The subgenus Bryophyllum belongs to genus Kalanchoe (Crassulaceae family) and includes about 25 plant species native to Madagascar, which are widely used in traditional medicine in vast regions throughout Africa, Asia and South Africa. Different formulations from bryophyllum (this term is proposed to be the common name to collectively refer to these species) have been employed for the treatment of several ailments, including infections, gynecological disorders, and chronic diseases, such as diabetes, neurological and neoplastic diseases. Two major families of secondary metabolites have been reported as responsible for these bioactivities: phenolic compounds and bufadienolides. These compounds are found in limited amounts in plants because they are biosynthesized in response to different biotic and abiotic stresses. Therefore, novel approaches should be undertaken with the aim of achieving the phytochemical valorization of Bryophyllum sp., allowing a sustainable production that prevents from a massive exploitation of wild plant resources. This review focuses on the study of phytoconstituents reported on bryophyllum; the application of plant tissue culture methodology as a reliable tool for the valorization of bioactive compounds; and the application of machine learning technology to model and optimize the full phytochemical potential of bryophyllum. As a result, bryophyllum species can be considered as a promising source of plant bioactive compounds, with enormous antioxidant and anticancer potential, which could be used for their large-scale biotechnological exploitation in cosmetic, food, and pharmaceutical industries.
- Bryophyllum
- traditional medicine
- secondary metabolism
- plant biotechnology
- plant polyphenols
- bufadienolides
- bioactive compounds
- antioxidants
- cytotoxic agents
- artificial intelligence
1. Introduction
The genus Kalanchoe (Adanson, 1736 [1]) belongs to the Crassulaceae family and comprises 150 to 200 succulent species native to Madagascar and naturalized across Africa, South America, and Asia [2][3]. Kalanchoe constitutes a complex genus with an intricate taxonomy, not yet clearly elucidated. Authors disagree whether the classification is based on a single genus called Kalanchoe (sensu lato) or three separate sections: Kalanchoe (sensu stricto), Bryophyllum Kahl. (Salisbury, 1805 [4]), and Kitchingia (Baker, 1881 [5]). However, other authors propose a three-subgenera classification of the genus Kalanchoe, due to different evolutive arguments, morphological traits [6] and molecular analyses [7], including Kalanchoe, Bryophyllum and Calophygia [8]. Amongst the different subgenera, the subgenus Bryophyllum includes around 25 species, endemic to Madagascar [9] that gained much interest on plant science research, as they are considered model plants for different physiological features: the Crassulacean Acid Metabolism (CAM) [10], vegetative reproduction [11], plant cell regeneration [12], and a source of therapeutical compounds [13]. Nevertheless, the most relevant feature associated to this subgenus is the use of their constitutive species in the traditional medicine worldwide, thus considering Bryophyllum sp. as medicinal plants, due to their associated bioactivities [13].
CAM photosynthesis is an advantageous adaptative strategy that enables plant adaptation to arid ecosystems, as it is the case of the whole Kalanchoe genus [14]. Bryophyllum species present a flexible CAM regime, with no time restriction on CO2 uptake, which is fixed at night [15]. On the other hand, Bryophyllum sp. present a highly specialized asexual reproductive mechanism, based on the symmetric plantlet development along the leaf margins or leaf tips of adult plants (Figure 1) [12][16]. Such clonal-spreading reproductive mechanism is driven by a complex phenomenon that combines both embryogenic and organogenetic events that has not been fully elucidated to date [17][18][19][20][21]. Both the metabolic and reproductive patterns found on Bryophyllum sp. contribute to the invasiveness of these species. It allows them a rapid colonization of unexplored territories with high adaptative efficiency, which has contributed to their worldwide naturalization [22][23].

Figure 1. In vitro-cultured plants of B. daigremontianum (left); B. × houghtonii (center); and B. tubiflorum (right). Bars = 1 cm; arrows indicate plantlets formed asexually on leaf margins. Original figure.
Bryophyllum and other Kalanchoe species have been widely used in the traditional medicine of vast regions throughout Africa, South America, and Asia [24]. Because of its wide distribution and ubiquitous medicinal use, much research on this subgenus has focused on Bryophyllum pinnatum (Lam.) Oken [25][26][27]; however, there is an extensive variety of other species that have also been exploited in Ethnomedicine, such as: B. daigremontianum (Raym.-Hamet et Perr.) Berg. [28], B. tubiflorum Harv. [29][30] and B. × houghtonii D.B. Ward (syn. B. daigremontianum × tubiflorum) [31]. Leaf and root-derived formulations have been mostly used for the treatment of several common illnesses such as burns, wounds, insect bites, skin diseases, cough, fever or several infections, and chronic diseases, such as diabetes, and neurological and neoplastic diseases (Table 1).
Table 1. Ethnobotanical uses of Bryophyllum species.
| Species | Ethnobotanical Uses | Plant Organ | Locations 1 | References |
|---|---|---|---|---|
| B. crenatum (Andr.) Baker | Wounds, smallpox, otitis, cough, asthma, palpitations, headache, abscesses, convulsions, general debility, diabetes, obstetrics and gynecology, vermifuge, abortion, antimicrobial treatment | Leaves Roots |
Africa | [32][33][34][35] |
| B. daigremontianum Raym.-Hamet et Perr. | Leucorrhea, dysmenorrheal, carminative, psychic agitation, anxiety, restlessness | Leaves | Bangladesh | [28][36] |
| B. fedtschenkoi Raym.-Hamet et Perr. | Analgesic, cytotoxic, antimicrobial treatment | Leaves Aerial parts Woody stems |
Brazil | [37][38][39] |
| B. mortagei (Raym.-Hamet et Perr.) G.E. Wickens | Digestive disorders, neoplastic diseases, vermifuge, antimicrobial treatment | Aerial parts Flowers Roots |
Mexico, Colombia, Indonesia | [37][40][41][42] |
Two major subfamilies of phenolic compounds have been widely reported for Bryophyllum sp.: phenolic acids and flavonoids [76][77], which have been recently found to accumulate inside highly specialized leaf cells, called idioblasts [78].
The antioxidant activity of Bryophyllum phenolic compounds, focused on the free-radical scavenging activity, has been largely determined [63][79]. Recently, the antioxidant capacity of Bryophyllum extracts for preventing the lipid oxidation of omega-3 enriched fish oil emulsions was reported, thus conferring a valuable approach for the application of Bryophyllum-derived by-products in the food and pharmacological industries [64]. In the same way, the polyphenols from Bryophyllum-derived extracts may be efficiently purified using environmental-friendly procedures, like the use of activated carbon [80]. These approaches have been developed in order to allow the industrial exploitation of Bryophyllum polyphenols, due to the increasing interest in the research of these medicinal plants.
The great diversity of bioactivities described for these compounds places the phenolic compounds of Bryophyllum sp. as one of the main families of plant secondary metabolites that boost the phytochemical potential of this subgenus [62][64][81].
2.1.1. Phenolic Acids
Three species of Bryophyllum present high content in phenolic acids: B. pinnatum, B. daigremontianum, and B. tubiflorum, mostly located in leaf tissues (Table 2) [76][82]. Both subfamilies of phenolic acids have identified compounds in either free or glycosylated forms. Caffeic acid and ferulic acid are the most abundant cinnamic acids, while within the benzoic acids it is protocatechuic acid. β-resorcylic and γ-rosorcylic acids have also been referenced, although these are more unusual. [63].
Table 2. Phenolic acids reported in Bryophyllum sp.
.
2.1.2. Flavonoids
Flavonoids are universally found in Bryophyllum sp. in O-glycosylated form. To a large extent, they have been reported in three species, namely: B. pinnatum, B. daigremontianum and B. tubiflorum (Table 3). The flavonol glycosides were shown as the most abundant subfamily of flavonoids, showing a restricted accumulation on leaf tissues [13][76][89]. Both kaempferol and quercetin glycosides were found in Bryophyllum species [39][94][95]. Other flavonoid subfamilies, such as flavones and catechins, have also been reported, and a number of anthocyanins have been isolated from the flowers of different species [39][96], which are stored in the foliar idioblasts of B. daigremontianum [82] and B. tubiflorum [78].
Table 3. Flavonoids reported in Bryophyllum sp.
| Subfamily | Compound 1 | Species 2 | References | |
|---|---|---|---|---|
| Flavanones | Naringenin | BT | [92] | |
| Flavones | Luteolin | BP | [89][94][97] | |
| Apigenin | BP, BT | [50][78] | ||
| 4’,5-dihydroxy-3’,8-dimethoxyflavone | BP | [98][99] | ||
| B. pinnatum (Lam.) Oken | Wounds, burns, coughs, earache, headache, muscle pain, asthma, bronchitis, pneumonia, arthritis, rheumatism, ulcers, diabetes, urinary bladder stones, dysentery, diarrhea, vermifuge, antibacterial, insect bites, fevers, menstrual disorders, nausea, tumors, gynecology | Leaves Roots |
Nigeria, Uganda, Madagascar, India, China, Vietnam, Bangladesh, Australia, Brazil, Peru, Trinidad and Tobago | [43][44][45][46][47][48][49][50][51][52] |
| B. serratum (Mann. and Boit.) Blanco | Pain, inflammation, fever, antiviral | Stems | Taiwan | [53][54] |
| B. tubiflorum Harv. | Wounds, epilepsy, vermifuge, neoplastic diseases | Leaves | Brazil, Ethiopia | [29][30] |
1 Locations where the ethnobotanical uses have been reported.
The great therapeutic potential reported on Bryophyllum sp. [39] has promoted in-depth phytochemical analysis to adequately evaluate its biological and pharmacological properties [55][56]. Several authors have demonstrated the whole bioactive potential of Bryophyllum-derived extracts, acting as multifaceted agents.
The anti-inflammatory activity of Bryophyllum extracts has been determined by different methods using both in vivo and in vitro models. For instance, aqueous extracts from B. pinnatum were shown to exert a relevant effect against croton oil-induced ear edema and carrageenan-induced paw edema in murine models, driven by a decrease in pro-inflammatory cytokines [57]. Moreover, different flavonoids produced by B. tubiflorum showed an inhibitory effect on nitric oxide production by lipopolysaccharide-induced macrophage in vitro RAW264.7 cell line [58].
