Biofilms are formed on surfaces inside the oral cavity covered by the acquired pellicle and develop into a complex, dynamic, microbial environment. Oral biofilm is a causative factor of dental and periodontal diseases. Accordingly, novel materials that can resist biofilm formation have attracted significant attention. Zwitterionic polymers (ZPs) have unique features that resist protein adhesion and prevent biofilm formation while maintaining biocompatibility. Recent literature has reflected a rapid increase in the application of ZPs as coatings and additives with promising outcomes.
- Zwitterionic polymers
- Biofilm
- Anti-biofouling
- Carboxybetaine methacrylate
- Sulfobetaine methacrylate
- 2-methacryloyloxyethyl phosphorylcholine
1. Introduction
Dental biomaterials form the backbone, which helps prevent, restore, and rehabilitate oral form and function. Designing and testing these materials produces various mechanical and biological properties, leading to their harmonious presence inside the oral cavity. The last few decades have seen notable developments in dental materials, and properties such as adhesion to the tooth surface and biomimetics have been extensively explored[1]. All the approved materials meet the biocompatibility standards, but they do not account for the interfacial interaction with oral microcosms[2][3].
Methacrylate-based resin composites and polymers are the main components of dental restorative materials used to address the restoration of carious and missing teeth[4]. These materials meet most of the standard requirements but continue to be afflicted by issues concerning premature mechanical and esthetic failures[5]. These failures are multifactorial, but interaction with the host-factors is considered as a key factor[6][7]. In the host-factors, the critical factor of microbiological interaction at the surface leads to biofilm formation [8]. The etiologic role of oral biofilms in dental caries and periodontal diseases has been previously documented [9]. The improved scientific evidence also indicates an active interaction at the dental materials and oral biofilm interface. Majority of the studies, including clinical trials concerning biofilm research, are related to stomatognathic diseases and address a wide range of issues such as caries, periodontitis, and demineralization[10]. Nevertheless, it is essential to note that the interfacial interaction between the dental material and biofilm is multifactorial. Subsequently, research on a variety of methods are focused on improving this interaction.
Dental biomaterials differ from the hard tissues in the oral cavity in terms of both their physical and chemical properties. Advancements in biomimetic restorative materials are rapidly occurring, but they exhibit a different surface interaction. The key surface properties of topography, roughness, surface energy, and the inherent chemical composition alter the biofilm interaction at the surface of the restorative materials[11][12][13]. Materials with low surface energies are associated with a higher affinity for bacterial adhesion, and many dental materials present higher surface energies than natural enamel [14][15]. Dental plaque is an ecologically dominant form of oral biofilm associated with cariogenic and periodontal infections. The increase in surface microroughness and topography is correlated to the surface energy and plays a defining role in microbial adhesion. A rough surface leads to an increase in the difficulty of removing dental plaque like biofilms with conventional methods (e.g., mechanical brushing) alone. Microroughness, expressed as the arithmetic mean (Ra) value, has been suggested for comparing the surface roughness of the materials, and a maximum threshold of 0.2 μm has been proposed to limit microbial adhesion[14]. Biofilm formation showed considerable variation, independent of the bacterial adhesion correlation to the prescribed threshold [16]. Hence, regardless of the surface finish, dental materials lack any inherent biofilm resistance and are prone to secondary infections.
Broadly, two approaches have been reported for the management of oral biofilm formation, comprised physical and chemical methods. While physical methods involve mechanical removal of the biofilm by actions such as tooth brushing, conventional chemical methods include dentifrices [17] and dental mouth rinses[18]. These approaches have focused on substituting or adding chemicals and have displayed good efficacy in an in vitro environment. However, these approaches have inherent limitations, such as dependence on user compliance, and a tendency to damage restorations (e.g., by esthetic and strength deterioration) and oral tissue (e.g., due to hypersensitivity, desiccation, and discoloration) [19].
