A central aspect of Brucella pathogenicity is its ability to invade, survive, and replicate in diverse phagocytic and non-phagocytic cell types, leading to chronic infections and chronic inflammatory phenomena. Adhesion to the target cell is a critical first step in the invasion process. Several Brucella adhesins have been shown to mediate adhesion to cells, extracellular matrix components (ECM), or both. These include the sialic acid-binding proteins SP29 and SP41, the BigA and BigB proteins that contain an Ig-like domain, the monomeric autotransporters BmaA, BmaB, and BmaC, the trimeric autotransporters BtaE and BtaF, and Bp26.
- Brucella
- pathogenicity
- adhesins
- autotransporter
- extracellular matrix
- host-bacteria interaction
- adhesion
1. Introduction
Brucella spp. are Gram-negative bacteria that infect several animal species and can be transmitted to humans by several routes, producing one of the most common zoonotic diseases worldwide. Brucella can invade, survive, and replicate in several phagocytic and non-phagocytic cell types. Several Brucella proteins have been shown to be involved in the adhesion of this bacterium to different cell types and/or to extracellular matrix (ECM) components.
With more than 500,000 new cases annually, human brucellosis continues to be one of the commonest zoonotic diseases worldwide. It still constitutes a public health problem in Latin America, the Middle East, North and East Africa, and South and Central Asia [1]Pappas, G.; Papadimitriou, P.; Akritidis, N.; Christou, L.; Tsianos, E. V The new global map of human brucellosis. Lancet Infect. Dis. 2006, 6, 91–99., and is still present in some European countries. Despite the great number of Brucella species identified, which may infect domestic animals and wild animals, only B. melitensis (goats and sheep), B. suis (pigs), B. abortus (cattle), and, to a minor extent, B. canis (dogs) are linked to human brucellosis. Different Brucella species can yield smooth (S) or rough (R) colonies according to the structure of the lipopolysaccharide (LPS). The LPS is divided into three regions: the lipid A (the innermost portion), a polysaccharidic core, and the O polysaccharide (the outermost portion). Smooth species (B. melitensis, B. suis, B. abortus, and others) produce a “complete” LPS (S-LPS) containing the three portions, whereas rough species (B. canis and B. ovis) produce a rough LPS (R-LPS) that lacks the O polysaccharide.
Brucella infection in humans is mainly acquired through the consumption of raw animal products, inhalation of contaminated aerosols in slaughterhouses, rural settings or laboratories [2], and contact of the abraded skin with contaminated tissues or materials. Accidental infection with attenuated vaccine strains and vertical transmission have been also reported [3][4].
The disease usually presents as a febrile illness accompanied by myalgia, arthralgia, and hepatomegaly, and may evolve with an uncomplicated course or may present complications involving particular organs or systems [5]. Osteoarticular involvement is the most common focal complication. The diversity of tissues that can be affected by Brucella is likely related to its ability to invade, survive, and replicate in several phagocytic and non-phagocytic cell types.
2. Adhesins
Most pathogenic bacteria interact with their hosts through adhesive molecules (adhesins) that are exposed on their cell surfaces. The adhesins can be grouped into two types: (1) filamentous (fimbrial) adhesins consisting of complex structures made up of multiple subunits and (2) non-fimbrial adhesins that can be monomeric or trimeric proteins. As mentioned below, Brucella spp. do not seem to express fimbrial adhesins.
Non-fimbrial adhesins include adhesins that belong to the RTX (repeat in toxin) protein family and those that correspond to type V secretion systems (T5SS), which are also called autotransporter proteins. RTX adhesins are secreted by a type 1 secretion system (T1SS) that has three components: an inner-membrane ABC (ATP binding cassette) transporter, a membrane fusion protein, and an outer-membrane pore from the TolC family. The RTX adhesins are usually loosely attached to the bacterial surface and have been implicated in bacteria-to-bacteria interactions during biofilm formation and adhesion to epithelial cells [6].