The antimicrobial activity attributed to Bryophyllum extracts was shown to present a high effectiveness against a wide range of both bacterial and fungal activities. In this sense, hydroethanolic extracts from B. fedtschenkoi showed a strong inhibitory effect against different antimicrobial resistant strains from the ESKAPE complex, including both Gram-negative and Gram-positive bacteria [37]. Similarly, the bactericidal effect of B. crenatum leaf juice against Bacillus subtilis and Klebsiella pneumoniae was also reported, as well as high effectiveness of methanol extracts from B. pinnatum to Gram-positive bacteria [34]. Moreover, different isolated fractions from B. daigremontianum ethanolic extracts promoted a potent activity against Safase S-04 yeast strain, fungi, such as Candida albicans and Aspergillus niger, and bacteria, including Staphylococcus aureus and Escherichia coli [59]. Furthermore, the antiviral activity of Bryophyllum extracts has been also assessed for relevant viral diseases. It is the case of the antiviral activity of kaempferol derivatives from B. daigremontianum against Herpes Simplex Virus (HSV) types 1 and 2 [60] and bryophyllin B from B. pinnatum as a potent inhibitor of Human Immunodeficiency Virus (HIV) [61].
Additionally, the analgesic and sedative properties of Bryophyllum extracts were evaluated using in vivo murine models, indicating that leaf extracts from B. crenatum showed a protective effect against formalin and acetic acid-induced pain and inhibited the manifestation of seizures under convulsant agents application [32].
The antioxidant properties of Bryophyllum extracts have been widely reported by a plethora of different methods. The radical scavenging activity against 2,2-diphenyl-picryl-hydrazyl (DPPH), superoxide anion and nitric oxide of B. daigremontianum, B. tubiflorum, B. × houghtonii, and B. pinnatum leaf and aerial part extracts was reported [62][63]. The inhibition of lipid peroxidation by hydromethanolic extracts from aerial parts of B. daigremontianum, B. tubiflorum, and B. × houghtonii, cultured in vitro was also determined [64]. Moreover, cell-based in vitro antioxidant assays have been performed for the inhibition of lipid peroxidation of root extracts from B. daigremontianum [24].
Bryophyllum extracts have been also shown to present insecticidal properties, as a consequence of bufadienolide production, as reviewed later. In this sense, methanolic leaf extracts from B. daigremontianum, B. pinnatum, and B. × houghtonii showed an intense effect against silkworm larvae (Bombyx mori) [65][66][67].
Moreover, cardioprotective and antihypertensive properties were attributed to different Bryophyllum sp. [68]. For instance, the aqueous extracts of B. pinnatum have been shown to exhibit in vivo antihypertensive activity on high salt-loaded rats models [69]. Furthermore, isolates from B. daigremontianum root extracts developed an in vitro anti-thrombotic activity [70].
Against all the bioactivities associated with Bryophyllum sp., the cytotoxic activity gained much interest during the phytochemical characterization of these species [71]. A great variety of in vitro models have been employed for the determination of cytotoxic and anti-cancer activities on different Bryophyllum species, whose extracts have been tested against a high number of cancer cell lines [13][68]. Due to the relevance of this bioactivity, the cytotoxic properties of Bryophyllum extracts are included during this review.
Finally, there are additional health-enhancing properties related to Bryophyllum sp., as it is the case of hepatoprotective, antidiabetic activities. Thus, the leaf juice and aqueous of B. pinnatum showed a marked in vivo hepatoprotective effect on carbon tetrachloride-induced hepatotoxicity in rats [72], as well as hypoglycemic and hypocholesterolemic effects in streptozotocin-induced diabetic rats [73].
As a result, the combination of all bioactivities attributed to Bryophyllum sp. aroused the interest in the study of their great therapeutic potential, which is a challenge, as it is an unexplored subgenus with countless potential as a health promoter.
2. Bryophyllum sp. Secondary Metabolites as Antioxidants and Anticancer Agents
It is now well-known that the full set of bioactivities attributed to Bryophyllum sp. is developed by a plethora of phytoconstituents, including phenolic compounds, bufadienolides, organic salts, terpenoids and fatty acids [55]. Phytoconstituents are considered secondary metabolites, since they are biosynthesized by induction of secondary metabolism, which is responsible for the defensive and adaptative plant response against environmental threads and biotic stress [74][75]. Phenolic compounds and bufadienolides are considered the two main families of secondary metabolites of Bryophyllum sp., widely distributed throughout the subgenus [13]. Furthermore, they are responsible for the bioactivity associated with Bryophyllum sp. and, consequently, a deeper insight into these compounds will be provided.
2.1. Phenolic Compounds
| Benzoic acids | |||
| p-Hydroxybenzoic acid | |||
| BD, BP, BT | |||
| Acacetin | BP | [85 | [87] |
| ] | Protocatechuic acid | BD, BP, BT | [26][63][82 |
| Diosmetin | BP | [85]][87][89] | |
| Vanillic acid | BT | [58][78] | |
| Gallic acid | BD, BP, BT | [63][78][82][88][87][89] | |
| Syringic acid | BD, BP, BT | [63][78][89] |
1 Compounds are named as their free-form to simplify the identification. 2 BD: B. daigremontianum; BP: B. pinnatum; BT: B. tubiflorum.
Concerning bioactivities, phenolic acids are considered powerful antioxidants whose activity depends on the number, position, and combination of hydroxyl groups within their structure [83]. Potential therapeutic properties for them have also been reported, as they promote antimicrobial, antiviral, cytotoxic, and anti-inflammatory activities [90][91][92][93]. Phenolic acids from Bryophyllum-derived extracts have already been related to the development of antibacterial and antifungal activity against a series of pathogenic microorganisms [86], antioxidant activity, and cytotoxicity against human lymphoblastic leukemia J45 and H9 T-cell lines [63]
| Afzelin | |||
| BP | |||
| [ | |||
| 100 | ] | ||
| Galangustin | BT | [58] | |
| Hispidulin | BT | [88] | |
| Flavonols | Quercetin | BD, BP, BT | [58][77][78][88][84][88][94][95][98] |
| Kaempferol | BD, BP, BT | [77][78][88][89][90][92][100][98][99] | |
| Quercitrin | BP | [98][99] | |
| Myricetin | BD, BP, BT | [77][90][92] | |
| Rutin | BP | [89][94] | |
| Isorhamnetin | BD, BP | [77][88] | |
| Kaempferitrin | BP | [100] | |
| Herbacetin | BT | [58] | |
| Patuletin | BD | [77] | |
| Isoquercetin | BT | [92] | |
| Aromadendrin | BT | [92] | |
| Galangin | BT | [92] | |
| Flavanols | Catechin | BP | [89] |
| Epicatechin | BT | [92] | |
| Epigallocatechin | BP | [97] |
1 Flavonoids are named as their free-form to simplify the identification. 2 BD: B. daigremontianum; BP: B. pinnatum; BT: B. tubiflorum.
The antioxidant activity of flavonoids is directly proportional to the number and position of hydroxyl groups in their structure [101], that assist in the dissipation of electrons generated after UV-overexposure [102]. Additionally, they also prevent lipid peroxidation [103] (by decomposing lipid peroxides and scavenging harmful free-radicals) and develop an effective metal chelation activity [104]. The free-radical scavenging [62][105][100] and lipid oxidation preventing activities [64] of Bryophyllum-derived extracts rich in flavonoids have already been reported. Other bioactivities, such as antibacterial [106], antiviral [107], cytotoxic [108], anti-inflammatory [109], cardioprotective [110], sedative and anti-diabetic activities [111] have been associated to flavonoids. These bioactivities have been extensively studied for Bryophyllum sp. and have also been related to flavonoid content, mainly using B. pinnatum as a plant model [88][94][95][101][104][112].
2.2. Bufadienolides
Bufadienolides constitute a subfamily within cardiac glycosides family of secondary metabolites and they are considered polyhydroxy C-24 steroids, presenting an α-pyrone ring at the C-17β position (Figure 2) [113]. Bufadienolides presence in Bryophyllum species is genotype and organ dependent [68], being four species the most representative sources of these compounds: B. daigremontianum, B. × houghtonii, B. tubiflorum, and B. pinnatum (Table 4). Universally-distributed bufadienolides, such as bersaldegenin and bryophyllin derivatives [77][114], can be found together with genotype-specific compounds, such as kalanchosides [115] and kalanhybrins [71].
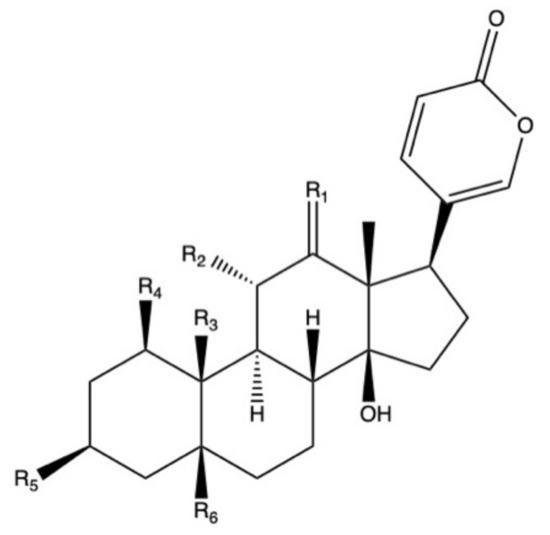
Figure 2. Basic molecular structure of bufadienolides.
Table 4. Bufadienolides identified in Bryophyllum sp. and their associated bioactivities.
