Natural killer (NK) cells play a significant and vital role in the first line of defense against infection through their ability to target cells without prior sensitization. They also contribute significantly to the activation and recruitment of both innate and adaptive immune cells through the production of a range of cytokines and chemokines. In the context of cytomegalovirus (CMV) infection, NK cells and CMV have co-evolved side by side to employ several mechanisms to evade one another. However, during this co-evolution the discovery of a subset of long-lived NK cells with enhanced effector potential, increased antibody-dependent responses and the potential to mediate immune memory has revolutionized the field of NK cell biology. The ability of a virus to imprint on the NK cell receptor repertoire resulting in the expansion of diverse, highly functional NK cells to this day remains a significant immunological phenomenon that only occurs in the context of CMV.
- natural killer cells
- cytomegalovirus
- viral infection
- transplantation
- vaccination
- cancer immunotherapy
Please note: Below is an entry draft based on your previous paper, which is wrirren tightly around the entry title. Since it may not be very comprehensive, we kindly invite you to modify it (both title and content can be replaced) according to your extensive expertise. We believe this entry would be beneficial to generate more views for your work. In addition, no worry about the entry format, we will correct it and add references after the entry is online (you can also send a word file to us, and we will help you with submitting).
1. Introduction
Cytomegalovirus (CMV) has an interesting and diverse relationship with the human immune system, co-evolving side by side for millions of years to produce a finely tuned symbiotic relationship under normal homeostatic conditions. However, while immunocompetent individuals rarely present with symptoms, CMV infection remains a serious threat to immunocompromised individuals such as transplant recipients and is the most common congenital infection that can lead to significant neurological deficiencies in newborns [1]. Natural killer (NK) cells play an important role in combating CMV infection, which has resulted in a dynamic interplay between NK cells and CMV evasion mechanisms. Arguably one of the most important consequences of this relationship is the emergence of a subset of NK cells known as adaptive NK cells. To date only identified in the context of CMV infection, the discovery of these NK cells has played a significant role in advancing our understanding of NK cell function and their ability to bridge the divide between innate and adaptive immune responses. Furthermore, adaptive NK cells have emerged as important players across several contexts from viral infections and vaccination to transplantation and cancer immunotherapy.
2. Biology of NK Cells
Discovered in the mid 1970s, NK cells are categorized as CD56+ CD3− cells that are unique in their ability to kill target cells without prior antigen sensitization [2]. This feature is critical for the rapid elimination or containment of infection, allowing the recruitment and activation of the adaptive immune system for a specific attack and the development of immune memory. NK cells are commonly split into two major subtypes based on the density of CD56. These subtypes are defined broadly by their distinct functions, delineated generally by cytotoxic effector capacity (CD56dim) and immunoregulatory cytokine production (CD56bright) [3]. CD56bright NK cells produce cytokines such as interferon gamma (IFNγ), tumor necrosis factor alpha (TNFα) and granulocyte-macrophage colony-stimulating factor (GM-CSF), soluble factors that are necessary for the recruitment of other immune cells during the initial innate immune response [4]. Whilst CD56dim NK cells are similarly capable of secreting cytokines, they are distinguished by their ability to induce target cell apoptosis through the release of lytic granules containing perforin and granzymes [5]. As such, NK cells play an important role in bridging the innate and adaptive immune systems, regulating the immune response to virally infected and tumorigenic cells.