The T5SSs (subfamilies Va–Ve) play important roles in the interaction of several pathogens with their hosts [7]. Other factors, such as chaperones and the BAM (β-barrel Assembly Machinery) system are required for secretion of these proteins. More recently, it was shown that the TAM (Translocation and Assembly Module) complex is also required for the correct translocation of autotransporters into the outer membrane [8]. The T5SS or autotransporter proteins share common structural and functional characteristics: (1) an N-terminal Sec-dependent signal peptide that mediates the transport from the cytoplasm to the periplasm, (2) a passenger (and functional) domain, and (3) a C-terminal β-barrel domain that forms a pore in the outer membrane through which the passenger domain is translocated to the cell surface [9]. In the subclass Va, the autotransporters are monomeric and the passenger and secretion domains are integrated into the same protein. Some of these autotransporters are important virulence factors, playing diverse functions in the interaction with the host. Some adhesins of the monomeric autotransporter family have been described in Brucella spp., as explained below. In the two-partner secretion systems (T5SS type Vb), the passenger and β-translocator domains are encoded by two different genes. Filamentous haemagglutinin adhesins are exported by this type of system. These adhesins are often involved in a tight interaction with a host cell receptor and also in biofilm formation [7]. All members of the trimeric autotransporter Type Vc group that have been characterized so far are implicated in adhesion functions. They usually bind to host receptors or to host ECM components. As detailed below, Brucella spp. express adhesins that belong to this subclass of autotransporters. While the overall organization of these proteins is similar to that of the monomeric autotransporters, they contain a shorter C-terminal translocation domain of 50–100 amino acids and the 12 β-strand pore is achieved by protein trimerization. Usually, the passenger domain harbors conserved structural elements named as head, connector, and stalk domains. Although the head domains typically mediate adhesion to host targets, the stalk domains can also participate in adhesion functions. The Type Ve of T5SS harbors a 12-stranded β-barrel domain and a secreted, monomeric passenger domain that remains attached after translocation. The main difference with type Va autotransporters is that the type Ve have an inverted domain order with the β-barrel at the N-terminal end and the passenger domain at the C-terminus, and, thus, are named as “inverse autotransporters” [10]. The passenger domains of this type of T5SS contain domains with Immunoglobulin (Ig)-like or lectin-like structures.
3. Adhesins of Brucella spp.
The genomes of Brucella spp. do not harbor loci associated with components of pili or curli that could function as fimbrial adhesins. However, several non-fimbrial adhesins have been identified that were shown to have a role in the interaction with the host.
