intracellular drug delivery. PolymerMeiying Liu; Xiqi Zhang; Bin Yang; Fengjie Deng; Zengfang Huang; Yang Yang; Zhen Li; Xiaoyong Zhang; Yen Wei; Ultrabright and biocompatible AIE dye based zwitterionic polymeric nanoparticles for biological imaging. RSC ChAdvancemistry 2015, 6, 4715-4718, 10.1039/C5PY00584A.4, 4, 35137-35143, 10.1039/c4ra06160h.
- Meiying Liu; Xiqi Zhang; Bin Yang; Fengjie Deng; Zengfang Huang; Yang Yang; Zhen Li; Xiaoyong Zhang; Yen Wei; Ultrabright and biocompatible AIE dye based zwitterionic polymeric nanoparticles for biological imaging. RSCYulin Zhu; Xuhan Xia; Sha Deng; Bin Yan; Yi Dong; Kaixiang Zhang; Ruijie Deng; Qiang He; Label-free fluorescent aptasensing of mycotoxins via aggregation-induced emission dye. Dyes Advanced Pigments 2014, 4, 35137-35143, 10.1039/c4ra06160h.9, 170, 107572, 10.1016/j.dyepig.2019.107572.
- Yulin Zhu; Xuhan Xia; Sha Deng; Bin Yan; Yi Dong; Kaixiang Zhang; Ruijie Deng; Qiang He; Label-free fluorescent aptasensing of mycotoxins via aggregation-induced emission dye. DyYong-Jiang Xu; Chengshu Wang; Wanxing Eugene Ho; Choon Nam Ong; Recent developments and applications of metabolomics in microbiological investigations. TrAC Trends and Pigments in Analytical Chemistry 2019, 170, 107572, 10.1016/j.dyepig.2019.107572.4, 56, 37-48, 10.1016/j.trac.2013.12.009.
- Yong-Jiang Xu; Chengshu Wang; Wanxing Eugene Ho; Choon Nam Ong; Recent developments and applications of metabolomics in microbiological investigations. TrAC Trends Natalie J. Serkova; Mark S Brown; Quantitative analysis in magnetic resonance spectroscopy: from metabolic profiling toin vivobiomarkers. Bioan Analytical Chemialysistry 2014, 56, 37-48, 10.1016/j.trac.2013.12.009.2, 4, 321-341, 10.4155/bio.11.320.
- Natalie J. Serkova; Mark S Brown; Quantitative analysis in magnetic resonance spectroscopy: from metabolic profiling toin vivobiomarkers. BiKarin Kleigrewe; Filiz Aydin; Kirstin Hogrefe; Patrick Piecuch; Klaus Bergander; Ernst-Ulrich Würthwein; Hans-Ulrich Humpf; Structure Elucidation of New Fusarins Revealing Insights in the Rearrangement Mechanisms of theFusariumMycotoxin Fusarin C. Journanalysl of Agricultural and Food Chemis try 2012, 4, 321-341, 10.4155/bio.11.320., 60, 5497-5505, 10.1021/jf3009469.
- Karin Kleigrewe; Filiz Aydin; Kirstin Hogrefe; Patrick Piecuch; Klaus Bergander; Ernst-Ulrich Würthwein; Hans-Ulrich Humpf; Structure Elucidation of New Fusarins Revealing Insights in the Rearrangement Mechanisms of theFusariumMycotoxin Fusarin C. JoRosalía López-Ruiz; Roberto Romero-González; Antonia Garrido Frenich; Metabolomics approaches for the determination of multiple contaminants in food. Currenal of Agricultural andt Opinion in Food ChSciencemistry 2012, 60, 5497-5505, 10.1021/jf3009469.9, 28, 49-57, 10.1016/j.cofs.2019.08.006.
- Rosalía López-Ruiz; Roberto Romero-González; Antonia Garrido Frenich; Metabolomics approaches for the determination of multiple contaminants in food. Current Opinion in Antoni Femenias; Ferran Gatius; Antonio J. Ramos; Vicente Sanchis; Sonia Marín; Use of hyperspectral imaging as a tool for Fusarium and deoxynivalenol risk management in cereals: A review. Food ScieConce trol 202019, 2, 108, 49-57, 10.1016/j.cofs.2019.08.006., 106819, 10.1016/j.foodcont.2019.106819.
