Curcumin is a polyphenolic natural compound with diverse and attractive biological properties, which may prevent or ameliorate pathological processes underlying age-related cognitive decline, Alzheimer’s disease (AD), dementia, or mode disorders. AD is a chronic neurodegenerative disorder that is known as one of the rapidly growing diseases, especially in the elderly population. Moreover, being the eminent cause of dementia, posing problems for families, societies as well a severe burden on the economy. There are no effective drugs to cure AD. Although curcumin and its derivatives have shown properties that can be considered useful in inhibiting the hallmarks of AD, however, they have low bioavailability. Furthermore, to combat diagnostic and therapeutic limitations, various nanoformulations have also been recognized as theranostic agents that can also enhance the pharmacokinetic properties of curcumin and other bioactive compounds. Nanocarriers have shown beneficial properties to deliver curcumin and other nutritional compounds against the blood-brain barrier to efficiently distribute them in the brain.
- neurodegenerative disorder
- curcumin
- nanoparticle
- Theranostic agents
1. Introduction
Globally, mental disorders are one of the main reasons for disability and pointing concerns for public health issues [1]. In 2010, 16.2% of the world population consisted of people aged 65 or over, a figure that is expected to rise up to 26.9% by 2050. Increasing life expectancy highlights the importance of physical and mental health in old age. Aging is a very complex process that alters an individual’s normal functioning resulting in deterioration of biological functions, especially brain and cognitive functions over a long period. Previous studies have generated inconsistent findings of the prevalence of mental illness among older adults [2]. Due to their higher prevalence, mental problems are contributing to significant health, economic and social burden [3]. Previous studies tended to focus on selective disorders such as dementia or depression, implying that the entire range of mental disorders has been insufficiently addressed [4]. Dementia is a syndrome (or group of symptoms) that causes deterioration in behavior, ability to perform everyday activities, thinking, memory, and learning capacity, language, and judgement. Dementia is not a normal part of aging but generally affects old age people [5]. Among the different kinds of dementia, the most common form is Alzheimer’s disease (AD) [6] and may contribute to 60–80% of cases [7]. According to WHO [5], AD and other types of dementia are the 5th prominent reason for deaths in the world and become a growing public health concern, with about 10 million new cases every year across the world and it is estimated that the frequency will be doubled by 2030 and tripled by 2050 [8]. The conditions worsen over the years, to the point, people suffering from AD may have difficulty in remembering events, names, or recent conversations and can have depression in very early stages. Later these symptoms lead to behavioral changes, confusion, impaired communication, poor judgment, disorientation, and ultimately patients are facing difficulty in walking, swallowing, and speaking [7]. There are two major hallmarks of AD as follows; (1) development of amyloidal plaques outside the cells nerve due to cleavage of membrane-embedded proteins (amyloid precursor proteins: APP) into the neurotoxic single amyloidal units (amyloid-beta: Aβ) peptides during proteolytic processing by secretases enzymes, such as β and γ-secretase, and (2) formation of neurofibrillary tangles (NFTs) inside the nerve cells due to accumulation of paired helical filaments of hyperphosphorylated tau proteins [6]. Aβ oligomers are the small and soluble aggregates of Aβ-peptide are considered as the key pathogenic structures in AD [9][10][11][9,10,11]. Despite Aβ-tau hypothesis; vascular abnormalities, oxidative stress, neuroinflammation, mitochondrial damage, etc. are also contributing to the pathogenesis of AD [12].