| Species 1 | Plant Organ | Bufadienolides | Bioactivities 2 | References |
|---|---|---|---|---|
| BD | Roots | 11α,19-dihydroksytelocinobufagin, bersaldegenin-1-acetate, bersaldegenin-1,3,5-orthoacetate, 19-(acetyloxy)-3β,5β,11α,14-tetrahydroxyl-12-oxo-bufa-20,22-dienolide and 19-(acetyloxy)-1b,3b,5b,14-tetrahydroxyl-bufa-20,22-dienolide | Moderate antioxidant activity using in vitro blood plasma model under peroxynitrite-induced oxidative stress. Effective for prevention of lipid hydroperoxides generation and thiobarbituric acid-reactive substances (TBARS) | [24] |
| BP | Leaves | Bryophyllin A and C | Insecticidal against silkworm larvae | [66] |
| BH | Leaves | Bryophyllin A and C, bersaldegenin-1-acetate, bersaldegenin-3-acetate, bersaldegenin-1,3,5-orthoacetate, daigremontianin, methyl daigremoniate | Insecticidal against silkworm larvae, except for bersaldegenin-1-acetate. Cytotoxic effect of bersaldegenin-1,3,5-orthoacetate and daigremontianin against induced Raji cell line (Burkitt’s lymphoma); inhibition of Epstein–Barr virus |
[31][67] |
| BH | Whole plant | Kalanhybrins A, B and C, bersaldegenin-1-acetate, bersaldegenin-3-acetate | Cytotoxic activity of bersaldegenin derivatives against human breast MCF-7 cancer cell line, human lung carcinoma NCI-H460 and glioblastoma SF-268 cell line | [71] |
| BD | Roots | Kalandaigremosides A-H | nd | [116] |
| BP | Whole plant | Bryophyllin A and B, bersaldegenin-3-acetate | Cytotoxic effect against keratin-forming tumor KB cell line, adenocarcinomic human alveolar basal epithelial A-549 cell line and human ileocecal carcinoma HCT-8 cell line | [117] |
| BP, BD, BT | Leaves (BD, BP) and stems (BT) | BP, BT: bersaldegenin-1-acetate, bersaldegenin-3-acetate, bersaldegenin-1,3,5-orthoacetate, bryophyllin A. BD: Bersaldegenin-1,3,5-orthoacetate |
nd | [114] |
| BD | Leaves | Bersaldegenin-1,3,5-orthoacetate, daigremontianin | Insecticidal against silkworm larvae | [65] |
| BP | Leaves | Bersaldegenin-1-acetate, bersaldegenin-3-acetate, bersaldegenin-1,3,5-orthoacetate, bryophyllin A | nd | [85] |
| BD, BP | Leaves | BD: Bersaldegenin-1-acetate, bersaldegenin-2-acetate, bersaldegenin-1,3,5-orthoacetate, bryophyllin A, daigremontianin. BP: Bersaldegenin-1-acetate, bersaldegenin-2-acetate, bersaldegenin-3-acetate, bersaldegenin-4-acetate, bersaldegenin-5-acetate, bersaldegenin-1,3,5-orthoacetate, bryophyllin A |
Cytotoxic activity against human ovarian cancer SKOV-3 cell line, cervical adenocarcinoma HeLa S3 cell line and malignant melanoma A375 cell line. Antimicrobial activity against Corynebacterium diphtheriae, Staphylococcus aureus, Staphylococcus epidermidis, and Enterococcus hirae |
[77][118] |
| BT | Whole plant | Kalantubosides A and B, bryophyllin A, bersaldegenin-1-acetate, bersaldegenin-1,3,5-orthoacetate | Cytotoxic effect against adenocarcinomic human alveolar basal epithelial A-549 cell line, promyelocytic leukemia HL-60 cell line, oral adenosquamous carcinoma Cal-27 cell line, and melanoma A2058 cell line | [119] |
1 BD: B. daigremontianum; BH: B. × houghtonii; BP: B. pinnatum; and BT: B. tubiflorum. 2 nd: not determined.
As cardiac glycosides, the original bioactivity attributed to bufadienolides is their cardiotonic activity, acting as inhibitors of the sodium pump at the myocardial tissue [120]. However, its reduced therapeutic window conditions its efficacy, allowing eventual cardiotoxic events due to overdosage [121]. In fact, the accidental consumption of Bryophyllum species by different mammals is one of the leading causes of cattle mortality in Africa [122], with reporting episodes of stroke, subendocardial hemorrhages, and heart tissue necrosis [123]. The biosynthesis of bufadienolides is a plant defensive mechanism against insect and herbivore attacks. They have already been reported as effective insecticidal compounds [31].
Bufadienolides have also been described as potent anticancer agents, as demonstrated by a number of in vitro studies with multiple cancer cell lines (Table 4) [124]. Nevertheless, their inherent toxicity difficult their administration in animal and human models [125]. Current research on these compounds is focused on finding effective and safer semi-synthetic derivatives [126].
Table 4 shows the associated bioactivities of identified bufadienolides in Bryophyllum sp., with a special focus on the cytotoxic activity of these compounds, being effective against relevant cancer cell lines, mainly those derived from breast, ovarian and lung carcinomas [71][115].
The bioactivity of phenolic and bufadienolides compounds reveals an unexploited phytochemical potential associated with Bryophyllum sp. However, research on these secondary metabolites is still very limited, since their concentration and activity depend on adaptive responses of plants, which is why low-yield extraction protocols have been reported [61][123]. Consequently, in order to explore the phytochemical properties of these medicinal plants, the establishment of efficient biotechnological approaches is required to achieve the valorization of Bryophyllum subgenus.
3. Plant Tissue Culture for Sustainable Valorization of Bioactive Compounds of Bryophyllum sp.
Currently, medicinal plants represent the source of more than 25% of drugs officially approved by the Food and Drug Administration (FDA) and the European Medicinal Agency (EMA) for the development of novel synthetic drugs [127]. Their derived by-products account for the 75–90% of the total used in the primary healthcare systems of economically developed nations [128]. However, only 6% of the plants have been studied from the pharmacological point of view and for 85% of them their phytochemical potential has not been evaluated [129], which represents a vast territory of families of plants with medicinal properties unexplored, such as Bryophyllum sp. Novel strategies, based on plant biotechnology methodologies, are required to meet the growing global demand for products derived from medicinal plants for industrial purposes in different sectors, such as the food, cosmetic, and pharmaceutical industries [130].
Since then, plant biotechnology has constantly evolved, and it currently provides a reliable methodology for the bioproduction of secondary metabolites with pharmacological value, by using plant in vitro systems [131]. Consequently, plant tissue culture (PTC) became a basic biotechnological methodology with countless applications in different areas of knowledge [132]. However, PTC must face its own limitations, as it involves a set of highly specialized, usually expensive, techniques that are extremely sensitive to multiple factors [133]. In this section, we will provide a deeper insight about the key aspects of PTC, with particular focus on the methodology applied to Bryophyllum sp. (Figure 3).
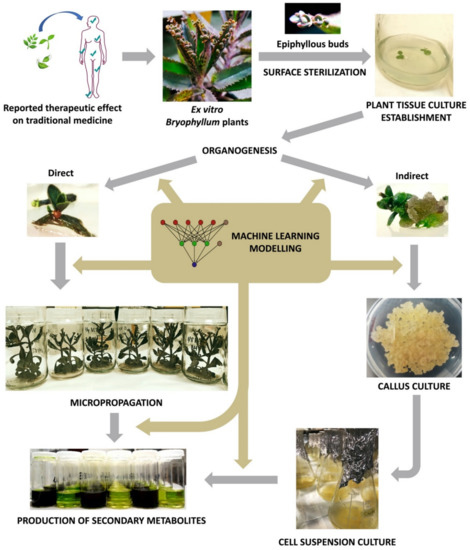
Figure 3. Workflow diagram of Bryophyllum sp. valorization via plant tissue culture (PTC).
3.1. PTC Establishment
The first step of a PTC protocol is the effective removal of pathogenic contaminants from the selected plant material (Figure 3) [134]. Therefore, the sterilization of the explant surface is required through a procedure that ensures convenient disinfection while maintaining its integrity, along with aseptic handling in laminar flow cabinets [135].
In particular, our research group has developed a simple and reliable method for the disinfection of epiphyllous buds of the adult plants of B. daigremontianum, B. × houghtonii and B. tubiflorum species grown in greenhouse, which involves the use of common, safe and environmental-friendly disinfectant agents [62][64][80][101][136]. The protocol includes an initial tap water wash of the buds overnight, followed by a two-step stage, where the buds are rinsed in 70% ethanol (v/v) for 1 min, washed with sterile distilled water and then rinsed in 0.4% (v/v) sodium hypochlorite with a few drops of Tween®-20 for 10 min. Finally, buds are gently washed with sterile distilled water and dried to remove persistent residues of disinfection agents. After the establishment under aseptic conditions, disinfected buds are placed and cultured in growth chambers under controlled conditions of photoperiod and temperature, thus enabling an adequate culture development. This procedure represents an improvement on the previously established disinfection protocols for Bryophyllum sp., in which slower procedures were performed [137] with more concentrated disinfectants [138]; with greater losses of viability [139] and explants integrity [140], or with more polluting agents, such as mercury chloride [141].
3.1.1. Plant Culture Media Composition
Plant culture media composition plays a crucial role in the success of PTC protocols, as the nutrition of cultured plant materials depends directly on its ingredients [142]. As a general rule, plant culture medium formulations contain a series of inorganic nutrients, divided into macro- and micronutrients according to their requirements for plant physiology, along with organic nutrients, such as vitamins [143]. Among the countless culture media formulations defined in PTC protocols, the formulation described by Murashige and Skoog in 1962 [144], mostly known as MS medium, is considered the universal medium to be applied as standard for different plant biotechnological applications [145]. The universality of the MS medium is based on its high levels of nitrogen sources, with a relatively high ratio of ammonium to nitrate [146]. However, it has recently been pointed out that the composition of the MS medium is supra-optimal for some species and therefore harmful due to an excessive concentration of ammonium ions [147][148].
Bryophyllum sp. are especially affected by excess of ammonium ion. This cation negatively affects the growth of these species, due to a deterioration of CAM photosynthetic efficiency [149][150]. Although PTC of Bryophyllum sp. has been established using MS medium [137][138], better growth and multiplication rates were achieved when the composition of the MS medium was modified, as it has been shown by reducing the concentration of macronutrients by half for B. daigremontianum, B. × houghtonii and B. tubiflorum [64][80][101].
3.2. Organogenesis and Plant Regeneration
Thus far, the information on the plant regeneration protocols for Bryophyllum sp. is limited. Most publications focus on the establishment of indirect regeneration protocols [137][138][139][140][151]. Recently, we have provided information on the effect of exogenous application of plant growth regulators (PGRs) on the in vitro organogenesis of B. daigremontianum, B. × houghtonii and B. tubiflorum [137], pointing at the concentration of the cytokinin 6-benzylaminopurine (BAP) as the most critical factor guiding this process. Specifically, it was demonstrated that at operational concentrations of BAP (0.375–0.75 mg L−1) both B. daigremontianum and B. × houghtonii present a higher frequency of direct shoot regeneration than B. tubiflorum. In turn, B. tubiflorum was revealed as the most efficient species for the induction of callus formation during indirect organogenesis [137]. These results highlight the complexity of the design of plant in vitro regeneration protocols and shed light into the organogenesis-related processes of Bryophyllum sp., facing to the large-scale exploitation of these medicinal plants.
3.3. Micropropagation
After the establishment of axenic cultures, PTC protocols normally are followed by multiplication, rooting and acclimatization stages, throughout the procedure called micropropagation (Figure 3), with the objective of achieving a large number of fully-developed true-to-type individuals [134]. The singular asexual reproduction that takes place at leaf margins of Bryophyllum, results in the clonal propagation of fully-developed epiphyllous buds, presenting individual aerial and root systems [11][12]. For this reason, Bryophyllum constitutes an outstanding subgenus for the micropropagation of different species. Nevertheless, the micropropagation of Bryophyllum is not exempt from difficulties due to its particular metabolism and poor nutritional requirements [150]. In this sense, it was recently reported that ammonium, sulfur, molybdenum, copper, and sodium play a crucial role on growth and plantlet formation on in vitro-cultured Bryophyllum in a species-dependent manner [152]. Therefore, multiple nutritional modifications may be required to achieve genotype-specific optimization, since mineral imbalances and interactions could directly influence the success of PTC protocols, by affecting micropropagation performance [153], and causing undesirable physiological disorders [147].
