Gestational diabetes mellitus (GDM) is a metabolic condition of increased maternal blood glucose level [1,2]. GDM affects up to 36% of pregnant women [3,4]. Mood dysregulations (MDs, e.g., depression, distress, and anxiety) are common among women with GDM [5]. Symptoms of depression and anxiety usually overlap with one another as well as with distress symptoms [6]; they develop in up to 27% and 24% of pregnancies, respectively [7,8].
- Mood Dysregulations
- gestational diabetes
- intermittent fasting/dietary interventions
1. Overview
The co-occurrence of GDM and MDs is high, and it is closely linked to poor glycemic control, poor self-care ability, functional disability, low quality of life, and premature death. Depression in diabetics is hard to treat given the complex nature of diabetes, especially when it occurs during pregnancy [1][9]. Both conditions share common risk factors, pathologies and adverse effects for mothers and their offspring[2] [5].
2. Adverse effects of GDM and MDs
GDM is associated with numerous morbid maternal and fetal outcomes: miscarriage, dystocia, cesarean section, neonatal death, premature birth, congenital anomalies, macrosomia (large for gestational age), respiratory distress, neonatal jaundice, hypoglycemia, hypocalcemia, and polycythemia [3][4][5][10-12]. Meanwhile, MDs increase the likelihood of prematurity, birth weight abnormalities, stunted growth, cognitive and neurodevelopmental deficits, infants’ temperament, anxiety, and depression, delays in fine motor and language development[6][7] [13,14], not initiating breastfeeding, early termination of breastfeeding, increased formula usage[8][9] [15,16], poor maternal antenatal attachment, and higher postpartum parenting stress[8] [15].
3. Risk factors and pathophysiological mechanisms common in GDM and MDs
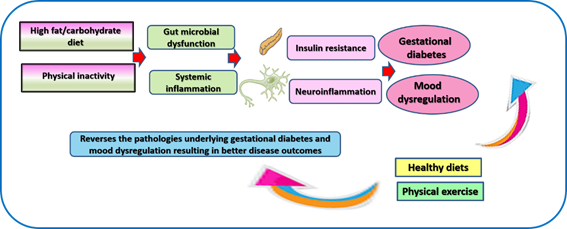
The accelerated prevalence of GDM and MDs is associated with common risk factors such as poverty, unhealthy diet and sedentary lifestyle, which contribute to chronic inflammatory conditions such as obesity and type 2 diabetes mellitus (T2DM)[10][11][12][13] [17-20]. Sleep disturbances, some of them develop in mood disorders (e.g., apnea, poor sleep quality, and short sleep), trigger the development of GDM[10][11] [17,18]. Both GDM and MDs share common mechanisms[2] [5] illustrated in Figure 1 and described in detail in this section.
Figure 1. Schematic summary of key risk factors and pathologies contributing to gestational diabetes and maternal mood dysregulation along with the therapeutic role of dietary interventions and physical activity in reverting these conditions.
Unhealthy food (rich in fat and refined carbohydrates and deficient in dietary fibers that exist in fruits and vegetables) and physical inactivity promote the propagation of toxic endobacteria and inhibit the growth of beneficial gut microflora[14][15][16] [21-23]. Such imbalance result in a deficiency of nutritive metabolites produced by beneficial bacteria, which are necessary for proper immune function and overall health[2][17][18][19] [5,24-26]. In addition, toxic bacterial metabolites cause injury to the intestinal wall to cause disseminated tissue damage e.g., in the brain leading to MDs and in the pancreas leading to GDM[2] [5].
The inflammatory reaction and associated oxidative stress conducive to GDM and MDs is accelerated by fetal tissue and placental-related molecules [4,27-29]. Secretions of these tissues bind the receptor of advanced glycation end products (RAGE) and toll-like receptors (TLR2 and TLR4), which indirectly activate C-X-C chemokine receptor type 4 (CXCR4) [20][21][22][23][4,27-29].
Estrogen is a key modulators of signal transduction that regulate cognition and mood[15][24] [22,30]. Alterations in estrogen are a key contributor to the high development of negative emotional states among women at different stages of life [24][25][30,31]. Estrogen dysfunction occurs in GDM due to oxidation of estrogen by cytochrome P450 enzymes resulting in genotoxic metabolites such as 2-hydroxyestrogen and 4-hydroxyestrogen. These metabolites exist in the blood, and they induce DNA damages in various tissues. They also bind insulin, neuroglobin, human serum albumin, and immunoglobulin. Their binding to insulin, a process known as insulin estrogenization, hinders insulin affinity of binding to insulin receptor resulting in vivid insulin resistance [26][32]. In the meantime, dysfunctional estrogen is uncapable of modulating brain structures
MDs in pregnancy may occur in response to the psychosocial stress imposed by pregnancy, hormonal fluctuations associated with pregnancy, as well as the inflammatory milieu generated by GDM and its underlying pathologies[2] [5].