The capacity of NK cells to recognize infected cells is determined by a balance of germline-encoded activating and inhibitory receptors. The combination of signals received by these receptors determines whether an NK cell is activated by the target cell. Inhibitory receptors on NK cells play an important role in self-recognition and NK cell education [6]. Prominent inhibitory receptors on NK cells are CD94/NKG2A, which recognizes the non-classical human leukocyte antigen (HLA)-E molecule, the killer immunoglobin-like receptors (KIRs) that recognize allelic epitopes present in certain HLA-A, -B and -C alleles and the leukocyte immunoglobulin-like receptors (LIRs) such as LIR-1 (CD85j) which binds HLA class I alleles with varying affinities [7]. NK cells gain functional competency during development through a process known as NK cell education [8]. When an educated NK cell encounters its HLA ligand on a target cell its activity is inhibited. Comparatively, the absence of this inhibitory signal will trigger activation through dominating activating signals, termed ‘missing self'’ [6]. Activating signals are received through a host of receptors, such as NKG2D, NKG2C, activating KIRs and the natural cytotoxicity receptors (NCRs); NKp30, NKp44 and NKp46, which bind to ligands upregulated on stressed or virally infected cells such as major histocompatibility complex class I chain-related A (MICA), MICB, UL16 binding proteins (ULPBs), B7-H6 and haemagglutinin [9][10][9,10]. The activating receptor FcγRIIIA (CD16) is able to induce antibody-dependent cellular cytotoxicity (ADCC), an apoptotic pathway where target cells are opsonized with IgG, triggering the release of perforin and granzyme B [11]. Activating receptors are often associated with co-receptors necessary for triggering this cytotoxic activity within the NK cell such as DNAX accessory molecule-1 (DNAM-1), CD96, NK-T-B antigen (NTBA) and CS1 (CD319). Together with inhibitory signals, NK cells rely on activating signals expressed by target cells to determine the degree of response to their cellular environment. This degree of response may differ between individuals as a function of the polymorphic nature of the varied NK cell receptor repertoires [12], in addition to lifetime exposure to pathogenic and other environmental factors [13].
3. NK Cells and CMV
A well-established case study where dynamic NK cell receptors and infection interact is in the context of CMV. CMV is a member of the Herpesviridae family of viruses and affects between 50–98% of human populations, depending on geographic locations and socio-economic backgrounds [14]. Once infected, the virus remains latent in the host for life with frequent reactivations. Although generally benign, CMV is damaging for immunocompromised individuals and newborns, with individuals deficient in either the function or number of NK cells experiencing severe disease, sometimes resulting in death [15]. In healthy individuals, CMV avoids elimination by the immune system through a variety of escape mechanisms likely developed over millions of years of coevolution with humans [16].
CMV encodes a set of genes that mediate these escape mechanisms, mostly through targeting HLA molecules on the surface of the infected cell by either interfering with the expression of HLA or encoding for HLA homologues [17]. US2, US3, US6 and US11 are inhibitory unique short region proteins expressed at different stages of infection [18]. US2 and US11 trigger proteasomal degradation of the HLA molecule, US3 promotes retention of the protein in the ER, while US6 inhibits peptide loading by blocking the transporter protein, TAP, binding to ATP [19]. Together, these proteins ensure the downregulation of HLA molecules to avoid CD8+ T cell detection. Under normal conditions, this downregulation of HLA molecules would trigger the ‘missing self'’ pathway of NK cell recognition and leave CMV-infected cells vulnerable to detection and elimination. However, CMV has evolved to express certain HLA homologues and peptide homologues to inhibit NK cell activation. The first CMV-encoded immune-evasive protein reported was UL18, a homologue of HLA [20]. Expressed in its place, UL18 has a high binding affinity to the NK cell inhibitory receptor LIR-1 in humans, up to 1000-fold more than when bound to its endogenous ligand, HLA class I [21].
Additional inhibitory mechanisms also exist, including the production of proteins UL16 and UL40. UL16 acts by inhibiting the production of MICA, MICB and ULBPs, ligands for the activating receptor NKG2D [22]. UL40 is a peptide produced by CMV homologous to the HLA-E binding protein [23]. UL40 allows for expression of HLA-E on the surface of infected cells by promoting its loading in a TAP-independent manner, bypassing inhibition by US6 [24]. UL40 bound to HLA-E binds to the inhibitory receptor NKG2A, providing the CMV infected cell with an effective NK cell escape mechanism [25]. However, the activating receptor NKG2C also recognizes HLA-E, the ramifications of which will be discussed in detail below. This interplay between the host and CMV allows CMV to persist in the body and shape the ensuing immune response for life.
4. History of Adaptive NK Cells
CMV plays a unique role in shaping the NK cell repertoire and driving the expansion of subset(s) of NK cells with memory-like properties, now routinely known as adaptive NK cells. First described in the context of mouse CMV (MCMV), these NK cells can expand and contract following infection [26]. NK cells expressing the activating receptor Ly49H directly recognize the MCMV protein, m157. Following MCMV infection, these NK cells proliferate over 100-fold in the spleen and 1000-fold in the liver before contracting once disease is cleared. Viral specific Ly49H+ NK cells however do not return to their pre-infection baseline and instead are maintained at an elevated level for several months. Upon rechallenge Ly49H+ NK cells respond and degranulate far more rapidly than CMV naïve NK cells and produce significantly more cytokines to protect against disease progression.