3.4. Establishment of Plant Suspension-Cultured Cells (PSCCs)
In the last decades, an increasing interest of plant biotechnology has been addressed to the evaluation and valorization of medicinal plants, with the aim of exploring their phytochemical potential and making it accessible to industrial applications [154]. In order to maximize the advantages of PTC for the production of secondary metabolites, plant suspension-cultured cells (PSCCs) emerged as a valuable biotechnological platform [155].
A single recent report is available for the establishment of PSCCs from B. × houghtonii [105]. In this work, the use of PSCCs from B. × houghtonii for the production of bioactive compounds was reported, with a special focus on the operational aspects required for the establishment of plant cell cultures, such as the determination of growth kinetics [105]. The typical four-phase growth behavior was reported only after 8 days of culture, starting with an initial lag phase where cells acclimatize to new culture conditions and no growth is observed. The lag phase is followed by the exponential phase, where cell divisions occur massively, reported by a severe increase in cell biomass. Afterward, a stationary phase is reached: cell growth stabilizes and the accumulation of secondary metabolites is observed, before reaching death phase, in which cell death takes place due to a lack of nutrients [156].
PSCCs are considered a valuable biological platform for the application of several approaches to enhance plant secondary metabolism, which have been widely exploited in the field of plant biotechnology for the production of bioactive compounds: elicitation, precursor feeding, two-phase culture system, and metabolic engineering [157]. Among them, elicitation is the most extended approach applied to PSCCs, although it can be applied to many other culture types [158]. Due to the importance of elicitation on the evaluation of medicinal plants and their phytochemical potential, the next section will be focused on this phenomenon, with a particular focus on the elicitation of Bryophyllum sp.
3.5. Enhancement of Phenolic Compounds Production from Bryophyllum sp. via Elicitation
In the last years, great efforts regarding the improvement of plant secondary metabolism have been made in the field of plant biotechnology, being the elicitation of PSCCs one of the most successful approaches applied for the large-scale production of plant bioactive compounds [159]. A review of the literature shows that the number of publications selected by Google Scholar® from the search “elicitation of plant cell culture” is close to 15,000 entries in the last five years.
The term elicitation, as recently defined by Narayani and Srivastava (2017) [160], refers to “the manipulation of biochemical and metabolic pathways, via stress induction, that can be implemented for enhancing secondary metabolite production and characterize the role of stress factors on plants using plant cell and/or tissue cultures as model systems”. On this basis, different types of culture may constitute precious biological platforms for the stimulation of plant secondary metabolism under controlled conditions, by the administration of elicitors (Figure 3). In all cases, obtaining the maximum viability and integrity of the elicited cultures is required in order to achieve an efficient and sustainable production system [161].
Little information about the elicited production of bioactive compounds by Bryophyllum sp. can be found in the literature. Recently, the elicitation of phenolic compounds from in vitro-cultured Bryophyllum sp. subjected to nutritional stress has been reported by García-Pérez and co-workers (2020) [62]. It was found that a decrease in the ammonium concentration in the culture medium causes a 50% overproduction and accumulation of phenolic compounds in the aerial parts of B. × houghtonii. The effect was less in magnitude in B. daigremontianum and B. tubiflorum [62]. In addition, the antioxidant efficiency of the derived Bryophyllum extracts was assessed in terms of their free-radical scavenging activity and lipid peroxidation inhibition [62][64], suggesting that in vitro-cultured B. × houghtonii can be considered a medicinal species with an improved phytochemical potential [80], in comparison to closely-related species, such as B. daigremontianum and B. tubiflorum. In this sense, due to its phytochemical potential, PSCCs from B. × houghtonii were subjected to elicitation by cyclodextrins (CDs) [105]. CDs are cyclic oligosaccharides able of forming inclusion complexes with hydrophobic molecules. The results suggested that CDs elicited the production of phenolic compounds in Bryophyllum PSCCs, as well as their associated free-radical scavenging activity. Specifically, it was shown that CDs favored the accumulation of total phenols and flavonoids in the culture medium (7.9 and 17.3-fold increases, respectively) after 7 days of culture, thus, preserving the integrity of the cellular fraction for subsequent elicitation cycles [105].
Altogether, the application of novel approaches should be developed in order to reveal the full phytochemical potential of Bryophyllum sp., based on the application of unexploited PTC strategies, taking benefit of the countless advantages provided by PSCCs, committed to the enhancement of plant secondary metabolite production.
4. Machine Learning for Optimizing the Biotechnological Valorization of Bryophyllum sp.
Along with this review, we provided evidence about the multifactorial behavior of PTC methodologies and the production of secondary metabolites (Figure 3). Therefore, the elucidation and characterization of such phenomena may require the development of complex experimental designs, to reveal relevant interactions between factors, which are not feasible due to cost and time. Furthermore, the analysis and interpretation of these complex experimental designs is difficult and, in many cases, limited or incomplete [162].
Machine Learning (ML) techniques stand out as a cutting-edge alternative to detect the critical factors behind a certain procedure, as well as a method to establish the influence of possible interactions between them [163]. The application of ML algorithms allows the modeling of complex processes, a powerful tool for making decisions and studying unknown phenomena [164]. Among the different ML tools, the combination of artificial neural networks with fuzzy logic, commonly known as neuro-fuzzy logic (NFL), constitutes a robust computational tool for the optimization and prediction of complex processes [165]. Furthermore, NFL offers another advantage regarding the efficacy of predictive models, thus providing direct knowledge from a detailed interpretation of the results, by the establishment of simple “IF-THEN” rules, that facilitate making conclusions [166].
Concerning Bryophyllum sp., the application of NFL was already applied to the identification of critical factors involved in plant in vitro nutrition [152] and organogenesis [136], as well as the production of phenolic compounds [62].
In this sense, ML was able to identify the key mineral nutrients and their interactions, in order to optimize the growth and reproduction of Bryophyllum sp. cultured in vitro. Among the 18 different mineral nutrients used on MS formulation, ML detected that only five nutrients were critical on Bryophyllum in vitro culture, in a genotype-dependent manner [152]. Specifically, ammonium, sulfate, sodium, molybdenum, and copper were selected by NFL as the critical factors guiding several growth-related parameters, and the interaction between sulfate and molybdenum was widely reported as responsible for most parameters: root length, plantlet formation, and aerial parts fresh weight [152].
ML was also employed for the modeling and predicting of Bryophyllum organogenesis in vitro [136]. BAP concentration was assessed as the critical factor guiding this phenomenon on B. daigremontianum, B. × houghtonii and B. tubiflorum; thus, predicting a minimal BAP concentration required for the development of different organogenetic responses (0.35 mg L−1). On the contrary, the application of auxins, such as indoleacetic acid (IAA), was outlined as an inhibitory factor on the indirect shoot regeneration on B. tubiflorum, whereas no IAA influence was reported on B. daigremontianum and B. × houghtonii [136].
Additionally, the production of phenolic compounds by in vitro-cultured Bryophyllum sp. was optimized using ML [62]. It was observed that phenolic compounds accumulation achieved the maximum concentrations in the aerial parts of cultured plants under low ammonium concentrations (<15 mM). Moreover, the extraction of total phenolic compounds was enhanced by the use of 55–85% aqueous methanol, whereas flavonoids were mostly extracted with higher methanol concentrations in water (>85%). In addition, the antioxidant potential of Bryophyllum extracts, in terms of radical-scavenging activity, was shown to be improved using 55–85% MeOH as solvent on B. × houghtonii cultured under low ammonium concentrations [62]. Furthermore, these experimental conditions for maximizing the antioxidant activity of B. × houghtonii were also validated in terms of preventing lipid oxidation [64]and plant in vitro growth [152]; thus, assessing the effectiveness of ML on the valorization of Bryophyllum.