Mitochondria is the essential component of a cell that plays role in cell energy production and pursuing the nutrient-sensing and growth signals within nerve cells [13]. Recently, studies have been exposed that oxidative stress in mitochondrial microglia has also played a part in the development of AD. Loss of mitochondrial membrane potential and elevated generation of reactive oxygen species (ROS) through various mechanisms have been observed in AD. Higher ROS in microglia cause inflammation and activating cell pathways of cell death [14]. Available medicinal treatments or strategies bring only symptomatic benefits, and still, there is no perfect/ideal cure for AD [15]. Therefore, the lack of effective pharmacology therapy has led researchers to seek alternative approaches to treat or prevent AD such as diet, because nutrients are important for everyday systematic function. Many epidemiological evidence suggest a strong correlation among lifestyle factors, diet, and the onset and consolidation of AD and other kinds of dementia. Moreover, it has been proven that metabolic syndromes and disorders like insulin resistance, obesity, cardiovascular diseases, diabetes, and AD are strongly related. Nutritional interventions and other preventive strategies can be effective approaches to stop or delay the risk of AD, cognitive decline and other non-psychiatric comorbidities. Many nutrients play a role in biochemical reactions; however, intake of a diet rich in probiotics, antioxidants, plant-based foods, ω-3 polyunsaturated fatty acids, soybeans, and nuts can be beneficial in mitigating AD. Furthermore, less consumption of animal-derived proteins, refined sugars, and low intake of saturated fats can also be useful in this regard [16]. Plant-based polyphenols (including curcumin) have been recognized as potent agents that can help lower down the effects of AD. Curcumin and its derivatives have potential to ameliorate the hallmarks of AD and other neurodegenerative diseases but still, clinical studies are required to prove this fact. This review summarizes the role of curcumin and its derivatives those have been beneficially associated with the upkeep of neurocognitive capacity and the risk of AD. Association of gut metabolism and curcumin to ameliorate the effects of AD and the effects of nanoformulations as theranostic agents (diagnosis and therapeutic) with the possible role and action in AD are also explored.
2. Curcumin
Curcumin (Curcuma longa L.) commonly known as turmeric belongs to Zingiberaceae (or ginger family) is a bright yellow plant pigment, a popular spice, and food additive widely used in South Asian and Middle Eastern countries [17]. Zingiberaceae is a family of flowering plants which is a rhizomatous herbaceous perennial known as Angiosperms. The powdered rhizome (of turmeric) is used in traditional medicine to cure various kinds of maladies as well as a coloring agent in beverage industries [18]. Compositionally, three main compounds of curcumin (1,7-bis[4-hydroxy-3-methoxyphenyl]-1,6-heptadiene-3,5-dione) are curcuminoid complex (80%), dimethoxy-curcumin (17%, 1,7-bis(3,4-dimethoxyphenyl)-1,6-heptadiene-3,5-dione) and bisdemethoxy-curcumin (3%, 1,7-Bis(4-hydroxyphenyl)-1,6-heptadiene-3,5-dione) [19]. Modern medicine has shown that curcumin exhibits a broad range of biological and pharmacological activities, including antioxidant [20], anti-inflammatory [21], anti-tumor and chemosensitizing [22], hepatoprotective [23], lipid-modifying [24] and neuroprotective [25] effects and are suggested to improve mental illnesses due to its ability to modulate numerous signaling molecules [8][26][27][8,26,27]. Many studies show that it is safe to consume 8 g per day of curcumin [28][29][28,29]. Therefore, many curcumin based products are available such as tablets, capsules, and as an additive to various energy drinks [27].