Apart from adhesive resins, the methacrylate-based resins used in the fabrication of prosthodontic, removable appliances are also limited by issues of plaque biofilm formation [20]. Plaque biofilm formation on acrylic-based appliances is the cause of secondary fungal and bacterial infections, leads to a degradation of the polymer constituents, and reduces the appliances' longevity[21]. The challenges mentioned above have laid the foundation for the development of novel approaches to counter biofilms. Many of these new methodologies, currently under research, involve modification of the dental materials by using antibiotics, incorporation of metal oxides [22], nanoparticles [23], and anti-adhesion coatings using hydrophilic polymers[24].
Amidst a large number of methods, the approach utilizing the resistance to biofilm formation due to anti-adhesion properties has attracted attention. This property is characteristically reported for zwitterionic polymers (ZPs). The innovation and application of ZPs is a close form of biomimicry against fouling, inspired by the mammalian cell phosphatidylcholine membrane, which is characteristically present on the outer surface [25][26]. These polymers are formed by an equal amount of anionic and cationic groups on their chains with a high dipole moment. With a net charge of zero, these polymers present superior surface lubrication, antifouling, and biocompatibility. This leads to a wide range of multi-disciplinary adaptations of these polymers, including dental biopolymers[27].
Therefore, it is imperative to provide an overview of current progress on the developing role of ZPs in dental biomaterial science. In this scoping review, ZPs are briefly introduced, followed by oral biofilm characterization, present trends in the research, and the development of zwitterionic dental materials reported in recent years. In the last section, the existing challenges and road to the future development of oral biofilm-resistant materials are highlighted. We hope that this review will stimulate more innovative ideas to advance research and point toward the persistent question of “what next?”.
2. Theoretical aspects of zwitterionic polymers
The use of hydrophilic polymers such as poly(vinyl alcohol) and poly(ethylene glycol) (PEG) has been suggested to address the problem of surface fouling. PEG has been used as the gold standard of nonfouling polymers as a biocompatible polymer but has poor stability due to autoxidation [28]. The degradation of the PEG chains further increases with an increase in temperature above 35 °C and an ionic environment, such as hemodynamics[29]. ZPs form the closest alternative to PEG when considering amphiphilicity. ZPs containing an equal number of cations and anions have been classified based on their chemical structure.
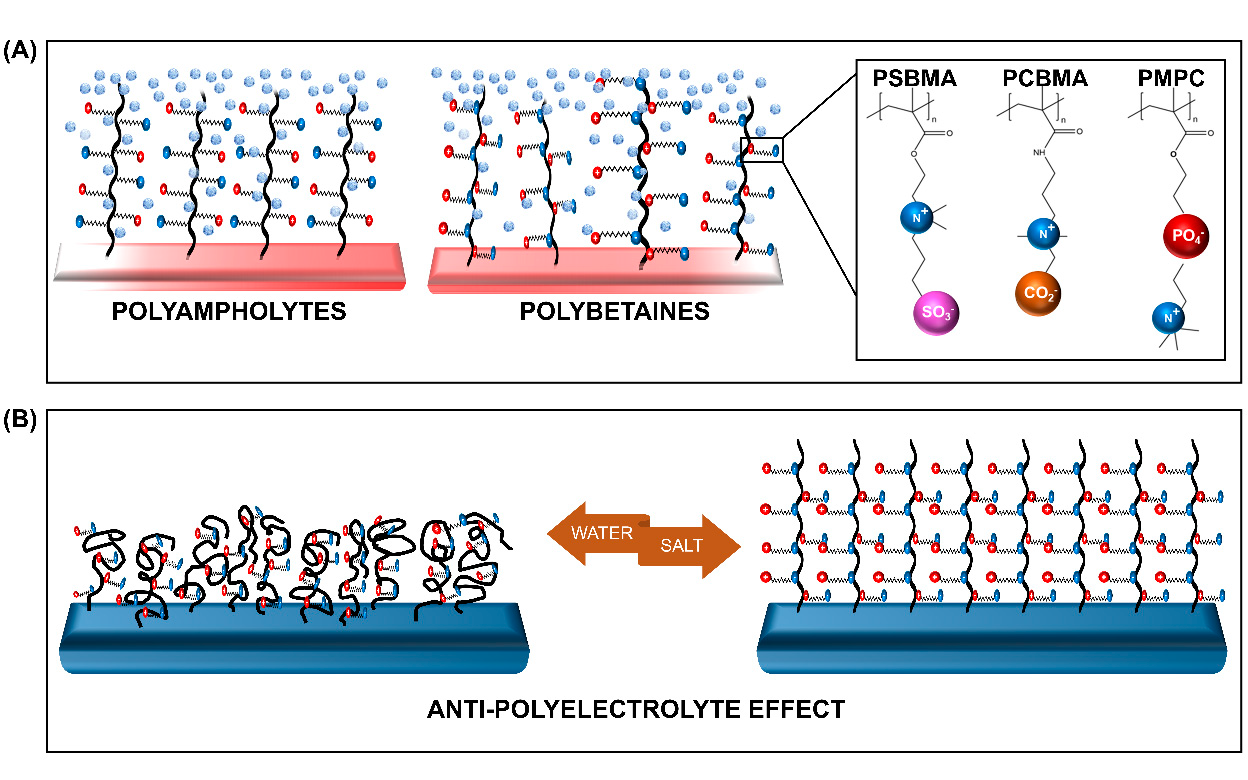