References
- Adanson, M. Familles des Plantes par M. Adanson; chez Vincent: Paris, France, 1763. [Google Scholar]
- Akulova-Barlow, Z. Kalanchoe. Cactus Succul. J. 2009, 81, 268–276. [Google Scholar] [CrossRef]
- Smith, G.; Volmer, P.A. Kalanchoe species poisoning in pets. Vet. Med. 2004, 99, 933–936. [Google Scholar]
- Salisbury, R.A. Crassulaceae Bryophyllum salisb. Parad. Londinensis 1805, 1, 3. [Google Scholar]
- Baker, J.G. Notes on a Collection of Flowering Plants made by L. Kitching, Esq., in Madagascar in 1879. Bot. J. Linn. Soc. 1881, 18, 264–281. [Google Scholar] [CrossRef]
- Chernetskyy, M.A. The role of morpho-anatomical traits of the leaves in the taxonomy of Kalanchoideae Berg. subfamily (Crassulaceae DC.). Mod. Phytomorphology 2012, 1, 15–18. [Google Scholar]
- Gehrig, H.H.; Rösicke, H.; Kluge, M. Detection of DNA polymorphisms in the genus Kalanchoe by RAPD-PCR fingerprint and its relationships to infrageneric taxonomic position and ecophysiological photosynthetic behaviour of the species. Plant Sci. 1997, 125, 41–51. [Google Scholar] [CrossRef]
- Descoings, B. Le genre Kalanchoe structure et définition par Bernard Descoings. Le J. Bot. 2006, 33, 3–28. [Google Scholar]
- Hamburger, M.; Potterat, O.; Fürer, K.; Simões-Wüst, A.P.; Von Mandach, U. Bryophyllum pinnatum - Reverse engineering of an anthroposophic herbal medicine. Nat. Prod. Commun. 2017, 12, 1359–1364. [Google Scholar] [CrossRef]
- Cushman, J.C. Crassulacean acid metabolism: Recent advances and future opportunities. Funct. Plant Biol. 2005, 32, 375–380. [Google Scholar] [CrossRef]
- Garcês, H.M.P.; Koenig, D.; Townsley, B.T.; Kim, M.; Sinha, N.R. Truncation of LEAFY COTYLEDON1 protein is required for asexual reproduction in Kalanchoë daigremontiana. Plant Physiol. 2014, 165, 196–206. [Google Scholar] [CrossRef]
- Garcês, H.; Sinha, N. The “Mother of Thousands” (Kalanchoë daigremontiana): A plant model for asexual reproduction and CAM studies. Cold Spring Harb. Protoc. 2009, 4, 1–9. [Google Scholar]
- García-Pérez, P.; Barreal, M.E.; Rojo-De Dios, L.; Cameselle-Teijeiro, J.F.; Gallego, P.P. Bioactive natural products from the genus Kalanchoe as cancer chemopreventive agents: A review. In Studies in Natural Products Chemistry; Rahman, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 61, pp. 49–84. ISBN 9780444641830. [Google Scholar]
- Boxall, S.F.; Kadu, N.; Dever, L.V.; Knerová, J.; Waller, J.L.; Gould, P.J.D.; Hartwell, J. Kalanchoë PPC1 is essential for crassulacean acid metabolism and the regulation of core circadian clock and guard cell signaling genes. Plant Cell 2020, 32, 1136–1160. [Google Scholar] [CrossRef] [PubMed]
- Gehrig, H.; Gaußmann, O.; Marx, H.; Schwarzott, D.; Kluge, M. Molecular phylogeny of the genus Kalanchoe (Crassulaceae) inferred from nucleotide sequences of the ITS-1 and ITS-2 regions. Plant Sci. 2001, 160, 827–835. [Google Scholar] [CrossRef]
- Kulka, R.G. Cytokinins inhibit epiphyllous plantlet development on leaves of Bryophyllum (Kalanchoë) marnierianum. J. Exp. Bot. 2006, 57, 4089–4098. [Google Scholar] [CrossRef]
- Garcês, H.M.P.; Champagne, C.E.M.; Townsley, B.T.; Park, S.; Malhó, R.; Pedroso, M.C.; Harada, J.J.; Sinha, N.R. Evolution of asexual reproduction in leaves of the genus Kalanchoë. Proc. Natl. Acad. Sci. USA 2007, 104, 15578–15583. [Google Scholar]
- Rodriguez, B.K. Daigremontiana as a Model Plant for the Study of Auxin Effects in Plant Morphology. J. Plant Biochem. Physiol. 2014, 02, 1–3. [Google Scholar] [CrossRef]
- Pasternak, T.; Dudits, D. Epigenetic clues to better understanding of the asexual embryogenesis In planta and In vitro. Front. Plant Sci. 2019, 10, 1–5. [Google Scholar] [CrossRef]
- Zhong, T.; Zhu, C.; Zeng, H.; Han, L. Analysis of gene expression in Kalanchoe daigremontiana leaves during plantlet formation under drought stress. Electron. J. Biotechnol. 2013, 16. [Google Scholar]
- Kulka, R.G. Hormonal control of root development on epiphyllous plantlets of Bryophyllum (Kalanchoë) marnierianum: Role of auxin and ethylene. J. Exp. Bot. 2008, 59, 2361–2370. [Google Scholar] [CrossRef]
- Herrera, I.; Nassar, J.M. Reproductive and recruitment traits as indicators of the invasive potential of Kalanchoe daigremontiana (Crassulaceae) and Stapelia gigantea (Apocynaceae) in a Neotropical arid zone. J. Arid Environ. 2009, 73, 978–986. [Google Scholar] [CrossRef]
- Herrando-Moraira, S.; Vitales, D.; Nualart, N.; Gómez-Bellver, C.; Ibáñez, N.; Massó, S.; Cachón-Ferrero, P.; González-Gutiérrez, P.A.; Guillot, D.; Herrera, I.; et al. Global distribution patterns and niche modelling of the invasive Kalanchoe × houghtonii (Crassulaceae). Sci. Rep. 2020, 10, 3143. [Google Scholar] [CrossRef]
- Kolodziejczyk-Czepas, J.; Nowak, P.; Wachowicz, B.; Piechocka, J.; Głowacki, R.; Moniuszko-Szajwaj, B.; Stochmal, A. Antioxidant efficacy of Kalanchoe daigremontiana bufadienolide-rich fraction in blood plasma in vitro. Pharm. Biol. 2016, 54, 3182–3188. [Google Scholar] [CrossRef] [PubMed]
- Ojewole, J.A.O. Antinociceptive, anti-inflammatory and antidiabetic effects of Bryophyllum pinnatum (Crassulaceae) leaf aqueous extract. J. Ethnopharmacol. 2005, 99, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, A.; Saluja, A.K. Bryophyllum pinnatum (Lam.) Kurz.: Phytochemical and pharmacological profile: A review. Pharmacogn. Rev. 2009, 3, 364–374. [Google Scholar]
- Akinpelu, D.A. Antimicrobial activity of Bryophyllum pinnatum leaves. Fitoterapia 2000, 71, 193–194. [Google Scholar] [CrossRef]
- Mawla, F.; Khatoon, S.; Rehana, F.; Jahan, S.; Moshiur, M.R.; Hossain, S.; Haq, W.M.; Rahman, S.; Debnath, K.; Rahmatullah, M. Ethnomedicinal plants of folk medicinal practitioners in four villages of natore and rajshahi districts, bangladesh. Am. J. Sustain. Agric. 2012, 6, 406–416. [Google Scholar]
- Hsieh, Y.J.; Yang, M.Y.; Leu, Y.L.; Chen, C.; Wan, C.F.; Chang, M.Y.; Chang, C.J. Kalanchoe tubiflora extract inhibits cell proliferation by affecting the mitotic apparatus. BMC Complement. Altern. Med. 2012, 12. [Google Scholar] [CrossRef]
- Abebe, W. An Overview of Ethiopian Traditional Medicinal Plants Used for Cancer Treatment. European J. Med. Plants 2016, 14, 1–16. [Google Scholar] [CrossRef]
- Supratman, U.; Fujita, T.; Akiyama, K.; Hayashi, H.; Murakami, A.; Sakai, H.; Koshimizu, K.; Ohigashi, H. Anti-tumor promoting activity of bufadienolides from Kalanchoe pinnata and K. daigremontiana × tubiflora. Biosci. Biotechnol. Biochem. 2001, 65, 947–949. [Google Scholar] [CrossRef]
- Nguelefack, T.B.; Nana, P.; Atsamo, A.D.; Dimo, T.; Watcho, P.; Dongmo, A.B.; Tapondjou, L.A.; Njamen, D.; Wansi, S.L.; Kamanyi, A. Analgesic and anticonvulsant effects of extracts from the leaves of Kalanchoe crenata (Andrews) Haworth (Crassulaceae). J. Ethnopharmacol. 2006, 106, 70–75. [Google Scholar] [CrossRef]
- Kamgang, R.; Youmbi Mboumi, R.; Foyet Fondjo, A.; Fokam Tagne, M.A.; Mengue N’dillé, G.P.R.; Ngogang Yonkeu, J. Antihyperglycaemic potential of the water-ethanol extract of Kalanchoe crenata (Crassulaceae). J. Nat. Med. 2008, 62, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Akinsulire, O.R.; Aibinu, I.E.; Adenipekun, T.; Adelowotan, T.; Odugbemi, T. In vitro antimicrobial activity of crude extracts from plants Bryophyllum pinnatum and Kalanchoe crenata. African J. Tradit. Complement. Altern. Med. 2007, 4, 338–344. [Google Scholar] [CrossRef]
- Malan, D.F.; Neuba, D.F.R. Traditional practices and medicinal plants use during pregnancy by Anyi-Ndenye women (Eastern Côte d’Ivoire). Afr. J. Reprod. Health 2011, 15, 85–93. [Google Scholar] [PubMed]
- Süsskind, M.; Thürmann, P.A.; Lüke, C.; Jeschke, E.; Tabali, M.; Matthes, H.; Ostermann, T. Adverse drug reactions in a complementary medicine hospital: A prospective, intensified surveillance study. Evidence-based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Richwagen, N.; Lyles, J.T.; Dale, B.L.F.; Quave, C.L. Antibacterial activity of Kalanchoe mortagei and K. fedtschenkoi against ESKAPE pathogens. Front. Pharmacol. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cumberbatch, A. An Ethonobotanical Survey of Medicinal Plant Usage in Salvador de Bahia, Brazil. CGI Gr. 2011. Available online: https://radar.auctr.edu/islandora/object/sc.gstem%3A2011_cumberbatch_ashli (accessed on 21 October 2020).
- Costa, S.S.; Muzitano, M.F.; Camargo, L.M.M.; Coutinho, M.A.S. Therapeutic potential of Kalanchoe species: Flavonoids and other secondary metabolites. Nat. Prod. Commun. 2008, 3, 2151–2164. [Google Scholar] [CrossRef]
- Suárez, F.S.; Ramirez, A.M.; López-Marure, R.; Gutiérrez, R.M.P. In vitro cytotoxic potential and apoptotic activity of bufadienolide-rich fraction from leaves of Kalanchoe mortagei against human HeLa cancer cells. Int. J. Ayurvedic Med. 2018, 9, 25–33. [Google Scholar]
- Vera-Marin, B.; Sánchez-Sáen, M. Plantas medicinales y predictibilidad de uso en algunas veredas del corregimiento de San Cristóbal (Antioquia), Colombia. Actual. Biológicas 2016, 38, 167–180. [Google Scholar]
- Herawati, M.H.; Husin, N. Berbagai jenis tumbuhan yang berkhasiat sebagai obat kecacingan. Media Penelit. dan Pengemb. Kesehat. 2000, 10. Available online: isis://www.neliti.com/publications/158068/berbagai-jenis-tumbuhan-yang-berkhasiat-sebagai-obat-kecacingan (accessed on 21 October 2020).