2.1. Curcumin and Aβ
It has been mentioned above and also evident from several studies that the formation of Aβ plaques is the beginning of the onset of AD. Thus, inhibition or hindrance of these plaques can be useful to deal with AD. Curcumin is proposed to be resistant to Aβ aggregation as has a higher binding affinity to Aβ [30] and its derivatives are also considered as principal candidates to prevent the aggregation of amyloid plaques. Qin et al. [31] in their work regarding detection strategies of AD disclosed that curcumin has potential to bind Aβ and iron in plaques through intermolecular hydrogen bonds without the combination of any other chemical linkages or bonding. Molecular chains of curcumin have symmetrical methoxyl and phenyl groups. These groups can easily bind amyloid plaques via hydrophobic interactions with nonpolar sections of the amyloid plaques. Further, these can be stabilized by di-ketone and hydroxyl groups of curcumin with polar sections of Aβ plaques by hydrogen bonding. Consequently, curcumin can be considered as potent candidate for binding and locating Aβ plaques in a brain suffering from AD [32]. Besides, in vivo studies have documented that curcumin inhibits the aggregation of Aβ and also reduces the size of deposits [33][34][33,34]. Curcumin destabilizes Aβ40 and Aβ42 [35] as well as pyrazoles and isoxazoles (derived from curcumin) upon binding with Aβ inhibits the metabolism of AβPP [36]. Curcumin also has the potential to block Aβ self-assembly by inhibiting Aβ formation [33]. Kim et al. [37] stated that curcumin protects human umbilical endothelial cells and PC12 cells (PC12 cell line is used in neuroscience research, such as studies on neuroprotection, neurotoxicity, and neuroinflammation [38]) from oxidative stress-induced by Aβ that helps to suppress the levels of oxidized proteins and interleukin-1β in the brains of APP mice. Fiala et al. [39] [39] proposed that bone marrow-derived dendritic cells may correct immune defects and offer immunotherapy to the person suffering from AD by enhancing the Aβ uptake by macrophages. Curcumin regulates Aβ metabolism and inhibits Aβ aggregation in several ways, as shown in Figure 1.
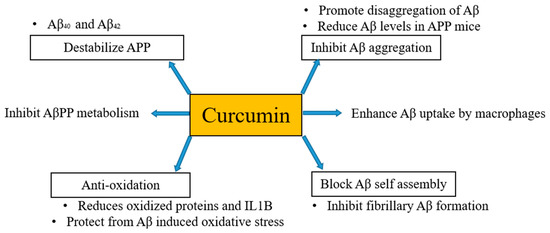
Figure 1.
2.2. Curcumin and Glial Cells
Glial cells are non-neuronal cells (consisting of astrocytes, oligodendrocyte lineage cells as their significant components and especially microglia) from the immune system of the central nervous system (CNS) play major roles in modulating neural plasticity, maintaining homeostasis, and shaping brain development [40][42]. Microglia, being the primary immune cell of the CNS and due to highly responsive cell, reacts immediately against local injury, a multiplicity of brain pathologies, neuroinflammation, and immune surveillance [41][43]. Recently conducted studies on genetics have highlighted more about the importance of these cells as with the discovery of many polymorphisms in microglial-enriched genes. They are related to various neurological disorders such as amyotrophic lateral sclerosis, frontotemporal dementia, AD, schizophrenia, and autism [40][42]. Glial cells originate an inflammatory cascade under the influence of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling. NF-κB seems to modulate the expression of many chemokines and cytokines, including interleukin, tumor, interferon, necrosis factor α, and inflammation reactive proteins. These proteins and cytokines through paracrine or autocrine pathways excite the glial cells for additional production of Aβ42, p-tau, and pro-inflammatory molecules that causing neurodegeneration (Figure 2).
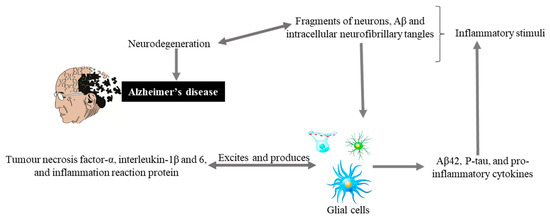
Figure 2. Hypothetical inflammation in AD. Inflammatory stimuli activate glial cells that produce tumor necrosis factor-α, interleukin-1β and 6, and inflammation reaction proteins. These can also excite glial cells that produce Aβ42, P-tau, and pro-inflammatory cytokines, and cycle is maintained that leads to neurodegeneration and Alzheimer’s disease.