ZPs with anion and cation groups existing on the same monomeric units form polybetaines (also known as polyzwitterions), such as poly(sulfobetaine methacrylate) (SBMA), poly(carboxybetaine methacrylate) (CBMA), and poly(2-methacryloyloxyethyl phosphorylcholine) (MPC). When both ion groups are present on different monomers, the ZPs are of the polyampholyte type, such as 2-(dimethylamino)ethyl methacrylate and methylmethacrylate block copolymer (DMAEMA-MAA). All ZPs can exist in different architectures, among which the membrane and brush type are the most commonly grafted types.
The distinctive feature of ZPs occurs due to its antipolyelectrolyte effect (APE) [30],[31]and super-hydration ability [24]. The salt-responsive state of ZPs, which also affects their viscosity in a solution with low-molecular salt (LMS), is regarded as the opposite of the poly-electrolyte effect, hence it is termed as the APE. This property, detailed by Georgiev et al. [30], has been extensively explored. Accordingly, the introduction of LMS is believed to reduce the interchain dipole interaction within the polymer. The change in the polymer's charge interaction promotes the extension of the chains, and subsequent swelling of the globalized polymer occurs. The reduction in the interchain interaction encourages the polymer-water interaction to achieve an entropy balance, resulting in the formation of a tightly-bound water layer[32]. This characteristic, bound, water layer formation is the second unique feature of ZPs, referred to as their super-hydration ability.
Figure 1. Schematic representation of the (A) zwitterionic polymers (ZPs) and (B) the salt-responsive antipolyelectrolyte effect (APE) of the ZP brushes. PSBMA—poly (sulfobetaine methacrylate), PCBMA—poly (carboxybetaine methacrylate), and PMPC—poly (2-methacryloyloxyethyl phosphorylcholine).
The super-hydration ability, characterized by the formation of the hydration shell via electrostatic interaction, is also influenced by the arrangement of water molecules within the shell. The arrangement mimicking free water causes an increase in the affinity to water[33]. Therefore, the formation of a compactly-bound, thick, energetic layer occurs on the ZP brush. This layer enforces a strong steric effect, facilitating a strong resistance to protein adsorption and subsequently to fouling [26]. In other words, the development of biofilm resistance takes place as a combination of surface hydration and steric repulsion [34].
3. Human oral biofilm: composition and properties
The microbiome has been described as the presence of pathogenic, symbiotic, and commensal microorganisms together in a community, and thus an oral biofilm can be considered a sub-microbiome[35]. Moreover, the biofilm is a microbially-derived, sessile community wherein cells that are irreversibly attached to the substrate or each other are embedded in an extracellular polymer substance (EPS) matrix[36]. Advancements in genomic studies have helped decode the oral microbiome, evidencing a broad heterogeneous nature of the oral microbiota with more than 600 different species coexisting [37][38].