In humans, adaptive NK cells are most commonly associated with expression of NKG2C. Guma and colleagues [27] first reported the observation that NK cells expressing NKG2C were increased in CMV seropositive (CMV+) healthy donors. A similar association was not detected in patients who were herpes simplex virus (HSV) or Epstein-Barr virus (EBV) seropositive, suggesting the expansion of NKG2C was specific to CMV infection and not to other Herpesviridae infections. In addition, NK cells expressing NKG2C had lower expression of the two activating receptors, NKp30 and NKp46 and increased proportions of KIR and LIR-1 expression. Building on these observations, Guma and colleagues [28] later demonstrated that NK cells expressing NKG2C preferentially expand following co-culture with CMV-infected fibroblasts using the AD169 or Towne strains of CMV. This expansion did not occur with virus alone nor was there any expansion of NKG2C+ NK cells in CMV seronegative donors. Importantly, blocking NKG2C on HCMV donors decreased NK cell expansion demonstrating that NKG2C was directly involved in mediating this phenomenon, although the mechanisms driving this remained unclear.
Unlike in MCMV, where there is a distinct population of MCMV-specific Ly49H NK cells that expand, contract and respond preferentially upon rechallenge [26], how CMV shapes the NK cell receptor repertoire in humans is more complex. Nevertheless, multiple studies have provided evidence for the possibility of a memory-like NK cell population in humans in the context of CMV infection [29][30][31][32][33][34][35][29,30,31,32,33,34,35]. NK cells co-expressing NKG2C and CD57 were first associated with CMV infection in solid organ transplant (SOT) recipients [33]. CD57 is expressed on mature NK cells and typically associated with high effector function [36]. Following CMV infection in these SOT recipients, CD57+NKG2C+ NK cells preferentially expanded over time. In the context of hematopoietic stem cell transplantation (HSCT), we similarly observed an expansion of NK cells expressing NKG2C with increased acquisition of CD57 over time [32]. During acute CMV reactivation in recipients of umbilical cord blood (UCB) transplants, NKG2C+ NK cells preferentially expand, peaking at four weeks post-infection. This population remained a significant proportion of each patient'’s NK cell receptor repertoire at one year post-transplant, accounting for approximately 50% of their NK cells. In addition, these NKG2C+ NK cells produced significantly more IFNγ compared to NKG2C− NK cells. NK cells are the first lymphocyte to reconstitute following UCB HSCT; however, they present with an immature phenotype and overall poor effector function that can take up to a year to be restored [37]. CMV reactivation appeared to dramatically accelerate the maturation of these NK cells, as demonstrated by a rapid decline in NKG2A expression, acquisition of KIR, CD57 and enhanced effector function [32]. Furthermore, increased mRNA expression of T-bet and IFNγ was detected six months and one year post-transplant, indicating their potential to respond more rapidly with cytokines and acquire a memory-like phenotype. In a follow-up study, we identified that NKG2C+ NK cells expand even in the absence of clinically-detectable CMV viraemia in CMV but only if both the donor and recipient were CMV+ [31]. This did not occur if the recipient was CMV seronegative, suggesting that the continued persistence of NKG2C+ NK cells requires the presence of latent (subclinical) expression of CMV antigen. Furthermore, in recipients who received a transplant from a CMV+ donor, we demonstrated the potential of NKG2C+ NK cells to represent memory-like NK cells with heightened effector function and expansion following CMV reactivation. Collectively, these studies demonstrate the profound effect CMV has on the NK cell receptor repertoire and the potential for memory-like NK cells to exist in humans.