Concerning this, many studies have reported that through inhibiting activation of the TLR4/MyD88/NF-κB signaling pathway and promoting M2 polarization, curcumin offers protection of neurons [42][44]. Liu et al. [43][45] documented that curcumin enhances the PPARγ activity and reduces the production of cytokines, astrocytes, and microglia through the inhibition of the NF-κB signaling that decreases the neuroinflammation in AD induced rats. Curcumin could regulate the production of CC motif ligand 2 and reduce c-Jun N-terminal kinase phosphorylation in astrocytoma cells [44][46]. Additionally, curcumin has the ability to enhance the RANTES (Regulated upon Activation, Normal T Cell Expressed and Presumably Secreted) expression in P1-3K signaling pathways, activated mitogen-activated protein kinase (MAPK), and in astrocytes so, ultimately shows neuroprotective activities in rats [45][47]. Tai et al. [46][48] exhibited that curcuminoid submicron particles can improve the pathological deficits and memory impairment in mouse with AD. Moreover, it decreases the astrogliosis and Aβ plaques in vivo and enhances the microglial Aβ phagocytosis in vitro. Thus, these are the main mechanisms that shows the beneficial properties of curcuminoid submicron particles in modulation of neuroinflammation. Curcumin at 160 ppm can reduce oxidative damage, significantly reduce Aβ and can inhibit markers of glial inflammation such as cytokine interleukin-1 and glial fibrillary acidic protein in AD transgenic mice [47][49]. Hence, curcumin has low toxicity and high efficacy, so can be a potential agent for AD treatment.
2.3. Curcumin and Tau Proteins
Tau proteins are associated with microtubules (major components of the cytoskeleton and abundantly present in neurons), found in the central and peripheral nervous system. Post-translational modifications (most notable phosphorylation) are deciding the localization of tau in neurons [48][50]. Same as other proteins associated with microtubule, tau also monitors intracellular trafficking and stabilizes microtubule integrity [49][51]. Conversely, in AD, tau losses their normal functioning to bind microtubules but start to bind each other’s which results in accumulated phosphorylation. Due to phosphorylated tau overall tubulin assembly starting to decrease that lead towards the development/formation of intraneuronal NFTs [50][52]. These NFTs remain intracellular and causing the death of neurons [51][53]. Moreover, impaired tau’s function increases the burden on the mitochondrial function that leads to ROS release and mitochondrial dysfunction [52][54]. Thus, therapeutic agents that can target tau pathology, not only repair abnormality in tau but also restrain a series of reactions caused by the abnormality of tau could play a vital role in the treatment of AD [53][55]. Studies have been confirmed that curcumin has these abilities that could decrease hyperphosphorylated tau aggregation and restrain tau hyperphosphorylation [54][56] by avoiding intracellular fibrillary tangles [55][56][57,58]. Curcumin can suppress tau protein dimmer formation and hyperphosphorylated tau protein oligomerization and prevents glycogen synthase kinase-3β activity in tau protein-induced AD mice. However, docosahexaenoic acid and curcumin (oral administration) inhibited insulin receptor substrate 1 and c-Jun N-terminal kinase (family of protein kinases that have a significant role in stress signaling pathways associated with neuronal plasticity, cell death, gene expression, and regulation of cellular senescence [57][59]) activities that suppressed hyperphophorylated tau protein levels [58][60]. Another mechanism of hyperphosphorylation inhibition by curcumin is reported by Huang et al. [59] [61] which showed that human neuroblastoma SHSY5Y cells via the phosphate and tensin homologue/protein kinase B (Akt)/GSK-3 pathway which is induced by Aβ inhibits the tau pathologies.