The development of oral biofilms is a spatiotemporal phenomenon that is composed of initial, physicochemical interactions. This initial contact establishes the foundation for the biofilm's growth and maturation, incorporating a nutrient, pH, and oxygen level gradient [39]. The multi-stage biofilm life cycle is described in detail in the review by Stoodley et al. [40], and the biofilm has also been recently discussed as an "emergent form of bacterial life" by Flemming et al.[41]. Therefore, to better understand the mechanisms that help combat biofilm formation in the oral cavity, it is important to understand the development of this emergent life form.
Figure 2. Cycle of biofilm development on the tooth surface. (1) Free swimming bacterial cells alight on the tooth surface, conditioned with pellicle derived from salivary proteins, and form clusters. (2) Collected cells begin the production of a gooey matrix and cellular signalizing (QS) promotes multiplication and colonization. (3) Growth of biofilm leads to the development of chemical and oxygen gradients, accompanied by new cell addition to the colony. Water channels develop and pass through the colony. (4) Cells and EPS detach from the existing colony. (5) Free swimming cells rejoin the microbiome or reinitiate the same process, and expansion of growth of the biofilm occurs.
Due to the complexity of the biofilm, Lin [35] proposed a categorization of the four attributes of the biofilm. First, the constituents, which describe the materials that comprise the biofilm, including the microorganisms, viruses and particles, extracellular matrix, signaling molecules and enzymes, and debris. Second, the quantity of the biofilm, referring to the overall biomass, number, and concentration of the heterogeneous biofilm. In addition, the amount and constituents of EPS are also included in this category. The third attribute is the biofilm structure, which is related to the arrangement of the constituents. It is correlated with both the QS and the resistance of the film to shear forces. Lastly, biofilm function characterizes the pathogenicity of the biofilm, which also entails the metabolic activity, gene expression, and mechanism of surface adhesion. Each of the above attributes is associated with a measurand, which can help to analyze the efficacy of the intervention being used to prevent/remove the biofilm, as shown in Figure 3.
Figure 3. Strategies to combat biofilm development on the surface. Intervention in the material properties can be made to draw an effect at various levels such that it can prevent adhesion and maturation or denatures and kills the constituent microorganism of the biofilm. Each intervention can be objectively analyzed by the corresponding measurands. A detailed analysis of a group of measurands provides a holistic view of the biofilm-resistant mechanism by factoring in the different characteristics of the biofilm.
4. Biofilm-resistant dental materials
In recent years, the dynamic nature of biofilm interaction with dental materials has been widely recognized. The increased focus on the longevity of dental materials and the challenge of secondary caries and other recalcitrant disease forms[42] have driven biomaterial research into the development of robust materials. While biofilm characteristics occupy a large area of the research focus, it is essential to state here that the underlying substrate acts as a significant factor in defining these characteristics [35].
The dental biomaterials which are undergoing active research to develop anti-biofouling characteristics can be summarized as:
- Restorative materials[43]
- Varnish and sealants[44][45][46]
- Adhesives[47][48]
- Endodontic cement [49][50]
- Materials for the fabrication of removable appliances (PMMA)[51][52]
- Hydroxyapatite and surgical membranes [53][54]
5. Summary
"Zwitterionization" of the biomaterial is seen at the cusp of advancements in biomaterial research with multiple, simultaneous, research paths taking place to improve clinical adaptation. Commonly-used dental materials surface-functionalized with ZPs show promise in combating biofilm growth with exceptional biocompatibility.
The development of newer monomeric units in combination with incorporated bioactive materials and collaborative efforts to assess the bio-interactive nature of biofilm at the interface of dental materials will propel future research in dental materials in a holistic manner.
References
- Bayne, S.C.; Ferracane, J.L.; Marshall, G.W.; Marshall, S.J.; Van Noort, R. The Evolution of Dental Materials over the Past Century: Silver and Gold to Tooth Color and Beyond. Journal of Dental Research 2019, 98, 257-265, doi:10.1177/0022034518822808.