Due to the abundance of carbohydrates in the surface of red cells, hemagglutination tests have been used for the detection and characterization of many lectin-like adhesins in bacterial pathogens. It has been shown that B. abortus and B. melitensis can agglutinate human (A+ and B+), hamster, and rabbit erythrocytes, and that this activity was associated with a bacterial 29-kDa surface protein (SP29) that binds to these cells [11]. This binding was abolished by neuraminidase treatment of red cells, indicating that SP29 binds to sialic acid-containing receptors. SP29 seems to be a D-ribose-binding periplasmic protein precursor in B. melitensis (BruAb2_0373) (Figure 1).
The first Brucella adhesin for which a functional role was fully characterized in vitro was SP41 [12] (Figure 1). This protein is the predicted product of the ugpB locus, which encodes a protein of 433 amino acids with similarity to a periplasmic glycerol-3-phosphate-binding ATP-binding cassette (ABC) transporter. SP41 is surface exposed, and antibodies directed to SP41 inhibit B. suis adherence to HeLa cells. A ΔugpB B. suis mutant exhibited a significant reduction in the adherence to epithelial cells, although the same phenotype was not observed for a B. ovis mutant [13]. Treatment of HeLa cells with neuraminidase abolished SP41 binding to these cells, suggesting the involvement of sialic acid residues in this interaction. (Figure 2)
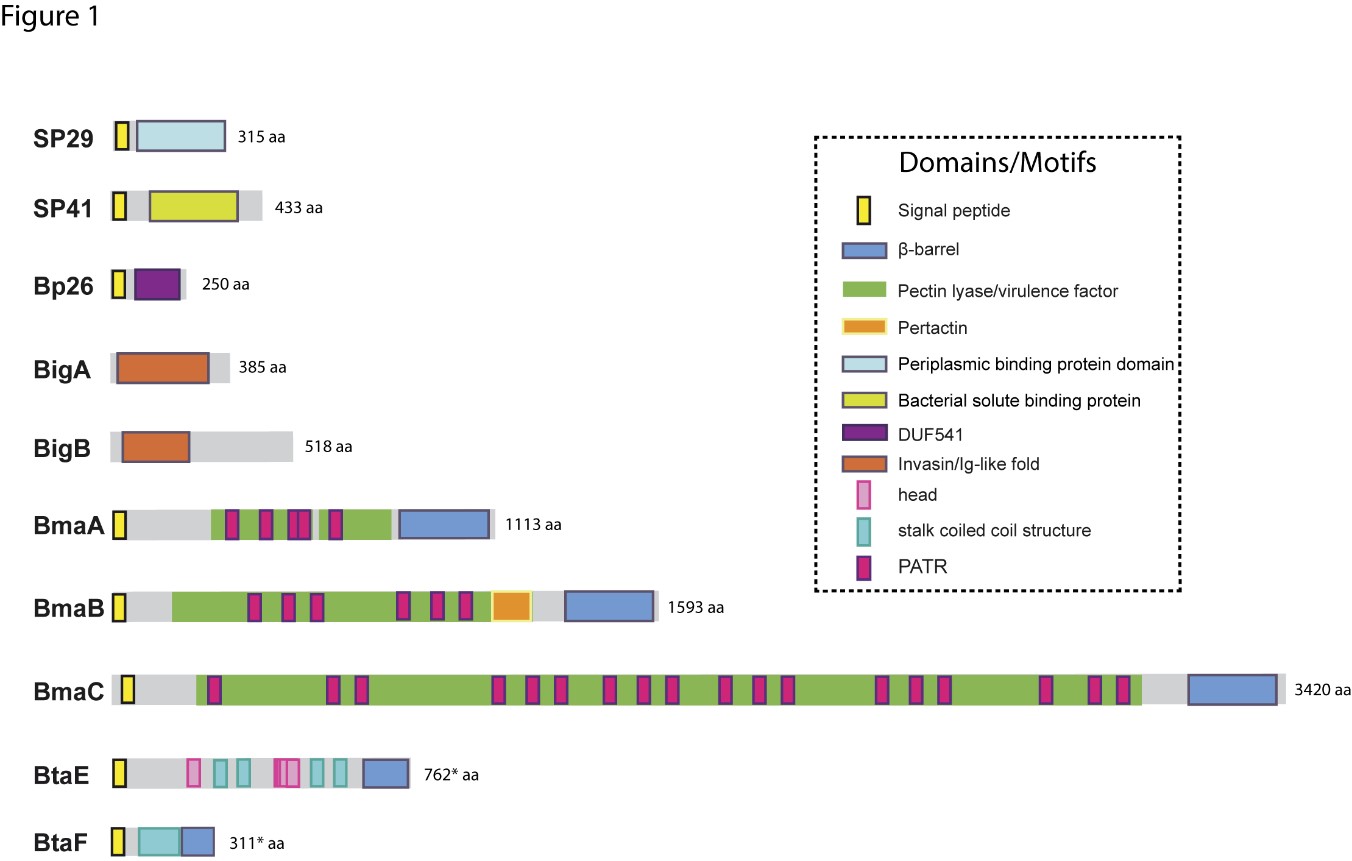
The potential role of the Brucella Bp26 protein as an adhesin was recently tested in vitro [14]. Bp26 is a 250 amino acid-predicted protein with a domain of unknown function (DUF541) (Figure 1). Bp26 binds to both immobilized and soluble type I collagen and vitronectin, and to soluble (but not immobilized) fibronectin, but does not bind to laminin (Figure 2). The relevance of Bp26 for in vitro adhesion of Brucella to cells or for the outcome of in vivo infections has not been tested.
A pathogenicity island in B. abortus (BAB1_2009-2012) whose deletion resulted in a reduced attachment of the bacterium to HeLa cells and a reduced capacity to colonize the spleen of mice after oral infection has been identified [15]. In particular, BAB1_2009 was found to encode a protein that harbors a bacterial Ig-like (BIg-like) domain present in adhesins from the invasin/intimin family [16] (Figure 1). A role of this protein (named as BigA) in adhesion to epithelial cells was demonstrated [17]. This study also revealed that BigA is an exposed outer membrane protein and that incubation of the bacteria with antibodies against the Ig-like domain of BigA before infection of HeLa cells reduces the number of intracellular bacteria. The BigA adhesin targets the bacteria to the cell-cell junction membrane in confluent epithelial cells and also induces cytoskeleton rearrangements (Figure 2). A recent study showed that other Ig-like (Blg-like) domain-containing protein (BAB1_2012, named BigB) (Figure 1), encoded by the same locus (BAB1_2009-2012), is also involved in adhesion to epithelial cells and targets proteins involved in cell-cell and cell-matrix interactions [18] (Figure 2). The ΔbigB mutant showed a significant reduction in intracellular bacteria at the early stages of infection both in HeLa cells and in polarized MDCK cells. Similar to BigA, recombinant BigB induced profound cytoskeleton rearrangements in HeLa cells.