2.4. Curcumin, Oxidative Stress and Metal Chelation
2.4. Curcumin, Oxidative Stress and Metal Chelation
It has been reported that oxidative stress plays a vital role in the pathogenesis of neurological and age-related diseases. Activation of nuclear factor erythroid 2-related factor 2 (Nrf2) occurs during oxidative stress that modulates the expression of various antioxidant dense enzymes and exhibits protective effects for free radical-mediated neuronal damage [60][62]. Further, Nrf2 also regulate genes related to nerve growth factor signaling and autophagy [61][63]. Several lines of evidence have demonstrated activation of the Nrf2 pathway as a promising novel strategy for the management and prevention of diseases related to the brain. Curcumin through its antioxidant effect raises the levels of glutathione that suppresses formation of 3-nitrotyrosine (indicator of nitric oxide production, inflammation, and neural damage) [62][64]. Gao et al. [63][65] demonstrated curcumin loaded T807/triphenylphosphine-red blood cell nanoparticles in AD model mice and found relieved symptoms of AD by mitigating mitochondrial oxidative stress and neural death in vitro and in vivo. Curcumin exhibits metal chelation properties that are due to its two methoxyphenol groups linked with a β di-ketone linker, which helps in scavenging hydroxyl radicals preserve glutathione and superoxide that ultimately suppresses oxidative damage. Additionally, it is also a selective activator of Nrf2/Keap1/ARE, which trigger heme oxygenase-1 (a redox-sensitive inducible protein) and relieves oxidative stress-mediated neuronal injury [64][66]. As well, curcumin shields astrocytes (as activation of astrocytes is one of the key pathological hallmarks of neural disorders) from oxidative stress and mitochondria impairment. It has a defensive action on astrocytes by its negative consequence on apoptosis and reactive astrogliosis [65][67]. It has been documented that curcumin prevents astrogliosis in astrocytes, mitochondrial dysfunction, and caspase 1-dependent inflammation that ultimately hampers the mitochondria-dependent/independent apoptosis caused by oxidative damage [66][68]. Maiti et al. [67] [69] observed that relative to natural curcumin, solid lipid curcumin particles were more permeable, useful on Aβ plaques and produced a more considerable decrease of pyknotic or tangle-like, neurons in the prefrontal cortex, CA1, and CA3 areas of the hippocampus.
3. Curcumin, Gut Microbiota and AD
Although AD is a neurodegenerative disease, however, scientists are considering a new hypothesis that despite the limited bioavailability of curcumin in gut, it has an indirect influence on the CNS that is due to microbiota-gut-brain axis [68][74]. It has been stated that gut bacterial metabolites employ their neuroprotective effects in several neurodegenerative disorders like AD [68][69][70][74,75,76]. Microbiota–gut-brain axis is a complex but bidirectional system in which gut microbiota (GM) and its composition represent a factor that preserves and determines the brain health [71][72][77,78]. Recently Sun et al. [73] [79] revealed that the interaction between curcumin and GM is bidirectional as GM transform curcumin while curcumin influences on the abundance of GM. It has been found that administration of curcumin tends to enhance the spatial learning and memory abilities as well as inhibits the Aβ plaques in the hippocampus of APP/PS1 mice. Apart from this, curcumin significantly alters the composition of GM taxa such as Prevotellaceae, Bacteroidaceae, Lactobacillaceae, and Rikenellaceae at family level Bacteroides, Prevotella, and Parabacteroides at the genus level, several of them are considered to be associated with AD development [73][79]. While the GM metabolite curcumin through demethylation, reduction, acetylation, hydroxylation, and demethoxylation [74][80]. The produced metabolites have been documented to show neuroprotective effects in AD-induced mice [73] [79] (Figure 3). It has been considered that gut metabolism can indirectly pose neuroprotective abilities via modulation of gut-brain axis (GBX) [69][75][75,81]. These findings not only enlighten the paradox between the low bioavailability of curcumin and its pharmacological effects, but also claim that GM might act as a useful source for microbiome-targeting therapies for AD. The low oral bioavailability of curcumin may be speculated as a plausible factor that limits its effects in humans [66][68]. Thus, utilization of several approaches can improve the bioavailability and its therapeutics as Annunziata et al. [76][82] proposed that fermentation is a natural strategy with minimum environmental impacts to increase the bioavailability of bioactive compounds (e.g., curcumin) that may help to cope up with AD. Fermentation mainly enhances the solubility and converts it to activated form that the body readily utilizes it for proper therapeutic effects. There are different types of fermentation (including lactic acid fermentation, alcohol fermentation, and acid fermentation), among them lactic acid fermentation considered as better because it is not contributing to cytotoxicity [77][83]. On the other hand, microencapsulation is a new strategy to improve the availability, delivery and therapeutic effects [78][84]. In microencapsulation, substances are incorporated in microscopic capsules that can measure from millimeter to micrometer, consist continuous films of coating material [79][85]. Microencapsulation enhances the solubility of curcumin in the aqueous solution and protect it during the gastrointestinal tract interact as well as increase the residence of curcumin and other substances in the intestine with the promotion of endocytosis [80][86]. Besides, Reddy et al. [69][75] suggested that nanoencapsulation is the encapsulation of substances at the nanoscale, improves the solubility, stability, target specificity, and drug release of the substance. It is considered advantageous as compared to microencapsulation as they have ultrathin layers that improve mass transport of substances to the islets and also reduces the volume of material [81][87]. Positive effects of these approaches in cell and animal models have shown positive results, but studies related to human are still lacking.