- Ferracane, J.L.L.; Hilton, T.J.J.; Stansbury, J.W.W.; Watts, D.C.C.; Silikas, N.; Ilie, N.; Heintze, S.; Cadenaro, M.; Hickel, R. Academy of Dental Materials guidance—Resin composites: Part II—Technique sensitivity (handling, polymerization, dimensional changes). Dental Materials 2017, 33, 1171-1191, doi:10.1016/j.dental.2017.08.188.
- Ilie, N.; Hilton, T.J.; Heintze, S.D.; Hickel, R.; Watts, D.C.; Silikas, N.; Stansbury, J.W.; Cadenaro, M.; Ferracane, J.L. Academy of Dental Materials guidance—Resin composites: Part I—Mechanical properties. Dental Materials 2017, 33, 880-894, doi:10.1016/j.dental.2017.04.013.
- Ferracane, J.L. Resin composite—State of the art. Dental Materials 2011, 27, 29-38, doi:10.1016/j.dental.2010.10.020.
- Demarco, F.F.; Collares, K.; Correa, M.B.; Cenci, M.S.; Moraes, R.R.D.; Opdam, N.J. Should my composite restorations last forever? Why are they failing? Brazilian Oral Research 2017, 31, doi:10.1590/1807-3107bor-2017.vol31.0056.
- Kreth, J.; Ferracane, J.L.; Pfeifer, C.S.; Khajotia, S.; Merritt, J. At the Interface of Materials and Microbiology: A Call for the Development of Standardized Approaches to Assay Biomaterial-Biofilm Interactions. J Dent Res 2019, 98, 850-852, doi:10.1177/0022034519854685.
- Demarco, F.F.; Corrêa, M.B.; Cenci, M.S.; Moraes, R.R.; Opdam, N.J.M. Longevity of posterior composite restorations: Not only a matter of materials. Dental Materials 2012, 28, 87-101, doi:10.1016/j.dental.2011.09.003.
- Hao, Y.; Huang, X.; Zhou, X.; Li, M.; Ren, B.; Peng, X.; Cheng, L. Influence of Dental Prosthesis and Restorative Materials Interface on Oral Biofilms. International Journal of Molecular Sciences 2018, 19, 3157, doi:10.3390/ijms19103157.
- Kleinberg, I. A Mixed-bacteriaEcologicalApproach toUnderstanding theRole of theOralBacteria inDentalCariesCausation: anAlternative toStreptococcus mutans and theSpecific-plaqueHypothesis. Critical Reviews in Oral Biology & Medicine 2002, 13, 108-125, doi:10.1177/154411130201300202.
- Rumbaugh, K.P. How well are we translating biofilm research from bench-side to bedside? Biofilm 2020, 2, 100028, doi:10.1016/j.bioflm.2020.100028.
- Tanner, J.; Robinson, C.; Soderling, E.; Vallittu, P. Early plaque formation on fibre-reinforced composites in vivo. Clin Oral Investig 2005, 9, 154-160, doi:10.1007/s00784-005-0317-4.
- Øilo, M.; Bakken, V. Biofilm and Dental Biomaterials. Materials 2015, 8, 2887-2900, doi:10.3390/ma8062887.
- Aykent, F.; Yondem, I.; Ozyesil, A.G.; Gunal, S.K.; Avunduk, M.C.; Ozkan, S. Effect of different finishing techniques for restorative materials on surface roughness and bacterial adhesion. The Journal of Prosthetic Dentistry 2010, 103, 221-227, doi:10.1016/s0022-3913(10)60034-0.
- Bollen L, C.M.L.; Lambrechts, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dental Materials 1997, 13, 258-269, doi:10.1016/S0109-5641(97)80038-3.