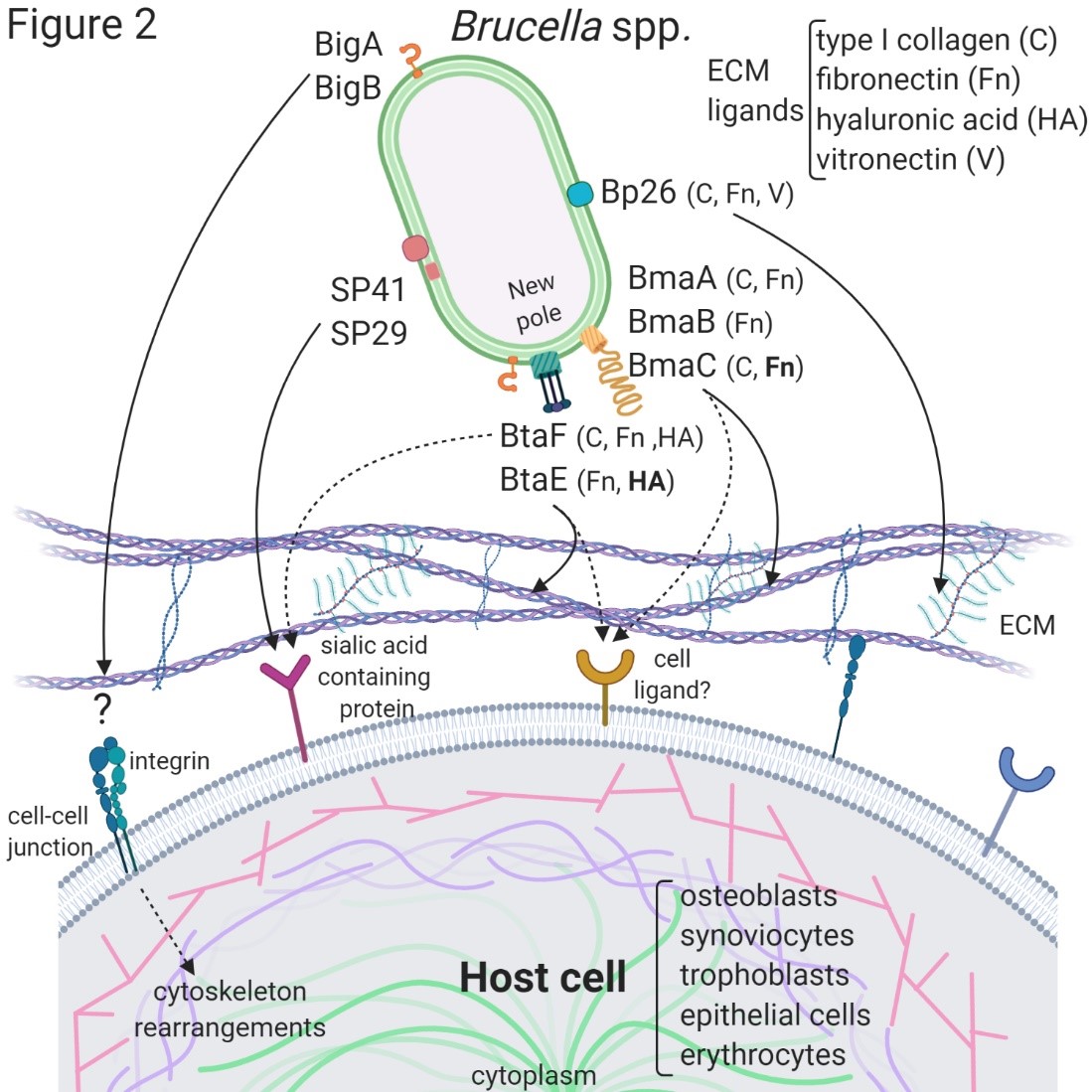
By panning an M13 phage display library of the B. suis 1330 genome against immobilized fibronectin, Posadas et al. [19] identified a fibronectin-binding protein that was predicted to be exposed on the cell surface. This protein corresponded to a monomeric autotransporter (BRA1148) and was named as BmaC for Bucella monomeric autotransporter. BmaC is a large 340 kDa-protein with a long N-terminal cleavable 72 amino acid signal peptide and several adhesion-related motifs within the passenger domain, an extended pectin lyase virulence factor domain, and several passenger-associated-transport-repeats (PATR) (Figure 1). The portion of BmaC expressed on the phage also exhibits affinity (although much less) for type I collagen, suggesting that this very large protein might contribute to the interaction of B. suis with other ECM ligands. Several lines of evidence have shown that BmaC of B. suis 1330 mediates the binding of B. suis to epithelial cells through cellular fibronectin. A bmaC deletion mutant was impaired in the attachment to immobilized fibronectin and to the surface of HeLa and A549 epithelial cells. Immunofluorescence microscopy showed that all bacteria with a detectable fluorescent signal displayed BmaC at only one pole, indicating that BmaC is polarly exposed on the cell surface (Figure 2). Occasionally, polar BmaC was located at the pole interacting with the cell, suggesting that the polar localization of BmaC could be relevant in the interaction with host cells in vivo [19].
The monomeric autotransporter proteins encoded by BR0173 and BR2013 (BmaA and BmaB, respectively) of B. suis 1330, although much smaller, share significant sequence similarities with BmaC (Figure 1). It was reported that a mutant of B. suis 1330, deficient in BmaB (previously called OmaA), is cleared from spleens of BALB/c mice faster than the wild-type strain [20]. Gain or loss of function studies indicated that, at least in B. suis strain 1330, BmaA, BmaB, and BmaC proteins contribute, to a greater or lesser degree, to bacterial adhesion to different cell types, such as epithelial (HT-29 and Caco2), synoviocytes, osteoblasts, and trophoblasts (Figure 2). A recent study indicated that the bmaB locus from all B. abortus strains analyzed and both the bmaA and bmaC loci from all B. melitensis strains seem to correspond to pseudogenes, while, in B. suis, all the Bma proteins could be functional in several strains of this species [21]. These observations show that there are variations in the repertoire of functional adhesins in Brucella spp. and open the possibility that these adhesins are involved in host preferences. BmaB was also found at the new pole generated after cell division [21].