The ability of CMV to imprint on the NK cell receptor repertoire was highlighted in a large cohort study of over 200 healthy donors [29]. In elegant detail, Beziat and colleagues demonstrated the ability of CMV infection to skew NK cell receptor repertoires towards an abundance of NKG2C+ CD57+ KIR+ self-educated NK cells. This general phenotype is strongly associated with those NK cells exhibiting the highest functional potential against target cells. In addition, other NK cell subsets identified lacked NKG2C and expressed activating KIR, suggesting NK cells expressing activating KIR also expand in the context of CMV infection. Indeed, adaptive NK cells have been shown to expand in individuals who lack NKG2C, clearly demonstrating that NKG2C is not required for the expansion of adaptive NK cells [38][39][40][41][38,39,40,41]. Beyond surface phenotypes, we and others have reported on the downregulation of the adaptor molecule FcεRIγ, the kinase Syk, the intracellular adaptor EAT-2 and the transcription factor PLZF (promyelocytic leukemia zinc finger) in CMV+ individuals [35][42][35,42]. Similar to our previous observations in HSCT recipients, NK cells lacking FcεRIγ, EAT-2 and Syk expanded during the first year after transplantation. However, what was most striking about these studies was the link between CMV infection and epigenetic modification of the NK cells, a notion first identified in 2014 with the identification of CMV infection epigenetically imprinting on the IFNG locus [34]. Compared with canonical NK cells, memory-like NK cells possess a methylation profile far more similar to CD8+ T cells [35]. This, coupled with their memory-like phenotype, led to their classification as adaptive NK cells, which to date have only been identified in CMV+ individuals. In the following sections we will provide an update on the development of adaptive NK cells, followed by their role in transplantation, viral infections, vaccination and cancer immunotherapy.
5. Current Status on the Development of Adaptive NK Cells
For many years there were two main overarching questions surrounding the development of adaptive NK cells: how is CMV driving the expansion of adaptive NK cells and why are these cells only found in approximately one-third of CMV+ donors? Following the first reports of an association between NKG2C and CMV, Guma and colleagues [28] attempted to determine how NKG2C+ NK cells were recognizing infected target cells. NKG2C recognizes the non-classical class I allele, HLA-E [43]. In CMV infection, HLA-E is not downregulated like other class I alleles and can be loaded with a peptide derived from the leader sequence of the CMV UL40 protein [23]. Presumably this has evolved to inhibit NK cell function through binding of HLA-E to the inhibitory NKG2A receptor. However, when using UL40 mutated viral strains there was only a minor effect on the expansion of NKG2C+ NK cells. It was not until 2018, when Hammer and colleagues [44] elegantly demonstrated the ability of the UL40 CMV peptide loaded in HLA-E to drive the expansion of NKG2C+ adaptive NK cells, that the role of CMV peptides was finally elucidated. Importantly, not all UL40 CMV peptides were equal in their ability to drive expansion. The VMAPRTLFL (found in ~1% of clinical isolates) peptide had the greatest ability to activate NKG2C+ adaptive NK cells whereas the VMAPRTLIL (found in ~40% of clinical isolates) and VMAPRTLVL (found in ~12% of clinical isolates) required co-stimulation through CD2-LFA-3 to achieve similar levels of activation. Arguably the most striking finding in this seminal publication was the ability to generate adaptive NK cells from CMV seronegative donors, which had previously never been reported. Peptide alone was not sufficient to drive expansion of NKG2C+ NK cells from CMV seronegative donors but required CD2 co-stimulation and was even greater in the presence of the proinflammatory cytokines interleukin (IL)-12 and IL-18. In addition to an expanded NKG2C+ population, these NK cells also exhibited other features of adaptive NK cells such as downregulation of FcεRIγ and hypomethylation of the IFNG locus. Hammer and colleagues [44] extended their findings to a cohort of HSCT recipients with CMV reactivation. The peptide-encoding UL40 region of the infecting viral strain was assessed for each patient and compared with the presence and/or expansion of adaptive NK cells. Recipients infected with strains harboring the VMAPRTLFL peptide expanded NK cells with an adaptive phenotype, whereas of those patients infected with strains harboring the VMAPRTLIL peptide, the presence of adaptive NK cells was far more varied. Interestingly, the VMAPRTLFL peptide can also be derived from the leader sequence of HLA-G, with Rolle and colleagues suggesting a role for increased HLA-G expression during CMV infection as another driver of the expansion of adaptive NK cells [45]. Clearly CMV peptide plays a significant role in the expansion of adaptive NK cells, however due to the great variation in the responses seen, other factors are likely to also be involved. Furthermore, adaptive NK cells do not all express NKG2C, which strongly suggests that other viral or potentially self-peptides also contribute to the generation of adaptive NK cells. Studies to identify these peptides, for example ones that may be recognized by activating KIR, are certainly warranted and will provide another important piece in the puzzle to understanding the unique relationship between CMV and adaptive NK cells.