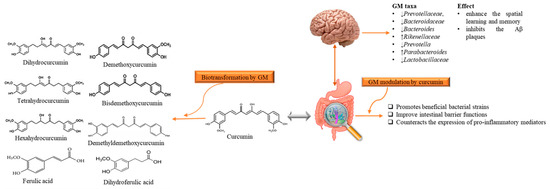
Figure 3. The reciprocal interaction between curcumin and Gut microbiota. Biotransformation of curcumin occurs due to gut microbiota that converts it into several metabolites through pathways like demethylation, reduction, acetylation, hydroxylation, and demethoxylation. These metabolites through gut-brain axis show antioxidant, anti-inflammatory, and neuroprotective effects. While, gut microbiota modulation alters the microbial abundance, diversity and composition, which also exert health benefits in Alzheimer disease-induced rats, indirectly. GM: gut microbiota.
4. Nanoformulations and Their Role in AD
4.1. Nanoparticles/Nanosensors as Theranostic Agents
Detection of AD during the early stages or the phase of the illness will offer great relief to patients suffering from AD, their friend and families, along with the economy [82][125]. Thus, it is the need of time to create multi-mode devices capable of simultaneous monitoring of many biomarkers that can stimulate fast, low cost, and reliable diagnosis [83][126]. Neurotransmitters are suitable biomarkers for various neurological disorders, such as AD. Several analytical techniques, such as microdialysis, capillary electrophoresis and electrochemistry are generally used to diagnose neurotransmitters of AD. However, researchers are still striving to discover an easy and accurate method to detect neurotransmitters of AD before devastating symptoms begin. In the last decade, biosensors have gained popularity as an attractive alternative method, and valuable efforts have been successfully made by scientists for the development of viable biosensors to propose a new analytical platform [84][127]. Biosensors are devices used for the detection of a chemical substance. They integrate a biochemical binding component with a signal conversion part and recently, several researchers have investigated the use of unique materials for developing biosensor for detection of different biomarkers of AD [85][86][128,129]. With the advancement in nanotechnology, nano-bio sensors have many prospective benefits as compared to other analytical or clinical methods. This advancement leads to improved assay efficiency, decrease in diagnostic testing costs, resourcefulness, and ability to deliver family general practitioners with molecular diagnostic devices [87][130]. Furthermore, the incorporation with nanocarriers has also been documented to enhance the conductivity and catalytic properties of the transducer. It happens while facilitating the immobilization of a large number of biological recognition elements because of the large surface area of nanoparticles [88][131]. The most common nanoparticle formulations that have a significant impact in the diagnosis and therapy of AD include polymeric nanoparticles, protein-based nanoparticle, gold nanoparticle, polysaccharide-based nanoparticle, selenium nanoparticle, gadolinium nanoparticle, etc. [89][132]. Recently, carbon nanomaterials, including carbon nanostructures, carbon dots, carbon nanotubes, graphite, graphene, and fullerene have received considerable significance [90][133]. After crossing the blood-brain barrier, nanoparticles can bind monomers and oligomers of Aβ so, specific inhibition of neurotoxicants can help to ameliorate AD. Surface functionalization or chemical modification of these nanocarriers may improve the aqueous solubility, and therefore, facilitating their utilization to treat the neurodegenerative disorders. Moreover, the concentration of drugs or conjugation of drugs with nanocarriers can be considerably used as an effective neuro-drug delivery system [91][134].