The search for conserved adhesion-associated domains/motifs in B. suis 1330 identified a group of trimeric autotransporters, including BR0072 and BR1846, which were named BtaE and BtaF, respectively. The B. suis BtaE trimeric autotransporter is a 740 amino acid protein that harbors several regions corresponding to the head and the neck subdomains in addition to a connector region and the β-barrel translocator domain (Figure 1). Different genetic approaches showed that BtaE of B. suis is involved in the adhesion to ECM components and host cells [22]. The BtaE-defective strain exhibited a decreased ability to adhere to HeLa and A549 epithelial cells. Expression of BtaE in a “non-adherent” E. coli strain increased the binding of this heterologous bacterium to immobilized hyaluronic acid and fibronectin. On the other hand, btaE deletion impaired bacterial adhesion to hyaluronic acid but had no effect in the adhesion to fibronectin, suggesting that other fibronectin-binding adhesins (such as BmaC) could compensate somehow for the absence of BtaE. In vivo experiments using the mouse model indicate that the BtaE adhesin is necessary for a successful infection; a significantly lower number of bacteria were recovered from spleens of animals inoculated through the intragastric route with the btaE mutant compared to those inoculated with the wild-type strain [22]. In a subsequent work, it was shown that the BtaE orthologue of B. abortus 2308 is also involved in adhesion to epithelial cells. Compared to B. suis BtaE, the ortologue of B. abortus is much larger and contains a higher number of repetitive adhesion motifs. Furthermore, the btaE gene of B. suis and B. abortus are under the regulation of different mechanisms [23].
The BtaF trimeric autotransporter of B. suis 1330, encoded by the BR1846 annotated locus, is a small protein that harbors an N-terminal peptide signal, a 170 amino acid passenger domain, and a YadA-like C-terminal translocator region (β-barrel translocator domain) (Figure 1). Unlike BtaE, the BtaF protein does not show the presence of conserved adhesion motifs. Most of the passenger domains correspond to a coiled coil stalk but none associated with the “head” structural region [24]. The btaF deletion mutant of B. suis showed a significant reduction in the ability to bind to fetuin, hyaluronic acid, and collagen I, and in the adhesion to an abiotic surface, even though the adhesion to fibronectin was not affected. Again, as it was observed for the btaE mutant, overlapping functions with other adhesins (such as Bma proteins and BtaE) may account for the lack of a ΔbtaF phenotype toward fibronectin (Figure 2). The BtaF-defective strain showed a significant reduction in the attachment to HeLa and A549 epithelial cells. Notably, BtaF was required for complete virulence in mice infected through the oral route (intragastric administration) [2425]. The strain lacking BtaF showed a reduction of about one log in the number of bacteria recovered from spleen at early stages of infection. The absence of both trimeric adhesins (BtaE and BtaF) resulted in a more severe phenotype in vivo compared with the attenuation observed for the single mutants. Recently, it was shown that BtaF is also required for virulence in mice after inoculation via a respiratory (intratracheal administration) route [2526]. It was proposed that, in addition to the LPS, other surface factors mediate the varied sensitivity of Brucella species to the bactericidal action of serum [2627]. The btaF mutant showed a significantly reduced survival in the presence of 50% porcine serum compared with the wild-type strain [24], suggesting that BtaF is involved in the resistance to complement-mediated serum killing.
Similar to BmaC and BmaB, BtaE and BtaF adhesins were found to be polarly localized on the bacterial surface [22][2425](Figure 2). As it was observed for BmaC and BmaB, they were found at the same pole as AidB-YFP (a new pole marker). An attractive hypothesis is that the initial adhesion of Brucella to the host cell would be mediated by adhesins located at the new pole and that adhesin expression only occurs in an infectious bacterial subpopulation. Polar localization could be a way of increasing the adhesive power by concentrating the adhesins in a particular region.
BR0049 of Brucella spp. shares a relatively low identity (around 22%) with TamB from Gammaproteobacteria, but, similarly to this protein, it contains a membrane anchor signal at the N-terminus, which is followed by a region with an abundant β-helix structure, and, at the C-terminus, a short β-barrel structure within the conserved DUF490 domain. It was demonstrated that BR0049 is required for the correct insertion in the OM of the B. suis BmaB monomeric autotransporter. In addition, BR0049 was required for complete virulence in mice infected through the intragastric route [2728]. The BR0049 mutant showed an increased sensitivity to polymyxin B, lysozyme, and Triton X-100, and, thus, BR0049 was named as MapB (Membrane altering protein).