- Rajsekhar, P.B.; Arvind Bharani, R.S.; Ramachandran, M.; Jini Angel, K.; Rajsekhar, S.P.V. The “wonder plant” Kalanchoe pinnata (linn.) pers.: A review. J. Appl. Pharm. Sci. 2016, 6, 151–158. [Google Scholar] [CrossRef]
- Rahmatullah, M.; Mollik, M.A.H.; Ali, M.; Abbas, M.F.B.; Jahan, R.; Khatun, A.; Seraj, S.; Ahsan, S. An ethnomedicinal survey of vitbilia village in sujanagar sub-district of pabna district, Bangladesh. Am. J. Sustain. Agric. 2010, 4, 302–308. [Google Scholar]
- Lans, C.A. Ethnomedicines used in Trinidad and Tobago for urinary problems and diabetes mellitus. J. Ethnobiol. Ethnomed. 2006, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, R.; Saha, M.R.; Rahman, M.A.; Islam, M.A.U. Ethnomedicinal Survey of Plants in the Southern District Noakhali, Bangladesh. Bangladesh Pharm. J. 2015, 17, 205–214. [Google Scholar] [CrossRef]
- Fernandes, J.M.; Cunha, L.M.; Azevedo, E.P.; Lourenço, E.M.G.; Fernandes-Pedrosa, M.F.; Zucolotto, S.M. Kalanchoe laciniata and Bryophyllum pinnatum: An updated review about ethnopharmacology, phytochemistry, pharmacology and toxicology. Rev. Bras. Farmacogn. 2019, 29, 529–558. [Google Scholar] [CrossRef]
- Sen, P.; Dollo, M.; Choudhury, M.D.; Choudhury, D. Documentation of traditional herbal knowledge of Khamptis of Arunachal Pradesh. Indian J. Tradit. Knowl. 2008, 7, 438–442. [Google Scholar]
- Khan, M.A.; Islam, M.K.; Siraj, M.A.; Saha, S.; Barman, A.K.; Awang, K.; Rahman, M.M.; Shilpi, J.A.; Jahan, R.; Islam, E.; et al. Ethnomedicinal survey of various communities residing in Garo Hills of Durgapur, Bangladesh. J. Ethnobiol. Ethnomed. 2015, 11. [Google Scholar] [CrossRef]
- Okwu, D.E.; Nnamdi, F.U. Two novel flavonoids from Bryophyllum pinnatum and their antimicrobial activity. J. Chem. Pharm. Res. 2011, 3, 1–10. [Google Scholar]
- Budi, V.; Sihotang, L. Ethnomedicinal study of the Sundanese people at the Bodogol area, Gede Pangrango Mountain National Park, West Java. Gard. Bull. Singapore 2011, 63, 527–534. [Google Scholar]
- Namukobe, J.; Kasenene, J.M.; Kiremire, B.T.; Byamukama, R.; Kamatenesi-Mugisha, M.; Krief, S.; Dumontet, V.; Kabasa, J.D. Traditional plants used for medicinal purposes by local communities around the Northern sector of Kibale National Park, Uganda. J. Ethnopharmacol. 2011, 136, 236–245. [Google Scholar] [CrossRef]
- Lai, Z.R.; Peng, W.H.; Ho, Y.L.; Huang, S.C.; Huang, T.H.; Lai, S.C.; Ku, Y.R.; Tsai, J.C.; Wang, C.Y.; Chang, Y.S. Analgesic and anti-inflammatory activities of the methanol extract of Kalanchoe gracilis (L.) DC stem in mice. Am. J. Chin. Med. 2010, 38, 529–546. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Huang, S.C.; Zhang, Y.; Lai, Z.R.; Kung, S.H.; Chang, Y.S.; Lin, C.W. Antiviral ability of Kalanchoe gracilis leaf extract against Enterovirus 71 and coxsackievirus A16. Evidence-based Complement. Altern. Med. 2012, 2012, 503165. [Google Scholar]
- Milad, R. Genus Kalanchoe (Crassulaceae): A review of its ethnomedicinal, botanical, chemical and pharmacological properties. European J. Med. Plants 2014, 4, 86–104. [Google Scholar] [CrossRef]
- Al-Snafi, A. The chemical constituents and pharmacological effects of Bryophyllum calycinum. A review. Int. J. Pharma Sci. Res. 2013, 4, 171–176. [Google Scholar]
- De Araújo, E.R.D.; Félix-Silva, J.; Xavier-Santos, J.B.; Fernandes, J.M.; Guerra, G.C.B.; de Araújo, A.A.; Araújo, D.F.d.S.; de Santis Ferreira, L.; da Silva Júnior, A.A.; Fernandes-Pedrosa, M.d.F.; et al. Local anti-inflammatory activity: Topical formulation containing Kalanchoe brasiliensis and Kalanchoe pinnata leaf aqueous extract. Biomed. Pharmacother. 2019, 113, 108721. [Google Scholar]
- Huang, H.C.; Huang, G.J.; Liaw, C.C.; Yang, C.S.; Yang, C.P.; Kuo, C.L.; Tseng, Y.H.; Wang, S.Y.; Chang, W.T.; Kuo, Y.H. A new megastigmane from Kalanchoe tubiflora (Harvey) Hamet. Phytochem. Lett. 2013, 6, 379–382. [Google Scholar] [CrossRef]
- Anisimov, M.M.; Gerasimenko, N.I.; Chaikina, E.L.; Serebryakov, Y.M. Biological activity of metabolites of the herb Kalanchoe daigremontiana (Hamet de la Bathie) Jacobs et Perr. Biol. Bull. 2009, 36, 568–574. [Google Scholar] [CrossRef]
- Ürményi, F.G.G.; Saraiva, G.d.N.; Casanova, L.M.; Matos, A.d.S.; de Magalhães Camargo, L.M.; Romanos, M.T.V.; Costa, S.S. Anti-HSV-1 and HSV-2 Flavonoids and a New Kaempferol Triglycoside from the Medicinal Plant Kalanchoe daigremontiana. Chem. Biodivers. 2016, 13, 1707–1714. [Google Scholar] [CrossRef]
- Mahata, S.; Maru, S.; Shukla, S.; Pandey, A.; Mugesh, G.; Das, B.C.; Bharti, A.C. Anticancer property of Bryophyllum pinnata (Lam.) Oken. leaf on human cervical cancer cells. BMC Complement. Altern. Med. 2012, 12, 15. [Google Scholar] [CrossRef]
- García-Pérez, P.; Lozano-Milo, E.; Landín, M.; Gallego, P.P. Combining medicinal plant in vitro culture with machine learning technologies for maximizing the production of phenolic compounds. Antioxidants 2020, 9, 210. [Google Scholar] [CrossRef]
- Bogucka-Kocka, A.; Zidorn, C.; Kasprzycka, M.; Szymczak, G.; Szewczyk, K. Phenolic acid content, antioxidant and cytotoxic activities of four Kalanchoë species. Saudi J. Biol. Sci. 2016, 25, 622–630. [Google Scholar] [CrossRef] [PubMed]
- García-Pérez, P.; Losada-Barreiro, S.; Bravo-Díaz, C.; Gallego, P.P. Exploring the use of Bryophyllum as natural source of bioactive compounds with antioxidant activity to prevent lipid oxidation of fish oil-in-water emulsions. Plants 2020, 9, 1012. [Google Scholar] [CrossRef] [PubMed]
- Maharani, R.; Fajriah, S.; Hardiawan, R.; Supratman, U. Insecticidal bufadienolides from the leaves of Kalanchoe daigremontiana (Crassulaceae). Proceeding Int. Semin. Chem. 2008, 11, 236–239. [Google Scholar]
- Supratman, U.; Fujita, T.; Akiyama, K.; Hayash, H. New insecticidal bufadienolide, bryophyllin C, from Kalanchoe pinnata. Biosci. Biotechnol. Biochem. 2000, 64, 1310–1312. [Google Scholar] [CrossRef]
- Supratman, U.; Fujita, T.; Akiyama, K.; Hayashi, H. Insecticidal compounds from Kalanchoe daigremontiana x tubiflora. Phytochemistry 2001, 58, 311–314. [Google Scholar] [CrossRef]
- Kolodziejczyk-Czepas, J.; Stochmal, A. Bufadienolides of Kalanchoe species: An overview of chemical structure, biological activity and prospects for pharmacological use. Phytochem. Rev. 2017, 16, 1155–1171. [Google Scholar] [CrossRef]
- Bopda, O.S.M.; Longo, F.; Bella, T.N.; Edzah, P.M.O.; Taïwe, G.S.; Bilanda, D.C.; Tom, E.N.L.; Kamtchouing, P.; Dimo, T. Antihypertensive activities of the aqueous extract of Kalanchoe pinnata (Crassulaceae) in high salt-loaded rats. J. Ethnopharmacol. 2014, 153, 400–407. [Google Scholar] [CrossRef]
- Kolodziejczyk-Czepas, J.; Sieradzka, M.; Moniuszko-Szajwaj, B.; Pecio, Ł.; Ponczek, M.B.; Nowak, P.; Stochmal, A. Bufadienolides from Kalanchoe daigremontiana as thrombin inhibitors—In vitro and in silico study. Int. J. Biol. Macromol. 2017, 99, 141–150. [Google Scholar] [CrossRef]
- Kuo, P.C.; Kuo, T.H.; Su, C.R.; Liou, M.J.; Wu, T.S. Cytotoxic principles and α-pyrone ring-opening derivatives of bufadienolides from Kalanchoe hybrida. Tetrahedron 2008, 64, 3392–3396. [Google Scholar] [CrossRef]
- Yadav, N.P.; Dixit, V.K. Hepatoprotective activity of leaves of Kalanchoe pinnata Pers. J. Ethnopharmacol. 2003, 86, 197–202. [Google Scholar] [CrossRef]
- Menon, N.; Sparks, J.; Omoruyi, F. Hypoglycemic and hypocholesterolemic activities of the aqueous preparation of Kalanchoe pinnata leaves in streptozotocin-induced diabetic rats. Asian Pac. J. Trop. Biomed. 2015, 5, 3–9. [Google Scholar] [CrossRef]
- Bartwal, A.; Mall, R.; Lohani, P.; Guru, S.K.; Arora, S. Role of Secondary Metabolites and Brassinosteroids in Plant Defense Against Environmental Stresses. J. Plant Growth Regul. 2013, 32, 216–232. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Fürer, K.; Simões-Wüst, A.P.; Von Mandach, U.; Hamburger, M.; Potterat, O. Bryophyllum pinnatum and related species used in anthroposophic medicine: Constituents, pharmacological activities, and clinical efficacy. Planta Med. 2016, 82, 930–941. [Google Scholar] [CrossRef]
- Stefanowicz-Hajduk, J.; Asztemborska, M.; Krauze-Baranowska, M.; Godlewska, S.; Gucwa, M.; Moniuszko-Szajwaj, B.; Stochmal, A.; Ochocka, J.R. Identification of flavonoids and bufadienolides and cytotoxic effects of Kalanchoe daigremontiana extracts on human cancer cell lines. Planta Med. 2020, 86, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Casanova, J.M.; dos Santos Nascimento, L.B.; Casanova, L.M.; Leal-Costa, M.V.; Costa, S.S.; Tavares, E.S. Differential distribution of flavonoids and phenolic acids in leaves of Kalanchoe delagoensis Ecklon & Zeyher (Crassulaceae). Microsc. Microanal. 2020, 1–8. [Google Scholar]
- Prasad, A.K.; Kumar, S.; Iyer, S.V.; Sudani, R.J.; Vaidya, S.K. Pharmacognostical, Phytochemical and Pharmacological Review on Bryophyllum pinnata. Int. J. Pharm. Biol. Arch. 2012, 3, 423–433. [Google Scholar]
- García-Pérez, P.; Losada-Barreiro, S.; Gallego, P.P.; Bravo-Díaz, C. Adsorption of gallic acid, propyl gallate and polyphenols from Bryophyllum extracts on activated carbon. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- García-Pérez, P.; Lozano-Milo, E.; Gallego, P.P.; Tojo, C.; Losada-Barreiro, S.; Bravo-Díaz, C. Plant antioxidants in food emulsions. In Some New Aspects of Colloidal Systems in Foods; Milani, J., Ed.; IntechOpen: Rijeka, Croatia, 2018; pp. 11–29. [Google Scholar]