4. Common non-pharmacological interventions for GDM and MDs
Physical exercise is well-known to increase the sensitivity of body tissue to insulin resulting into glycemic control[16][19] [23,26]. It is also an effective treatment for mood disorders[27][28] [33,34] . Its therapeutic effects take place through the regulation of key signaling molecules involved in cellular activities as well as correction of gut dysbiosis, thus preventing gut-related systemic inflammation[16][19][29][30] [23,26,35,36].
A plethora of research emphasize the role of functional foods in the control of GDM and MDs[28][31][32] [34,37,38]. Such foods correct gut microbiome dysfunction, modulate signaling involved in metabolism, immune function, antioxidant production, autophagy, etc.[17][18][33][34] [24,25,39,40]. A considerable attention is paid to refraining from high fat/high carbohydrate diets and adopting dietary patterns that involve the intake of fresh fruits, vegetables, sea foods, complex carbohydrates (whole grains and bran of wheat and rice), bee products, etc.[2][16][35][36] [5,23,41,42]. Intermittent fasting (IF) as a form of dietary restriction has been effectively used to correct pathologies that underlie T2DM and MDs. We have recently demonstrated a case of GDM properly managed by IF indicating that this modality may prove to be effective if investigated on a large scale[2] [5].
5. Conclusion
Both GDM and mood disorders are common in pregnancy, and their co-occurrence leads to grave effects on mothers and their fetus. Both conditions can be exacerbated by unhealthy diet and sedentary behaviors while dietary patterns such as IF, the intake of functional foods, and physical exercise can reverse the pathologies that underlie these conditions leading to better outcomes.
References
- Lorenzo, P.I.; Martín-Montalvo, A.; Cobo Vuilleumier, N.; Gauthier, B.R. Molecular Modelling of Islet β-Cell Adaptation to Inflammation in Pregnancy and Gestational Diabetes Mellitus. Int J Mol Sci 2019, 20, doi:10.3390/ijms20246171.
- Allman, B.R.; Andres, A.; Børsheim, E. The Association of Maternal Protein Intake during Pregnancy in Humans with Maternal and Offspring Insulin Sensitivity Measures. Curr Dev Nutr 2019, 3, nzz055, doi:10.1093/cdn/nzz055.
- Hernandez, T.L.; Brand-Miller, J.C. Nutrition Therapy in Gestational Diabetes Mellitus: Time to Move Forward. Diabetes Care 2018, 41, 1343-1345, doi:10.2337/dci18-0014.
- Santangelo, C.; Filardi, T.; Perrone, G.; Mariani, M.; Mari, E.; Scazzocchio, B.; Masella, R.; Brunelli, R.; Lenzi, A.; Zicari, A., et al. Cross-talk between fetal membranes and visceral adipose tissue involves HMGB1-RAGE and VIP-VPAC2 pathways in human gestational diabetes mellitus. Acta Diabetol 2019, 56, 681-689, doi:10.1007/s00592-019-01304-x.
- Ali, A.M.; Kunugi, H. Intermittent fasting, dietary modifications, and exercise for the control of gestational diabetes and maternal mood dysregulation: a review and a case report. Int. J. Environ. Res. Public Health 2020, 17, 9379, doi:10.3390/ijerph17249379.
- Ali, A.M.; Green, J. Factor structure of the depression anxiety stress Scale-21 (DASS-21): Unidimensionality of the Arabic version among Egyptian drug users. Substance Abuse Treatment, Prevention, and Policy 2019, 14, 40, doi:10.1186/s13011-019-0226-1.
- Ecklund-Flores, L.; Myers, M.M.; Monk, C.; Perez, A.; Odendaal, H.J.; Fifer, W.P. Maternal depression during pregnancy is associated with increased birth weight in term infants. Dev Psychobiol 2017, 59, 314-323, doi:https://doi.org/10.1002/dev.21496.
- Alqahtani, A.H.; Al Khedair, K.; Al-Jeheiman, R.; Al-Turki, H.A.; Al Qahtani, N.H. Anxiety and depression during pregnancy in women attending clinics in a University Hospital in Eastern province of Saudi Arabia: prevalence and associated factors. International journal of women's health 2018, 10, 101-108, doi:10.2147/IJWH.S153273.
- Wang, D.; Wang, H.; Gao, H.; Zhang, H.; Zhang, H.; Wang, Q.; Sun, Z. P2X7 receptor mediates NLRP3 inflammasome activation in depression and diabetes. Cell Biosci 2020, 10, 28, doi:10.1186/s13578-020-00388-1.