An essential step in establishing a successful infection is the adhesion of microorganisms to eukaryotic cells, resulting in colonization of the tissue involved. Therefore, the molecules involved in this initial interaction have been widely studied as targets for the development of vaccines against various pathogens. However, there are few studies on the potential of Brucella adhesins as vaccine candidates. In a study by Al-Mariri et al. [2829], intramuscular (i.m.) administration of pCISP41, a plasmid construct for SP41 expression in mammalian cells, induced SP41-specific serum immunoglobulin G (IgG) antibodies and also a specific T cells response (spleen cells proliferation and IFN-γ secretion after in vitro stimulation with lysed B. melitensis). After an intraperitoneal challenge with B. melitensis 16M, mice immunized with pCISP41 exhibited a moderate reduction in their spleen bacterial burden as compared to vaccination with attenuated B. melitensis Rev-1 strain.
Brucella infection is frequently acquired through the oral and respiratory routes, and adhesins are anticipated to have a relevant role in these infectious processes. We assessed in BALB/c mice the immunogenic and protective potential of recombinant BtaF when administered intranasally with the mucosal adjuvant c-di-AMP [2526]. The immunization induced high levels of serum BtaF-specific IgG, IgA, IgG1, and IgG2a. In vitro, these serum antibodies reduced B. suis infection of human lung epithelial cells (A549 cell line), reduced bacterial binding to fetuin (a protein rich in sialic acid previously described as a ligand for BtaF), and enhanced B. suis phagocytosis by murine macrophages. In addition, immunization led to a significant production of specific IgA antibodies in the airways and in the gastrointestinal and genital mucosae. BtaF immunization induced a systemic and pulmonary Th1 immune response, shown by the secretion of high levels of IFN-γ by splenocytes and lung cells. BtaF-vaccination also triggered the differentiation of specific CD4+ T cells to central memory cells in cervical lymph nodes, and differentiation of T cells to a Th17 profile in the spleen. Mice vaccination with BtaF plus c-di-AMP demonstrated a high level of protection against B. suis oral infection, reducing the splenic burden of B. suis by 3.28 log. However, intranasal vaccination with BtaF failed to protect against respiratory infection with B. suis.
4. Summary and Future Directions
For a long time, the process of Brucella adhesion to host cells has received little attention. Adhesins of Brucella spp. identified to date have been studied with varying degrees of depth. Some of them have been analyzed regarding their roles in the binding to components of the ECM and to different cell types as well as their in vivo role, while, in other cases, their functions have been only evaluated in vitro. Still, for various adhesins, it will be necessary to identify their ligands or cell receptors. The possible use of Brucella adhesins as vaccine candidates was tested only in two cases, and one of them showed encouraging results. Therefore, the in vivo role of many of the Brucella adhesins identified so far, and their possible applications as a basis for acellular vaccines, remains to be evaluated. It is expected that, in the future, new adhesins will be identified that mediate the initial adhesion of Brucella to the remarkable variety of cell types that this pathogen can invade. An interesting perspective will be to characterize the roles of the different adhesin variants from different species/strains and to determine whether they play a role in host preferences.
References
- Pappas, G.; Papadimitriou, P.; Akritidis, N.; Christou, L.; Tsianos, E. V The new global map of human brucellosis. Lancet Infect. Dis. 2006, 6, 91–99.
- Traxler, R.M.; Lehman, M.W.; Bosserman, E.A.; Guerra, M.A.; Smith, T.L. A Literature Review of Laboratory-Acquired Brucellosis. J. Clin. Microbiol. 2013, 51, 3055–3062, doi:10.1128/JCM.00135-13.
- Strausbaugh, L.J.; Berkelman, R.L. Human Illness Associated with Use of Veterinary Vaccines. Clin. Infect. Dis. 2003, 37, 407–414, doi:10.1086/375595.
- Moreno, E. Retrospective and prospective perspectives on zoonotic brucellosis. Front. Microbiol. 2014, 5, 213.
- Buzgan, T.; Karahocagil, M.K.; Irmak, H.; Baran, A.I.; Karsen, H.; Evirgen, O.; Akdeniz, H. Clinical manifestations and complications in 1028 cases of brucellosis: a retrospective evaluation and review of the literature. Int. J. Infect. Dis. 2010, 14, e469-78.
- Satchell, K.J.F. Structure and function of MARTX toxins and other large repetitive RTX proteins. Annu. Rev. Microbiol. 2011, 65, 71–90.
- Meuskens, I.; Saragliadis, A.; Leo, J.C.; Linke, D. Type V secretion systems: An overview of passenger domain functions. Front. Microbiol. 2019, 10, 1163.
- Kiessling, A.R.; Malik, A.; Goldman, A. Recent advances in the understanding of trimeric autotransporter adhesins. Med. Microbiol. Immunol. 2020, 209, 233–242.
- Van Ulsen, P.; Rahman, S. ur; Jong, W.S.P.; Daleke-Schermerhorn, M.H.; Luirink, J. Type V secretion: From biogenesis to biotechnology. Biochim. Biophys. Acta - Mol. Cell Res. 2014, 1843, 1592–1611.