4.2. Curcumin Loaded with Nanoformulations and Their Therapeutic Effects in AD
Nano-delivery systems such as polymeric nanoparticles, peptide carriers, micelles, liposomes, cyclodextrins, conjugates, lipidic nanoparticles, emulsions, and solid dispersions have been extensively studied for improving the overall bioavailability of curcumin for enhanced brain delivery [92][149]. The main characteristic and transport mechanism for brain uptake and blood-brain barrier crossing of the main curcumin conjugated nanocarriers are exhibited in Table 13. Curcumin loaded with poly(lactic-co-glycolic acid; PLGA)-poly(ethylene glycol) nanoparticles permeate through the impaired blood-brain barrier and diffused efficiently through the brain parenchyma. Additionally, were localized in brain regions with AD, and exhibited a protecting impact in the injured neonatal brain [93][150]. Recently, a study stated the neuroprotective effect of red blood cell membrane-coated PLGA particles bearing T807 molecules attached to the red blood cell membrane surface (T807/RPCNP) loaded with curcumin. A stabilized and sustained curcumin release was observed. Synergistic effects of T807, T807/RPCNP showed effective penetration across the blood-brain barrier, and they also exhibited a high binding affinity to hyperphosphorylated tau in nerve cells where they block multiple critical pathways in tau-associated AD pathogenesis. Additionally, curcumin loaded T807/RPCNP nanoparticles could relieve AD symptoms by reducing phosphorylated-tau levels and inhibiting the neuronal death both in vitro and in vivo [94][151]. Curcumin nanoformulation by altering the surface of the PLGA polymer and encapsulation of selenium nanoparticles could decrease the Aβ load and inflammations in the brains samples of AD mice and treated the memory loss of the model mice. Moreover, the histopathological images of animal tissues displayed no deceptive abnormalities [32]. Zhang et al. [95] [152] developed hydroxypropyl-β-cyclodextrin-encapsulated curcumin complexes (CUR/HP-β-CD inclusion complexes), and curcumin encapsulated chitosan-coated PLGA nanoparticles (CUR-CS-PLGA-NPs) and compared their effects through intranasal administration. CUR/HP-β-CD inclusion complexes (in vitro) showed stability under physiological parameters and also showed a higher cellular uptake level of curcumin as compared to CUR-CS-PLGA-NPs. Further, both formulations behaved to reduce cellular cytotoxicity and exhibited antioxidant and anti-inflammatory effects. in vivo, after intranasal administration, the area under cover values of curcumin in the brain and plasma of the CUR/HP-β-CD inclusion complex group also showed higher values. So, CUR/HP-β-CD inclusion complexes presented better properties for application in AD than CUR-CS-PLGA-NPs.
Table 13. Characteristic and the transport mechanism of the main curcumin-conjugated nanocarriers for BBB.