- Quirynen, M.; Marechal, M.; Busscher, H.J.; Weerkamp, A.H.; Darius, P.L.; Steenberghe, D. The influence of surface free energy and surface roughness on early plaque formation. Journal of Clinical Pharmacy and Therapeutics 1992, 17, 138-144, doi:10.1111/j.1365-2710.1992.tb00751.x.
- Dutra, D.; Pereira, G.; Kantorski, K.; Valandro, L.; Zanatta, F. Does Finishing and Polishing of Restorative Materials Affect Bacterial Adhesion and Biofilm Formation? A Systematic Review. Operative Dentistry 2018, 43, E37-E52, doi:10.2341/17-073-l.
- Marinho, V.C.C. Evidence-based Effectiveness of Topical Fluorides. Advances in Dental Research 2008, 20, 3-7, doi:10.1177/154407370802000102.
- Ribeiro, L.G.M.; Hashizume, L.N.; Maltz, M. The effect of different formulations of chlorhexidine in reducing levels of mutans streptococci in the oral cavity: A systematic review of the literature. Journal of Dentistry 2007, 35, 359-370, doi:10.1016/j.jdent.2007.01.007.
- Patil, S.; Gs, V.; Baeshen, H.; Ali Sumayli, M.A.; Saeed AlShahrani, M.A.; Alkhallaf Najmi, A.I.; Jafer, M.A.; Vishwanathaiah, S.; Khan, S. Current trends and future prospects of chemical management of oral biofilms. J Oral Biol Craniofac Res 2020, 10, 660-664, doi:10.1016/j.jobcr.2020.08.017.
- Wen, J.; Jiang, F.; Yeh, C.-K.; Sun, Y. Controlling fungal biofilms with functional drug delivery denture biomaterials. Colloids and Surfaces B: Biointerfaces 2016, 140, 19-27, doi:10.1016/j.colsurfb.2015.12.028.
- Nikawa, H.; Jin, C.; Makihira, S.; Egusa, H.; Hamada, T.; Kumagai, H. Biofilm formation of Candida albicans on the surfaces of deteriorated soft denture lining materials caused by denture cleansers in vitro. J Oral Rehabil 2003, 30, 243-250, doi:10.1046/j.1365-2842.2003.01024.x.
- Hasegawa, T.; Takenaka, S.; Ohsumi, T.; Ida, T.; Ohshima, H.; Terao, Y.; Naksagoon, T.; Maeda, T.; Noiri, Y. Effect of a novel glass ionomer cement containing fluoro-zinc-silicate fillers on biofilm formation and dentin ion incorporation. Clin Oral Investig 2020, 24, 963-970, doi:10.1007/s00784-019-02991-0.
- Mangal, U.; Kim, J.Y.; Seo, J.Y.; Kwon, J.S.; Choi, S.H. Novel Poly(Methyl Methacrylate) Containing Nanodiamond to Improve the Mechanical Properties and Fungal Resistance. Materials (Basel) 2019, 12, 3438-3438, doi:10.3390/ma12203438.
- Choi, W.; Jin, J.; Park, S.; Kim, J.Y.; Lee, M.J.; Sun, H.; Kwon, J.S.; Lee, H.; Choi, S.H.; Hong, J. Quantitative Interpretation of Hydration Dynamics Enabled the Fabrication of a Zwitterionic Antifouling Surface. ACS Appl Mater Interfaces 2020, 12, 7951-7965, doi:10.1021/acsami.9b21566.
- Kanno, K.; Wu, M.K.; Scapa, E.F.; Roderick, S.L.; Cohen, D.E. Structure and function of phosphatidylcholine transfer protein (PC-TP)/StarD2. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 2007, 1771, 654-662, doi:10.1016/j.bbalip.2007.04.003.
- He, M.; Gao, K.; Zhou, L.; Jiao, Z.; Wu, M.; Cao, J.; You, X.; Cai, Z.; Su, Y.; Jiang, Z. Zwitterionic materials for antifouling membrane surface construction. Acta Biomaterialia 2016, 40, 142-152, doi:10.1016/j.actbio.2016.03.038.