- Chernetskyy, M.; Woźniak, A.; Skalska-Kamińska, A.; Żuraw, B.; Blicharska, E.; Rejdak, R.; Donica, H.; Weryszko-Chmielewska, E. Structure of leaves and phenolic acids in Kalanchoë daigremontiana Raym.-Hamet & H. Perrier. Acta Sci. Pol. Hortorum Cultus 2018, 17, 137–155. [Google Scholar]
- Bonache, M.A.; Moreno-Fernández, S.; Miguel, M.; Sabater-Muñoz, B.; González-Muñiz, R. Small Library of Triazolyl Polyphenols Correlating Antioxidant Activity and Stability with Number and Position of Hydroxyl Groups. ACS Comb. Sci. 2018, 20, 694–699. [Google Scholar] [CrossRef]
- Omojokun, O.S.; Oboh, G.; Ademiluyi, A.O.; Oladele, J.O.; Boligon, A.A. Impact of drying processes on Bryophyllum pinnatum phenolic constituents and its anti-inflammatory and antioxidative activities in human erythrocytes. J. Food Biochem. 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fürer, K.; Raith, M.; Brenneisen, R.; Mennet, M.; Simões-Wüst, A.P.; Von Mandach, U.; Hamburger, M.; Potterat, O. Two new flavonol glycosides and a metabolite profile of Bryophyllum pinnatum, a phytotherapeutic used in obstetrics and gynaecology. Planta Med. 2013, 79, 1565–1571. [Google Scholar] [CrossRef]
- El Abdellaoui, S.; Destandau, E.; Toribio, A.; Elfakir, C.; Lafosse, M.; Renimel, I.; André, P.; Cancellieri, P.; Landemarre, L. Bioactive molecules in Kalanchoe pinnata leaves: Extraction, purification, and identification. Anal. Bioanal. Chem. 2010, 398, 1329–1338. [Google Scholar] [CrossRef]
- Bä, W.; Dettner, K.; Pfeifer, P. Intra- and Interspecific Allelochemical Effects in Three Kalanchoe-Species (Crassulaceae). Zeitschrift fur Naturforsch. Sect. C J. Biosci. 1997, 52, 441–449. [Google Scholar]
- Katrucha, E.M.; Lopes, J.; Paim, M.; dos Santos, J.C.; Siebert, D.A.; Micke, G.A.; Vitali, L.; Alberton, M.D.; Tenfen, A. Phenolic profile by HPLC-ESI-MS/MS and enzymatic inhibitory effect of Bryophyllum delagoense. Nat. Prod. Res. 2020, 0, 1–4. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Ali, H.M.; Elshikh, M.S.; Abdel-Salam, E.M.; El-Esawi, M.; El-Ansary, D.O. Bioactivities of Traditional Medicinal Plants in Alexandria. Evidence-Based Complement. Altern. Med. 2018, 2018. [Google Scholar] [CrossRef]
- Özçelik, B.; Kartal, M.; Orhan, I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2011, 49, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Cueva, C.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Rivas, C.L.; Requena, T.; Rodríguez, J.M.; Bartolomé, B. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 2010, 161, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Mudnic, I.; Modun, D.; Rastija, V.; Vukovic, J.; Brizic, I.; Katalinic, V.; Kozina, B.; Medic-Saric, M.; Boban, M. Antioxidative and vasodilatory effects of phenolic acids in wine. Food Chem. 2010, 119, 1205–1210. [Google Scholar] [CrossRef]
- Gomes, C.A.; Girão Da Cruz, T.G.; Andrade, J.L.; Milhazes, N.; Borges, F.; Marques, M.P.M. Anticancer Activity of Phenolic Acids of Natural or Synthetic Origin: A Structure-Activity Study. J. Med. Chem. 2003, 46, 5395–5401. [Google Scholar] [CrossRef] [PubMed]
- Chibli, L.A.; Rodrigues, K.C.M.; Gasparetto, C.M.; Pinto, N.C.C.; Fabri, R.L.; Scio, E.; Alves, M.S.; Del-Vechio-Vieira, G.; Sousa, O.V. Anti-inflammatory effects of Bryophyllum pinnatum (Lam.) Oken ethanol extract in acute and chronic cutaneous inflammation. J. Ethnopharmacol. 2014, 154, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, L.B.d.S.; de Aguiar, P.F.; Leal-Costa, M.V.; Coutinho, M.A.S.; Borsodi, M.P.G.; Rossi-Bergmann, B.; Tavares, E.S.; Costa, S.S. Optimization of Aqueous Extraction from Kalanchoe pinnata Leaves to Obtain the Highest Content of an Anti-inflammatory Flavonoid using a Response Surface Model. Phytochem. Anal. 2018, 29, 308–315. [Google Scholar]
- Nielsen, A.H.; Olsen, C.E.; Møller, B.L. Flavonoids in flowers of 16 Kalanchoë blossfeldiana varieties. Phytochemistry 2005, 66, 2829–2835. [Google Scholar] [CrossRef] [PubMed]
- Ogungbamila, F.O.; Onawunmi, G.O.; Adeosun, O. A new acylated flavan-3-ol from Bryophyllum pinnatum. Nat. Prod. Lett. 1997, 10, 201–203. [Google Scholar] [CrossRef]
- Muzitano, M.F.; Tinoco, L.W.; Guette, C.; Kaiser, C.R.; Rossi-Bergmann, B.; Costa, S.S. The antileishmanial activity assessment of unusual flavonoids from Kalanchoe pinnata. Phytochemistry 2006, 67, 2071–2077. [Google Scholar] [CrossRef]
- Nascimento, L.B.D.S.; Leal-Costa, M.V.; Menezes, E.A.; Lopes, V.R.; Muzitano, M.F.; Costa, S.S.; Tavares, E.S. Ultraviolet-B radiation effects on phenolic profile and flavonoid content of Kalanchoe pinnata. J. Photochem. Photobiol. B Biol. 2015, 148, 73–81. [Google Scholar] [CrossRef]
- Tatsimo, S.J.N.; Tamokou, J.D.D.; Havyarimana, L.; Csupor, D.; Forgo, P.; Hohmann, J.; Kuiate, J.R.; Tane, P. Antimicrobial and antioxidant activity of kaempferol rhamnoside derivatives from Bryophyllum pinnatum. BMC Res. Notes 2012, 5, 1–6. [Google Scholar] [CrossRef]
- Wang, T.Y.; Li, Q.; Bi, K.S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Carotenoids and flavonoids contribute to nutritional protection against skin damage from sunlight. Mol. Biotechnol. 2007, 37, 26–30. [Google Scholar] [CrossRef]
- Mandić, L.; Sadžak, A.; Strasser, V.; Baranović, G.; Jurašin, D.D.; Sikirić, M.D.; Šegota, S. Enhanced protection of biological membranes during lipid peroxidation: Study of the interactions between flavonoid loaded mesoporous silica nanoparticles and model cell membranes. Int. J. Mol. Sci. 2019, 20, 2709. [Google Scholar] [CrossRef]
- Cherrak, S.A.; Mokhtari-Soulimane, N.; Berroukeche, F.; Bensenane, B.; Cherbonnel, A.; Merzouk, H.; Elhabiri, M. In vitro antioxidant versus metal ion chelating properties of flavonoids: A structure-activity investigation. PLoS ONE 2016, 11, 1–21. [Google Scholar] [CrossRef]
- García-Pérez, P.; Losada-Barreiro, S.; Gallego, P.P.; Bravo-Díaz, C. Cyclodextrin-elicited Bryophyllum suspension cultured cells: Enhancement of the production of bioactive compounds. Int. J. Mol. Sci. 2019, 20, 5180. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Orhan, D.D.; Özçelik, B.; Özgen, S.; Ergun, F. Antibacterial, antifungal, and antiviral activities of some flavonoids. Microbiol. Res. 2010, 165, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Plochmann, K.; Korte, G.; Koutsilieri, E.; Richling, E.; Riederer, P.; Rethwilm, A.; Schreier, P.; Scheller, C. Structure-activity relationships of flavonoid-induced cytotoxicity on human leukemia cells. Arch. Biochem. Biophys. 2007, 460, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Lai, C.S.; Ho, C.T. Anti-inflammatory activity of natural dietary flavonoids. Food Funct. 2010, 1, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Testai, L. Flavonoids and mitochondrial pharmacology: A new paradigm for cardioprotection. Life Sci. 2015, 135, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.V.A.; Liu, D.; Gilbert, E.R. Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J. Nutr. Biochem. 2013, 24, 1777–1789. [Google Scholar] [CrossRef]
- Ferreira, R.T.; Coutinho, M.A.S.; Malvar, D.D.C.; Costa, E.A.; Florentino, I.F.; Costa, S.S.; Vanderlinde, F.A. Mechanisms underlying the antinociceptive, antiedematogenic, and anti-inflammatory activity of the main flavonoid from Kalanchoe pinnata. Evidence-Based Complement. Altern. Med. 2014, 2014. [Google Scholar] [CrossRef]
- Henn, D.; Venter, A.; Botha, C. In vitro cytotoxicity induced by the bufadienolides 1α,2α-epoxyscillirosidine and lanceotoxin b on rat myocardial and mouse neuroblastoma cell lines. Toxins (Basel). 2019, 11, 14. [Google Scholar] [CrossRef]
- Oufir, M.; Seiler, C.; Gerodetti, M.; Gerber, J.; Fürer, K.; Mennet-von Eiff, M.; Elsas, S.M.; Brenneisen, R.; Von Mandach, U.; Hamburger, M.; et al. Quantification of Bufadienolides in Bryophyllum pinnatum Leaves and Manufactured Products by UHPLC-ESIMS/MS. Planta Med. 2015, 81, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.L.; Hsu, Y.L.; Wu, T.S.; Bastow, K.F.; Lee, K.H. Kalanchosides A-C, new cytotoxic bufadienolides from the aerial parts of Kalanchoe gracilis. Org. Lett. 2006, 8, 5207–5210. [Google Scholar] [CrossRef] [PubMed]
- Moniuszko-Szajwaj, B.; Pecio, Ł.; Kowalczyk, M.; Stochmal, A. New bufadienolides isolated from the roots of Kalanchoe daigremontiana (Crassulaceae). Molecules 2016, 21, 243. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, T.; Haruna, M.; Yan, X.-Z.; Chang, J.-J.; Lee, K.-H. Antitumor agents, 110, Bryophyllin B, a novel potent cytotoxic bufadienolide from Bryophyllum pinnatum. J. Nat. Prod. 1989, 52, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Stefanowicz-Hajduk, J.; Hering, A.; Gucwa, M.; Hałasa, R.; Soluch, A.; Kowalczyk, M.; Stochmal, A.; Ochocka, R. Biological activities of leaf extracts from selected Kalanchoe species and their relationship with bufadienolides content. Pharm. Biol. 2020, 58, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Lin, M.K.; Yang, H.L.; Hseu, Y.C.; Liaw, C.C.; Tseng, Y.H.; Tsuzuki, M.; Kuo, Y.H. Cardenolides and bufadienolide glycosides from Kalanchoe tubiflora and evaluation of cytotoxicity. Planta Med. 2013, 79, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lin, X.; Yang, Z.; Zhang, W.; Ren, T.; Qu, F.; Wang, Y.; Zhang, N.; Tang, X. A bufadienolide-loaded submicron emulsion for oral administration: Stability, antitumor efficacy and toxicity. Int. J. Pharm. 2015, 479, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Bick, R.J.; Poindexter, B.J.; Sweney, R.R.; Dasgupta, A. Effects of Chan Su, a traditional Chinese medicine, on the calcium transients of isolated cardiomyocytes: Cardiotoxicity due to more than Na, K-ATPase blocking. Life Sci. 2002, 72, 699–709. [Google Scholar] [CrossRef]