| Nano-Carrier Type | Shape/Size | Most Investigated Components | Mechanism in BBB | References |
|---|---|---|---|---|
| Solid Lipid Nanoparticles | Spherical (50–300) | Triglycerides, monoglycerides, complex glyceride mixtures, hard fats, cetyl alcohol, stearic acid, emulsifying wax and cholesterol butyrate. Surfactants are used to stabilise the lipid core (about 1–5% w/v) or co-surfactant (such as poloxamer 188 and/or Tween® 80) | Uptake by the paracellular pathway through brain microvasculature, endocytosis and passive diffusion, tight junctions opening. Active targeting with apolipoprotein E | [96][97][156,157] |
| Liquid Crystalline Nanocarriers | Inverted hexagonal (hexosomes), bicontinuous cubic (cubosome), or sponge phases (20–200 nm) | Unsaturated monoglycerides, phospholipids, glycolipids and surfactants | Adsorption-mediated transcytosis, passive targeting or receptor-mediated endocytosis | [98][99][158,159] |
| Liposome | Globular/lamellar (20–200 nm) | Lipids: PEGylated 1,2-distearoyl-sn-glycero-3-phospho-ethanolamine-PEG 2000, phosphatidylcholine, ethyl-phosphatidyl-choline, 2-dipalmitoyl-sn-glycero-3-phospho-choline, lecithin, sphingomyelin, cholesterol, | Passive targeting, receptor-mediated endocytosis or Adsorption-mediated transcytosis Active targeting with receptors glutathione, glucose, transferrin, lactoferrin, apolipoprotein E, and phosphatidic acid |
[100][101][160,161] |
| Micelles | Spherical (20–100 nm) | PLGA-PEG-PLGA triblock and PLGA-PEG diblock copolymers | Uptake by endocytosis and/or transcytosis. Targeting ligands with surface conjugation improve the transcytosis | [102][103][162,163] |
| Polymer Nanoparticles | Globular (10–200 nm) | PLGA, PBCA, PLA, chitosan and alginate. | Uptake by transcytosis and/or endocytosis through the endothelial cells and tight junctions opening | [89][96][132,156] |
| Cyclodextrins | Cyclic (150–500 nm) | Mainly the β-cyclodextrin derivatives | The direct action of cyclodextrin by extracting lipids like phospholipids and cholesterol, and proteins Modify the properties of the lipid bilayers and molecular composition |
[104][105][164,165] |
BBB: Blood-brain barrier, PLGA: Poly (lactic-co-glycolic acid), PEG: Polyethyleneglycol, PBCA: Poly(butyl)cyanoacrylate, PLA: poly (lactic acid).
4.3. Curcumin Derivatives, Curcumin and Its Nanoformulations Based Diagnostic Properties
Curcumin has properties like natural fluorescence, lipophilicity, and high binding affinity to Aβ, making it able for early diagnostic probe/plaque labelling fluorochrome [106][166]. It has been documented that it fluoresces yellow/green under a violet/blue (436 nm) light. These natural fluorescent qualities of curcumin have been explored in many studies for diagnostic purpose, which make it to absorb light at about 420 nm and discharges fluorescence at around 530 nm in aqueous solutions [107][167]. During the last two decades, comprehensive research studies have been conducted to develop curcumin-based probes for targeting Aβ with positron emission tomography, with existing imaging modalities, magnetic resonance imaging, near-infrared fluorescence and two-photon microscopy [108][168]. Si et al. [109][169] worked on the near-infrared fluorescence imaging probes of Aβ plaques for the early diagnosis of AD. Curcumin derivative dyes showed a considerably significant improvement in its fluorescence intensity after binding to Aβ plaques. Furthermore, in vitro and in vivo fluorescence imaging of Aβ plaques strained with one of the dyes exhibited that the compound was a potential probe to detect Aβ plaques in AD. Chibhabha et al. [110][170] developed anionic and water-soluble DSPE-PEG2000 curcumin polymeric micelles (also referred as curcumin micelles) those can detect both brain and retinal Aβ plaques. Another study showed that the curcumin-Ni electrode reported high sensitivity and wide detection range from 0.001 to 10 nM Aβ oligomer. This electropolymerized curcumin pledge the detection of Aβ oligomer at lower concentration and therefore, can be a prospective tool for the early detection of AD [31]. Singh et al. [111] [171] proposed curcumin encapsulated Pluronic F127 nanoparticles (FCur NPs) and compared their blood-brain barrier penetration of free curcumin and FCur NPs. The FCur NPs displayed 6.5-fold more potent fluoresce intensity in the brain of mice than free curcumin. Future, in vitro comparison with Congo red revealed that encapsulated curcumin maintains its ability to bind to Aβ plaques. FCur NPs also exhibited antioxidant and antiapoptotic activity when compared to free curcumin. The combination of in vitro and in vivo results suggests the potential utility of the inexpensive FCur NPs as a theranostic agent for AD. Hence, it can be proposed from the mentioned studies that curcumin and its nanoformulations based techniques cannot only be used for therapeutic purpose but also for diagnostic purpose.