- Zheng, L.; Sundaram, H.S.; Wei, Z.; Li, C.; Yuan, Z. Applications of zwitterionic polymers. Reactive and Functional Polymers 2017, 118, 51-61, doi:10.1016/j.reactfunctpolym.2017.07.006.
- Cao, Z.; Jiang, S. Super-hydrophilic zwitterionic poly(carboxybetaine) and amphiphilic non-ionic poly(ethylene glycol) for stealth nanoparticles. Nano Today 2012, 7, 404-413, doi:10.1016/j.nantod.2012.08.001.
- Leckband, D.; Sheth, S.; Halperin, A. Grafted poly(ethylene oxide) brushes as nonfouling surface coatings. J Biomater Sci Polym Ed 1999, 10, 1125-1147, doi:10.1163/156856299x00720.
- Georgiev, G.S.; Kamenska, E.B.; Vassileva, E.D.; Kamenova, I.P.; Georgieva, V.T.; Iliev, S.B.; Ivanov, I.A. Self-assembly, antipolyelectrolyte effect, and nonbiofouling properties of polyzwitterions. Biomacromolecules 2006, 7, 1329-1334, doi:10.1021/bm050938q.
- Xiao, S.; Ren, B.; Huang, L.; Shen, M.; Zhang, Y.; Zhong, M.; Yang, J.; Zheng, J. Salt-responsive zwitterionic polymer brushes with anti-polyelectrolyte property. Current Opinion in Chemical Engineering 2018, 19, 86-93, doi:10.1016/j.coche.2017.12.008.
- Chen, H.; Yang, J.; Xiao, S.; Hu, R.; Bhaway, S.M.; Vogt, B.D.; Zhang, M.; Chen, Q.; Ma, J.; Chang, Y., et al. Salt-responsive polyzwitterionic materials for surface regeneration between switchable fouling and antifouling properties. Acta Biomaterialia 2016, 40, 62-69, doi:10.1016/j.actbio.2016.03.009.
- Schlenoff, J.B. Zwitteration: Coating Surfaces with Zwitterionic Functionality to Reduce Nonspecific Adsorption. Langmuir 2014, 30, 9625-9636, doi:10.1021/la500057j.
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283-5293, doi:10.1016/j.polymer.2010.08.022.
- Lin, N.J. Biofilm over teeth and restorations: What do we need to know? Dental Materials 2017, 33, 667-680, doi:10.1016/j.dental.2017.03.003.
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clinical Microbiology Reviews 2002, 15, 167-193, doi:10.1128/cmr.15.2.167-193.2002.
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.R.; Yu, W.-H.; Lakshmanan, A.; Wade, W.G. The Human Oral Microbiome. Journal of Bacteriology 2010, 192, 5002-5017, doi:10.1128/jb.00542-10.
- Bjarnsholt, T.; Jensen, P.Ø.; Moser, C.; Høiby, N. Biofilm Infections; 2011; 10.1007/978-1-4419-6084-9.
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments. Trends in Microbiology 2018, 26, 229-242, doi:10.1016/j.tim.2017.09.008.
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as complex differentiated communities. Annu Rev Microbiol 2002, 56, 187-209, doi:10.1146/annurev.micro.56.012302.160705.
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: an emergent form of bacterial life. Nature Reviews Microbiology 2016, 14, 563-575, doi:10.1038/nrmicro.2016.94.
- Hannig, C.; Hannig, M. The oral cavity--a key system to understand substratum-dependent bioadhesion on solid surfaces in man. Clin Oral Investig 2009, 13, 123-139, doi:10.1007/s00784-008-0243-3.
- Lee, M.J.; Kwon, J.S.; Kim, J.Y.; Ryu, J.H.; Seo, J.Y.; Jang, S.; Kim, K.M.; Hwang, C.J.; Choi, S.H. Bioactive resin-based composite with surface pre-reacted glass-ionomer filler and zwitterionic material to prevent the formation of multi-species biofilm. Dental Materials 2019, 10.1016/j.dental.2019.06.004, doi:10.1016/j.dental.2019.06.004.