- Pintaudi, B.; Fresa, R.; Dalfrà, M.; Dodesini, A.R.; Vitacolonna, E.; Tumminia, A.; Sciacca, L.; Lencioni, C.; Marcone, T.; Lucisano, G., et al. The risk stratification of adverse neonatal outcomes in women with gestational diabetes (STRONG) study. Acta Diabetol 2018, 55, 1261-1273, doi:10.1007/s00592-018-1208-x.
- Filardi, T.; Panimolle, F.; Lenzi, A.; Morano, S. Bisphenol A and Phthalates in Diet: An Emerging Link with Pregnancy Complications. Nutrients 2020, 12, doi:10.3390/nu12020525.
- Nguyen-Ngo, C.; Jayabalan, N.; Haghvirdizadeh, P.; Salomon, C.; Lappas, M. Role of adipose tissue in regulating fetal growth in gestational diabetes mellitus. Placenta 2020, doi:https://doi.org/10.1016/j.placenta.2020.05.006.
- Aoyagi, S.S.; Tsuchiya, K.J. Does maternal postpartum depression affect children's developmental outcomes? J Obstet Gynaecol Res 2019, 45, 1809-1820, doi:10.1111/jog.14064.
- Sadurni, C.; Schneider, B.; Perez Delgadillo, P.; Rodriguez, M.; Tourgerman, I. A-72The Effects of Maternal Prenatal Mental Health Stress on Neurodevelopmental Deficits. Arch Clin Neuropsychol 2016, 31, 611-611, doi:10.1093/arclin/acw043.72.
- Galbally, M.; Watson, S.J.; Boyce, P.; Nguyen, T.; Lewis, A.J. The mother, the infant and the mother-infant relationship: What is the impact of antidepressant medication in pregnancy. J Affect Disord 2020, 272, 363-370, doi:10.1016/j.jad.2020.03.116.
- Stuebe, A.M.; Meltzer-Brody, S.; Propper, C.; Pearson, B.; Beiler, P.; Elam, M.; Walker, C.; Mills-Koonce, R.; Grewen, K. The Mood, Mother, and Infant Study: Associations Between Maternal Mood in Pregnancy and Breastfeeding Outcome. Breastfeed Med 2019, 14, 551-559, doi:10.1089/bfm.2019.0079.
- Kalmbach, D.A.; Cheng, P.; Sangha, R.; O'Brien, L.M.; Swanson, L.M.; Palagini, L.; Bazan, L.F.; Roth, T.; Drake, C.L. Insomnia, Short Sleep, And Snoring In Mid-To-Late Pregnancy: Disparities Related To Poverty, Race, And Obesity. Nature and science of sleep 2019, 11, 301-315, doi:10.2147/NSS.S226291.
- Mendez, D.D.; Thorpe, R.J.; Amutah, N.; Davis, E.M.; Walker, R.E.; Chapple-McGruder, T.; Bodnar, L. Neighborhood racial composition and poverty in association with pre-pregnancy weight and gestational weight gain. SSM - Population Health 2016, 2, 692-699, doi:https://doi.org/10.1016/j.ssmph.2016.09.008.
- Kampmann, U.; Knorr, S.; Fuglsang, J.; Ovesen, P. Determinants of Maternal Insulin Resistance during Pregnancy: An Updated Overview. J Diabetes Res 2019, 2019, 5320156, doi:10.1155/2019/5320156.
- Filardi, T.; Panimolle, F.; Crescioli, C.; Lenzi, A.; Morano, S. Gestational Diabetes Mellitus: The Impact of Carbohydrate Quality in Diet. Nutrients 2019, 11, doi:10.3390/nu11071549.
- Ali, A.M.; Kunugi, H. Apitherapy for Parkinson's disease: A focus on the effects of propolis and royal jelly. Oxid Med Cell Longev 2020, 2020, 1727142, doi:https://doi.org/10.1155/2020/1727142.
- Ali, A.M.; Kunugi, H. Royal jelly as an intelligent anti-aging—a focus on cognitive aging and Alzheimer's disease: a review. Antioxidants 2020, 9, E937, doi:10.3390/antiox9100937.
- Ali, A.M.; Kunugi, H. Apitherapy for age-related skeletal muscle dysfunction (sarcopenia): A review on the effects of royal jelly, propolis, and bee pollen. Foods 2020, 9, E1362, doi:10.3390/foods9101362.
- Ali, A.M.; Ali, E.M.; Ahmed, M.S.; Hendawy, A.O. Targeting gut microbiome and the recovery of muscle loss associated with cancer (cachexia): An overview of the possible effect of bee products. Medico Legal Update 2021, 21.
- Ali, A.M.; Hendawy, A.O. So, Antidepressant Drugs have Serious Adverse Effects, but what are the Alternatives? Nov Appro Drug Des Dev 2018, 4, 555636, doi:10.19080/NAPDD.2018.04.555636.