Additional research is also required to elucidate how CMV infection remodels the epigenetic landscape of adaptive NK cells. We know that at least three signals are required to induce epigenetic changes that result in the formation of adaptive NK cells: ligand engagement in the context of an appropriate peptide, co-stimulation and proinflammatory cytokine stimulation [44] (Figure 1). The peptide is crucial as proinflammatory cytokines alone do not generate adaptive NK cells, rather they generate a population of cytokine induced memory-like NK cells with their own distinct phenotype and functional capacity [46]. It would be interesting to examine whether every NK cell has the potential to become an adaptive NK cell or whether this is exclusive to a specific subset. One may speculate the existence of a population of NK cells that are more poised to undergo epigenetic remodeling than other NK cells, awaiting a strong engagement with a viral peptide during infection to drive them towards an adaptive phenotype. Answering these questions will be challenging, yet a deeper understanding of the potential of NK cells to gain an adaptive phenotype will allow researchers greater capacity to exploit these cells for future NK cell immunotherapies.
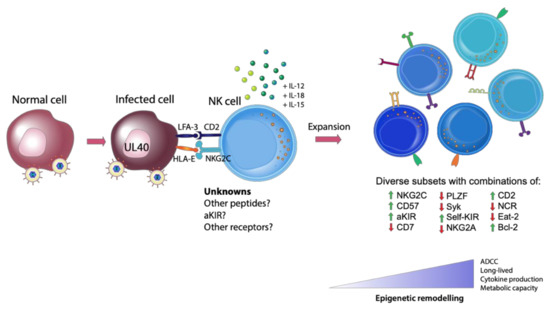
Figure 1. Generation of Adaptive natural killer (NK) cells. The expansion of adaptive NK cells requires three signals: (1) appropriate receptor engagement in the context of a viral peptide (such as HLA-E and NKG2C, with other unknown viral or self-peptides and receptors likely involved), (2) co-stimulation and (3) proinflammatory cytokines. This engagement with a virally infected cell leads to the generation of diverse, highly functional subsets of NK cells with differing degrees of epigenetic remodeling.
6. Concluding Remarks
The profound effect CMV has on modulating the human immune system remains a fascinating and mysterious immunological phenomenon. Why this commonly asymptomatic virus drives the expansion of NK cells that undergo significant epigenetic reprogramming is still not clear and understanding these drivers will only further increase the potential of these cells to be used therapeutically. Due to their increased functional capacity and adaptive-like features, including their potential to be long-lived, adaptive NK cells provide much promise across a range of contexts. However, there is a fine balance between effective and detrimental NK cell-mediated immune responses and careful consideration is needed when investigating adaptive NK cells and the host response. With the advent of and improved access to genomic technology, we will continue to gain a greater understanding of adaptive NK cells allowing us to further unlock the mysteries of the important role CMV plays in dictating NK cell fate (Figure 2).
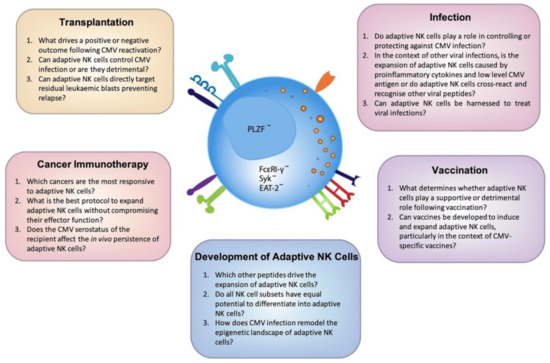
Figure 2. Outstanding questions to unlock the full potential of adaptive NK cells. Cytomegalovirus (CMV) has the unique ability to imprint on the NK cell repertoire resulting in the expansion of diverse, highly functional adaptive NK cells. A greater understanding of the mechanisms that drive the development of these cells is clearly warranted. Here we have identified the most pressing questions across a number of emerging fields which, upon further investigation, will elucidate the maximal clinical potential of adaptive NK cells.