- Antoni Femenias; Ferran Gatius; Antonio J. Ramos; Vicente Sanchis; Sonia Marín; Use of hyperspectral imaging as a tool for Fusarium and deoxynivalenol risk management in cereals: A review. Food Control 2020, 108, 106819, 10.1016/j.foodcont.2019.106819.ElMasry, G.; Sun, D.W. Principles of hyperspectral imaging technology. In Hyperspectral Imaging for Food Quality Analysis and Control; Sun, D.W., Ed.; Elsevier: London, UK, 2010; pp. 3–43.
- ElMasry, G.; Sun, D.W. Principles of hyperspectral imaging technology. In Hyperspectral Imaging for Food Quality Analysis and Control; Sun, D.W., Ed.; Elsevier: London, UK, 2010; pp. 3–43.Jianwei Qin; Moon Kim; Kuanglin Chao; Diane E. Chan; Stephen R. Delwiche; Byoung-Kwan Cho; Line-Scan Hyperspectral Imaging Techniques for Food Safety and Quality Applications. Applied Sciences 2017, 7, 125, 10.3390/app7020125.
- Jianwei Qin; Moon Kim; Kuanglin Chao; Diane E. Chan; Stephen R. Delwiche; Byoung-Kwan Cho; Line-Scan Hyperspectral Imaging Techniques for Food Safety and Quality Applications. AppGamal Elmasry; Mohammed Kamruzzaman; Da-Wen Sun; Paul Allen; Principles and Applications of Hyperspectral Imaging in Quality Evaluation of Agro-Food Products: A Review. Critical Reviews in Food Sciences and Nutrition 2017, 7, 125, 10.3390/app7020125.2, 52, 999-1023, 10.1080/10408398.2010.543495.
- Gamal Elmasry; Mohammed Kamruzzaman; Da-Wen Sun; Paul Allen; Principles and Applications of Hyperspectral Imaging in Quality Evaluation of Agro-Food Products: A Review. Selamawit Tekle; Ingrid Måge; Vegard H. Segtnan; Asmund Bjørnstad; Near-Infrared Hyperspectral Imaging of Fusarium-Damaged Oats (Avena sativa L.). Ceritical Reviews in Food Science and Nutrition eal Chemistry 2012, 55, 92, 999-1023, 10.1080/10408398.2010.543495., 73-80, 10.1094/cchem-04-14-0074-r.
- Selamawit Tekle; Ingrid Måge; Vegard H. Segtnan; Asmund Bjørnstad; Near-Infrared Hyperspectral Imaging of Fusarium-Damaged Oats (Avena sativa L.). CJayme Garcia Arnal Barbedo; Casiane Salete Tibola; Maria Imaculada Pontes Lima; Deoxynivalenol screening in wheat kernels using hyperspectral imaging. Biosysterms Engineal Chemistry ering 2015, 92, 73-80, 10.1094/cchem-04-14-0074-r.7, 155, 24-32, 10.1016/j.biosystemseng.2016.12.004.
- Jayme Garcia Arnal Barbedo; Casiane Salete Tibola; Maria Imaculada Pontes Lima; Deoxynivalenol screening in wheat kernels using hyperspectral imaging. BEwa Ropelewska; Piotr Zapotoczny; Classification of Fusarium-infected and healthy wheat kernels based on features from hyperspectral images and flatbed scanner images: a comparative analysis. Zeiotsystems Engineerichrift für Lebensmittel-Untersuchung und -Forschung 2017, 155, 24-32, 10.1016/j.biosystemseng.2016.12.004.8, 244, 1453-1462, 10.1007/s00217-018-3059-7.
- Ewa Ropelewska; Piotr Zapotoczny; Classification of Fusarium-infected and healthy wheat kernels based on features from hyperspectral images and flatbed scanner images: a comparative analysis. ZeitschK. Liang; Q. X. Liu; J. H. Xu; Y. Q. Wang; C. S. Okinda; M. X. Shena; Determination and Visualization of Different Levels of Deoxynivalenol in Bulk Wheat Kernels by Hyperspectral Imaging. Jouriftnal für Lebensmittel-Untersuchung und -Forschung of Applied Spectroscopy 2018, 244, 1453-1462, 10.1007/s00217-018-3059-7., 85, 953-961, 10.1007/s10812-018-0745-y.
- Stefania Valenzano; Vincenzo Lippolis; Michelangelo Pascale; Agostino De Marco; Chris M. Maragos; Michele Suman; Angelo Visconti; Determination of Deoxynivalenol in Wheat Bran and Whole-Wheat Flour by Fluorescence Polarization Immunoassay. Food Analytical Methods 2013, 7, 806-813, 10.1007/s12161-013-9684-7.