- Ali, A.M.; Kunugi, H. Corona Virus Disease 2019 (COVID-19): A pandemic that threatens physical and mental health by promoting physical inactivity. Sports Medicine and Health Science 2020, doi:https://doi.org/10.1016/j.smhs.2020.11.006.
- Liu, B.; Gan, X.; Zhao, Y.; Yu, H.; Gao, J.; Yu, H. Inhibition of HMGB1 Promotes Osseointegration under Hyperglycemic Condition through Improvement of BMSC Dysfunction. Oxid Med Cell Longev 2019, 2019, 1703709, doi:10.1155/2019/1703709.
- Lu, H.Y.; Ma, J.L.; Shan, J.Y.; Zhang, J.; Wang, Q.X.; Zhang, Q. High-mobility group box-1 and receptor for advanced glycation end products in preterm infants with brain injury. World J Pediatr 2017, 13, 228-235, doi:10.1007/s12519-016-0077-z.
- Thakur, V.; Sadanandan, J.; Chattopadhyay, M. High-Mobility Group Box 1 Protein Signaling in Painful Diabetic Neuropathy. Int J Mol Sci 2020, 21, doi:10.3390/ijms21030881.
- Ali, A.M.; Ahmed, A.H.; Smail, L. Psychological Climacteric Symptoms and Attitudes toward Menopause among Emirati Women. Int. J. Environ. Res. Public Health 2020, 17, 5028;, doi:10.3390/ijerph17145028
- Meers, J.M.; Nowakowski, S. Sleep, premenstrual mood disorder, and women’s health. Current Opinion in Psychology 2020, 34, 43-49, doi:https://doi.org/10.1016/j.copsyc.2019.09.003.
- Khan, W.A.; Malik, A.; Khan, M.W.A. Estrogenization of insulin by catecholestrogen produced high affinity autoantibodies and altered the normal function of insulin in type 1 diabetes. Life Sci 2020, 256, 117910, doi:10.1016/j.lfs.2020.117910.
- Kołomańska, D.; Zarawski, M.; Mazur-Bialy, A. Physical Activity and Depressive Disorders in Pregnant Women-A Systematic Review. Medicina (Kaunas) 2019, 55, 212, doi:10.3390/medicina55050212.
- Mijatovic-Vukas, J.; Capling, L.; Cheng, S.; Stamatakis, E.; Louie, J.; Cheung, N.W.; Markovic, T.; Ross, G.; Senior, A.; Brand-Miller, J.C., et al. Associations of Diet and Physical Activity with Risk for Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, doi:10.3390/nu10060698.
- Ali, A.M.; Kunugi, H. Skeletal Muscle Injury in Corona Virus Disease 2019 (COVID-19): a brief mechanistic review. In progress 2020.
- Ali, A.M.; Kunugi, H. Age-related skeletal muscle failure (sarcopenia)—a detrimental challenge during the Coronavirus Disease 2019 (COVID-19) era: A review. In progress 2020.
- Vall Castelló, J.; Tubianosa, C. Linking Mediterranean Diet and Lifestyle with Cardio Metabolic Disease and Depressive Symptoms: A Study on the Elderly in Europe. Int J Environ Res Public Health 2020, 17, doi:10.3390/ijerph17197053.
- Olmedo-Requena, R.; Gómez-Fernández, J.; Amezcua-Prieto, C.; Mozas-Moreno, J.; Khan, K.S.; Jiménez-Moleón, J.J. Pre-Pregnancy Adherence to the Mediterranean Diet and Gestational Diabetes Mellitus: A Case-Control Study. Nutrients 2019, 11, doi:10.3390/nu11051003.
- Ali, A.M.; Hendawy, A.O. Royal Jelly Acid, 10-Hydroxy-Trans-2-Decenoic Acid, for Psychiatric and Neurological Disorders: How helpful could it be. Edelweiss Journal of Food Science and Technology 2019, 1, 1-4, doi:https://doi.org/10.33805/2765-8821.101.
- Ali, A.M.; Hendawy, A.O. Bee Honey as a Potentially Effective Treatment for Depression: A Review of Clinical and Preclinical Findings. JOJ Nurse Health Care 2018, 9, 555764, doi:10.19080/JOJNHC.2018.09.555764.
- Ali, A.M.; Kunugi, H. Bee honey protects astrocytes against oxidative stress: A preliminary in vitro investigation. Neuropsychopharmacol Rep 2019, 39, 312–314, doi:https ://doi.org/10.1002/npr2.12079.
- Kunugi, H.; Ali, A.M. Royal Jelly and Its Components Promote Healthy Aging and Longevity: From Animal Models to Humans. Int. J. Mol. Sci 2019, 20, 4662, doi:https://doi.org/10.3390/ijms20194662.