- Kwon, J.S.; Lee, M.J.; Kim, J.Y.; Kim, D.; Ryu, J.H.; Jang, S.; Kim, K.M.; Hwang, C.J.; Choi, S.H. Novel anti-biofouling light-curable fluoride varnish containing 2-methacryloyloxyethyl phosphorylcholine to prevent enamel demineralization. Sci Rep 2019, 9, 1432, doi:10.1038/s41598-018-38255-2.
- Kim, D.; Lee, M.-J.; Kim, J.-Y.; Lee, D.; Kwon, J.-S.; Choi, S.-H. Incorporation of zwitterionic materials into light-curable fluoride varnish for biofilm inhibition and caries prevention. Scientific Reports 2019, 9, doi:10.1038/s41598-019-56131-5.
- Lee, M.J.; Mangal, U.; Kim, S.J.; Yoon, Y.P.; Ahn, E.S.; Jang, E.S.; Kwon, J.S.; Choi, S.H. Improvement in the Microbial Resistance of Resin-Based Dental Sealant by Sulfobetaine Methacrylate Incorporation. Polymers (Basel) 2020, 12, doi:10.3390/polym12081716.
- Song, L.; Ye, Q.; Ge, X.; Misra, A.; Tamerler, C.; Spencer, P. Probing the neutralization behavior of zwitterionic monomer-containing dental adhesive. Dental Materials 2017, 33, 564-574, doi:10.1016/j.dental.2017.03.013.
- Ge, X.; Ye, Q.; Song, L.; Spencer, P.; Laurence, J.S. Effect of crosslinking density of polymers and chemical structure of amine-containing monomers on the neutralization capacity of dentin adhesives. Dental Materials 2015, 31, 1245-1253, doi:10.1016/j.dental.2015.08.153.
- Kwon, J.S.; Lee, M.J.; Kim, J.Y.; Kim, D.; Ryu, J.H.; Jang, S.; Kim, K.M.; Hwang, C.J.; Choi, S.H. Novel anti-biofouling bioactive calcium silicate-based cement containing 2-methacryloyloxyethyl phosphorylcholine. PLoS One 2019, 14, e0211007, doi:10.1371/journal.pone.0211007.
- Wang, L.; Xie, X.; Li, C.; Liu, H.; Zhang, K.; Zhou, Y.; Chang, X.; Xu, H.H.K. Novel bioactive root canal sealer to inhibit endodontic multispecies biofilms with remineralizing calcium phosphate ions. Journal of Dentistry 2017, 60, 25-35, doi:10.1016/j.jdent.2017.02.011.
- Jin, J.; Kim, J.-Y.; Choi, W.; Lee, M.-J.; Seo, J.-Y.; Yu, J.; Kwon, J.-S.; Hong, J.; Choi, S.-H. Incorporation of carboxybetaine methacrylate into poly(methyl methacrylate) to prevent multi-species biofilm formation. Journal of Industrial and Engineering Chemistry 2020, 86, 194-204, doi:10.1016/j.jiec.2020.03.003.
- Takahashi, N.; Iwasa, F.; Inoue, Y.; Morisaki, H.; Ishihara, K.; Baba, K. Evaluation of the durability and antiadhesive action of 2-methacryloyloxyethyl phosphorylcholine grafting on an acrylic resin denture base material. The Journal of Prosthetic Dentistry 2014, 112, 194-203, doi:10.1016/j.prosdent.2013.08.020.
- Sánchez-Salcedo, S.; Colilla, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Design and preparation of biocompatible zwitterionic hydroxyapatite. J. Mater. Chem. B Mater. Biol. Med. 2013, 1, 1595, doi:10.1039/c3tb00122a.
- Prajatelistia, E.; Sanandiya, N.D.; Nurrochman, A.; Marseli, F.; Choy, S.; Hwang, D.S. Biomimetic Janus chitin nanofiber membrane for potential guided bone regeneration application. Carbohydr. Polym. 2021, 251, doi:10.1016/j.carbpol.2020.117032.