- McKenzie, R.A.; Franke, F.P.; Dunster, P.J. The toxicity to cattle and bufadienolide content of six Bryophyllum species. Aust. Vet. J. 1987, 64, 298–301. [Google Scholar] [CrossRef]
- McKenzie, R.A.; Franke, F.P.; Dunster, P.J. The toxicity for cattle of bufadienolide cardiac glycosides from Bryophyllum tubiflorum flowers. Aust. Vet. J. 1989, 66, 374–376. [Google Scholar] [CrossRef]
- Gao, H.; Popescu, R.; Kopp, B.; Wang, Z. Bufadienolides and their antitumor activity. Nat. Prod. Rep. 2011, 28, 953–969. [Google Scholar] [CrossRef]
- Li, F.; Weng, Y.; Wang, L.; He, H.; Yang, J.; Tang, X. The efficacy and safety of bufadienolides-loaded nanostructured lipid carriers. Int. J. Pharm. 2010, 393, 204–212. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhao, C.; Wu, W.Y.; Fan, T.Y.; Li, N.G.; Chen, M.; Duan, J.A.; Shi, Z.H. Total synthesis, chemical modification and structure-activity relationship of bufadienolides. Eur. J. Med. Chem. 2020, 189, 112038. [Google Scholar] [CrossRef]
- Marchev, A.S.; Yordanova, Z.P.; Georgiev, M.I. Green (cell) factories for advanced production of plant secondary metabolites. Crit. Rev. Biotechnol. 2020, 0, 1–16. [Google Scholar] [CrossRef]
- El Sheikha, A.F. Medicinal plants: Ethno-uses to biotechnology era. In Biotechnology and Production of Anti-Cancer Compounds; Malik, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–38. [Google Scholar]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef]
- Eibl, R.; Meier, P.; Stutz, I.; Schildberger, D.; Hühn, T.; Eibl, D. Plant cell culture technology in the cosmetics and food industries: Current state and future trends. Appl. Microbiol. Biotechnol. 2018, 102, 8661–8675. [Google Scholar] [CrossRef]
- Karuppusamy, S. A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J. Med. Plants Res. 2009, 3, 1222–1239. [Google Scholar]
- Thorpe, T.A. History of plant tissue culture. Mol. Biotechnol. 2007, 37, 169–180. [Google Scholar] [CrossRef]
- Su, Y.H.; Tang, L.P.; Zhao, X.Y.; Zhang, X.S. Plant cell totipotency: Insights into cellular reprogramming. J. Integr. Plant Biol. 2020, 00. [Google Scholar]
- George, E.F.; Hall, M.A.; De Klerk, G.-J. Plant tissue culture procedure-background. In Plant Propagation by Tissue Culture; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–28. [Google Scholar]
- Da Silva, J.A.T.; Tanaka, M. Thin cell layers: The technique. In Plant Cell Culture: Essential Methods.; Davey, M.R., Anthony, P., Eds.; John Wiley & Sons: Chichester, UK, 2010; pp. 25–37. [Google Scholar]
- García-Pérez, P.; Lozano-Milo, E.; Landin, M.; Gallego, P.P. Machine Learning technology reveals the concealed interactions of phytohormones on medicinal plant in vitro organogenesis. Biomolecules 2020, 10, 746. [Google Scholar] [CrossRef] [PubMed]
- Kulus, D. Micropropagation of Kalanchoe tubiflora (Harvey) Hamet. Nauk. Przyr. Technol. 2015, 9. [Google Scholar] [CrossRef]
- Naz, S.; Javad, S.; Ilyas, S.; Ali, A. An efficient protocol for rapid multiplication of Bryophyllum pinnatum and Bryophyllum daigremontianum. Pakistan J. Bot. 2009, 41, 2347–2355. [Google Scholar]
- Frello, S.; Venerus, E.; Serek, M. Regeneration of various species of Crassulaceae, with special reference to Kalanchoë. J. Hortic. Sci. Biotechnol. 2002, 77, 204–208. [Google Scholar] [CrossRef]
- Mohammed, S.U.B.; Choi, K.-S.; Kim, T.-R.; In, J.-G.; Yang, D.-C. Plant regeneration from leaf explants of Kalanchoe daigremontiana Hamet & Perrier. Korean J. Med. Crop Sci. 2006, 14, 293–298. [Google Scholar]
- Kefu, Z.; Hai, F.; San, Z.; Jie, S. Study on the salt and drought tolerance of Suaeda salsa and Kalanchoe daigremontiana under iso-osmotic salt and water stress. Plant Sci. 2003, 165, 837–844. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; De Klerk, G.-J. The components of plant tissue culture media I: Macro-and micro-nutrients. In Plant Propagation by Tissue Culture; Springer: Berlin/Heidelberg, 2008; pp. 65–113. [Google Scholar]
- Nezami-Alanagh, E.; Garoosi, G.A.; Haddad, R.; Maleki, S.; Landín, M.; Gallego, P.P. Design of tissue culture media for efficient Prunus rootstock micropropagation using artificial intelligence models. Plant Cell. Tissue Organ Cult. 2014, 117, 349–359. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Ikenganyia, E.E.; Anikwe, M.A.N.; Omeje, T.E.; Adinde, J.O. Plant tissue culture regeneration and aseptic techniques. Asian J. Biotechnol. Bioresour. Technol. 2017, 1–6. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Murashige, T.; Thorpe, T.A.; Vasil, I.K. Plant tissue culture media. In Vitro 1976, 12, 473–478. [Google Scholar] [CrossRef]
- Nezami-Alanagh, E.; Garoosi, G.A.; Landín, M.; Gallego, P.P. Computer-based tools provide new insight into the key factors that cause physiological disorders of pistachio rootstocks cultured in vitro. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Phillips, G.C.; Garda, M. Plant tissue culture media and practices: An overview. Vitr. Cell. Dev. Biol. Plant 2019, 55, 242–257. [Google Scholar] [CrossRef]
- Pereira, P.N.; Cushman, J.C. Exploring the relationship between crassulacean acid metabolism (CAM) and mineral nutrition with a special focus on nitrogen. Int. J. Mol. Sci. 2019, 20, 4363. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.N.; Smith, J.A.C.; Mercier, H. Nitrate enhancement of CAM activity in two Kalanchoë species is associated with increased vacuolar proton transport capacity. Physiol. Plant. 2017, 160, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.R.A.; Ferreira, M.G.R.; Guimarães, M.C.M.; Lima, R.A.; Oliveira, C. Callogenesis in leaves of Kalanchoe pinnata Lam. by 2, 4-D and BA action. Rev. Bras. Plantas Med. 2014, 16, 760–764. [Google Scholar] [CrossRef]
- García-Pérez, P.; Lozano-Milo, E.; Landin, M.; Gallego, P.P. Machine Learning unmasked nutritional imbalances on the medicinal plant Bryophyllum sp. cultured in vitro. Front. Plant Sci. 2020, 11, 576177. [Google Scholar] [CrossRef]
- Niedz, R.P.; Evens, T.J. A solution to the problem of ion. Nat. Methods 2006, 3, 34945. [Google Scholar] [CrossRef]
- Wilson, S.A.; Roberts, S.C. Recent advances towards development and commercialization of plant cell culture processes for the synthesis of biomolecules. Plant Biotechnol. J. 2012, 10, 249–268. [Google Scholar] [CrossRef]
- Namdeo, A.G. Plant cell elicitation for production of secondary metabolites: A review. Rev. Lit. Arts Am. 2007, 1, 69–79. [Google Scholar]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef]
- Yue, W.; Ming, Q.L.; Lin, B.; Rahman, K.; Zheng, C.J.; Han, T.; Qin, L.P. Medicinal plant cell suspension cultures: Pharmaceutical applications and high-yielding strategies for the desired secondary metabolites. Crit. Rev. Biotechnol. 2016, 36, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Giri, C.C.; Zaheer, M. Chemical elicitors versus secondary metabolite production in vitro using plant cell, tissue and organ cultures: Recent trends and a sky eye view appraisal. Plant Cell. Tissue Organ Cult. 2016, 126, 1–18. [Google Scholar] [CrossRef]
- Vasconsuelo, A.; Boland, R. Molecular aspects of the early stages of elicitation of secondary metabolites in plants. Plant Sci. 2007, 172, 861–875. [Google Scholar] [CrossRef]
- Narayani, M.; Srivastava, S. Elicitation: A stimulation of stress in in vitro plant cell/tissue cultures for enhancement of secondary metabolite production. Phytochem. Rev. 2017, 16, 1227–1252. [Google Scholar] [CrossRef]
- Ochoa-Villarreal, M.; Howat, S.; Hong, S.M.; Jang, M.O.; Jin, Y.W.; Lee, E.K.; Loake, G.J. Plant cell culture strategies for the production of natural products. BMB Rep. 2016, 49, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Gago, J.; Martínez-Núñez, L.; Landín, M.; Gallego, P.P. Artificial neural networks as an alternative to the traditional statistical methodology in plant research. J. Plant Physiol. 2010, 167, 23–27. [Google Scholar] [CrossRef]
- Gago, J.; Martínez-Núñez, L.; Landin, M.; Flexas, J.; Gallego, P.P. Modeling the effects of light and sucrose on in vitro propagated plants: A multiscale system analysis using artificial intelligence technology. PLoS ONE 2014, 9, e85989. [Google Scholar] [CrossRef]
- Olden, J.D.; Lawler, J.J.; Poff, N.L. Machine learning methods without tears: A primer for ecologists. Q. Rev. Biol. 2008, 83, 171–193. [Google Scholar] [CrossRef]
- Landin, M.; Rowe, R.C. Artificial neural networks technology to model, understand, and optimize drug formulations. In Formulation Tools for Pharmaceutical Development; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 7–37. ISBN 9781907568992. [Google Scholar]
- Gallego, P.P.; Gago, J.; Landín, M. Artificial Neural Networks Technology to Model and Predict Plant Biology Process. In Artificial Neural Networks; Suzuki, K., Ed.; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar]