- Oberhettinger, P.; Leo, J.C.; Linke, D.; Autenrieth, I.B.; Schütz, M.S. The inverse autotransporter intimin exports its passenger domain via a hairpin intermediate. J. Biol. Chem. 2015, 290, 1837–1849, doi:10.1074/jbc.M114.604769.
- Rocha-Gracia, R. del C.; Castañeda-Roldán, E.I.; Giono-Cerezo, S.; Girón, J.A. Brucella sp. bind to sialic acid residues on human and animal red blood cells. FEMS Microbiol. Lett. 2002, 213, 219–224, doi:10.1111/j.1574-6968.2002.tb11309.x.
- Castañeda-Roldán, E.I.; Ouahrani-Bettache, S.; Saldaña, Z.; Avelino, F.; Rendón, M.A.; Dornand, J.; Girón, J.A. Characterization of SP41, a surface protein of Brucella associated with adherence and invasion of host epithelial cells. Cell. Microbiol. 2006, 8, 1877–1887, doi:10.1111/j.1462-5822.2006.00754.x.
- Sidhu-Muñoz, R.S.; Sancho, P.; Vizcaíno, N. Brucella ovis PA mutants for outer membrane proteins Omp10, Omp19, SP41, and BepC are not altered in their virulence and outer membrane properties. Vet. Microbiol. 2016, 186, 59–66, doi:10.1016/j.vetmic.2016.02.010.
- Eltahir, Y.; Al-Araimi, A.; Nair, R.; Autio, K.J.; Tu, H.; Leo, J.C.; Al-Marzooqi, W.; Johnson, E. Binding of Brucella protein, Bp26, to select extracellular matrix molecules. BMC Mol. Cell Biol. 2019, 20, 55, doi:10.1186/s12860-019-0239-7.
- Czibener, C.; Ugalde, J.E. Identification of a unique gene cluster of Brucella spp . that mediates adhesion to host cells. Microbes Infect. 2012, 14, 79–85, doi:10.1016/j.micinf.2011.08.012.
- Bodelón, G.; Palomino, C.; Fernández, L.Á. Immunoglobulin domains in Escherichia coli and other enterobacteria: From pathogenesis to applications in antibody technologies. FEMS Microbiol. Rev. 2013, 37, 204–250.
- Czibener, C.; Merwaiss, F.; Guaimas, F.; Del Giudice, M.G.; Serantes, D.A.R.; Spera, J.M.; Ugalde, J.E. BigA is a novel adhesin of Brucella that mediates adhesion to epithelial cells. Cell. Microbiol. 2016, 18, 500–513, doi:10.1111/cmi.12526.
- Lopez, P.; Guaimas, F.; Czibener, C.; Ugalde, J.E. A genomic island in Brucella involved in the adhesion to host cells: Identification of a new adhesin and a translocation factor. Cell. Microbiol. 2020, e13245, doi:10.1111/cmi.13245.
- Posadas, D.M.; Ruiz-Ranwez, V.; Bonomi, H.R.; Martín, F.A.; Zorreguieta, A. BmaC, a novel autotransporter of Brucella suis, is involved in bacterial adhesion to host cells. Cell. Microbiol. 2012, 14, 965–982, doi:10.1111/j.1462-5822.2012.01771.x.
- Bandara, A.B.; Sriranganathan, N.; Schurig, G.G.; Boyle, S.M. Putative outer membrane autotransporter protein influences survival of Brucella suis in BALB/c mice. Vet. Microbiol. 2005, 109, 95–104, doi:10.1016/j.vetmic.2005.05.012.
- Bialer, M.G.; Ferrero, M.C.; Delpino, M.V.; Ruiz-Ranwez, V.; Posadas, D.M.; Baldi, P.C.; Zorreguieta, A. Adhesive functions or pseudogenization of monomeric autotransporters in Brucella species. unpublished.
- Ruiz-Ranwez, V.; Posadas, D.M.; Van der Henst, C.; Estein, S.M.; Arocena, G.M.; Abdian, P.L.; Martín, F.A.; Sieira, R.; De Bolle, X.; Zorreguieta, A. BtaE, an Adhesin That Belongs to the Trimeric Autotransporter Family, Is Required for Full Virulence and Defines a Specific Adhesive Pole of Brucella suis. Infect. Immun. 2013, 81, 996–1007, doi:10.1128/IAI.01241-12.
- Sieira, R.; Bialer, M.G.; Roset, M.S.; Ruiz-Ranwez, V.; Langer, T.; Arocena, G.M.; Mancini, E.; Zorreguieta, A. Combinatorial control of adhesion of Brucella abortus 2308 to host cells by transcriptional rewiring of the trimeric autotransporter btaE gene. Mol. Microbiol. 2017, 103, 553–565, doi:10.1111/mmi.13576.
- Ruiz-Ranwez, V.; Posadas, D.M.; Estein, S.M.; Abdian, P.L.; Martin, F.A.; Zorreguieta, A. The BtaF trimeric autotransporter of Brucella suis is involved in attachment to various surfaces, resistance to serum and virulence. PLoS One 2013, 8, e79770, doi:10.1371/journal.pone.0079770.Ferrero, M.C.; Hielpos, M.S.; Carvalho, N.B.; Barrionuevo, P.; Corsetti, P.P.; Giambartolomei, G.H.; Oliveira, S.C.; Baldi, P.C. Key role of toll-like receptor 2 in the inflammatory response and major histocompatibility complex class ii downregulation in Brucella abortus-infected alveolar macrophages. Infect. Immun. 2014, 82, 626–639
- Muñoz González, F.; Sycz, G.; Alonso Paiva, I.M.; Linke, D.; Zorreguieta, A.; Baldi, P.C.; Ferrero, M.C. The BtaF Adhesin Is Necessary for Full Virulence During Respiratory Infection by Brucella suis and Is a Novel Immunogen for Nasal Vaccination Against Brucella Infection. Front. Immunol. 2019, 10, 1775, doi:10.3389/fimmu.2019.01775.Ruiz-Ranwez, V.; Posadas, D.M.; Estein, S.M.; Abdian, P.L.; Martin, F.A.; Zorreguieta, A. The BtaF trimeric autotransporter of Brucella suis is involved in attachment to various surfaces, resistance to serum and virulence. PLoS One 2013, 8, e79770, doi:10.1371/journal.pone.0079770.
- Fernandez-Prada, C.M.; Nikolich, M.; Vemulapalli, R.; Sriranganathan, N.; Boyle, S.M.; Schurig, G.G.; Hadfield, T.L.; Hoover, D.L. Deletion of wboA enhances activation of the lectin pathway of complement in Brucella abortus and Brucella melitensis. Infect. Immun. 2001, 69, 4407–4416, doi:10.1128/IAI.69.7.4407-4416.2001.Muñoz González, F.; Sycz, G.; Alonso Paiva, I.M.; Linke, D.; Zorreguieta, A.; Baldi, P.C.; Ferrero, M.C. The BtaF Adhesin Is Necessary for Full Virulence During Respiratory Infection by Brucella suis and Is a Novel Immunogen for Nasal Vaccination Against Brucella Infection. Front. Immunol. 2019, 10, 1775, doi:10.3389/fimmu.2019.01775.
- Bialer, M.G.; Ruiz-Ranwez, V.; Sycz, G.; Estein, S.M.; Russo, D.M.; Altabe, S.; Sieira, R.; Zorreguieta, A. MapB, the Brucella suis TamB homologue, is involved in cell envelope biogenesis, cell division and virulence. Sci. Rep. 2019, 9, 2158, doi:10.1038/s41598-018-37668-3.Fernandez-Prada, C.M.; Nikolich, M.; Vemulapalli, R.; Sriranganathan, N.; Boyle, S.M.; Schurig, G.G.; Hadfield, T.L.; Hoover, D.L. Deletion of wboA enhances activation of the lectin pathway of complement in Brucella abortus and Brucella melitensis. Infect. Immun. 2001, 69, 4407–4416, doi:10.1128/IAI.69.7.4407-4416.2001.
- Al-Mariri, A.; Abbady, A.Q. Evaluation of the immunogenicity and the protective efficacy in mice of a DNA vaccine encoding SP41 from Brucella melitensis. J. Infect. Dev. Ctries. 2013, 7, 329–337, doi:10.3855/jidc.2296.Bialer, M.G.; Ruiz-Ranwez, V.; Sycz, G.; Estein, S.M.; Russo, D.M.; Altabe, S.; Sieira, R.; Zorreguieta, A. MapB, the Brucella suis TamB homologue, is involved in cell envelope biogenesis, cell division and virulence. Sci. Rep. 2019, 9, 2158, doi:10.1038/s41598-018-37668-3.
- Pappas, G.; Papadimitriou, P.; Akritidis, N.; Christou, L.; Tsianos, E. V The new global map of human brucellosis. Lancet Infect. Dis. 2006, 6, 91–99.Al-Mariri, A.; Abbady, A.Q. Evaluation of the immunogenicity and the protective efficacy in mice of a DNA vaccine encoding SP41 from Brucella melitensis. J. Infect. Dev. Ctries. 2013, 7, 329–337, doi:10.3855/jidc.2296.
- Ferrero, M.C.; Hielpos, M.S.; Carvalho, N.B.; Barrionuevo, P.; Corsetti, P.P.; Giambartolomei, G.H.; Oliveira, S.C.; Baldi, P.C. Key role of toll-like receptor 2 in the inflammatory response and major histocompatibility complex class ii downregulation in Brucella abortus-infected alveolar macrophages. Infect. Immun. 2014, 82, 626–639
