Recent advances in lab-on-a-chip technology establish solid foundations for wearable biosensors. These newly emerging wearable biosensors are capable of non-invasive, continuous monitoring by miniaturization of electronics and integration with microfluidics. The advent of flexible electronics, biochemical sensors, soft microfluidics, and pain-free microneedles have created new generations of wearable biosensors that explore brand-new avenues to interface with the human epidermis for monitoring physiological status. However, these devices are relatively underexplored for sports monitoring and analytics, which may be largely facilitated by the recent emergence of wearable biosensors characterized by real-time, non-invasive, and non-irritating sensing capacities.
- wearable biosensors
- biomedical microfluidics
- healthcare monitoring
- sports analytics
- wearable biosensors,biomedical microfluidics,healthcare monitoring,sports analytics
1. Introduction
Miniaturization of laboratory apparatus into microscale devices is a promising technology called lab-on-a-chip (LOC) [1]. About 30 years ago the concept of micro total analysis systems (μTAS) emerged from the field of semiconductor fabrication and was enhanced by microelectromechanical systems (MEMS) technologies [2][3][4][2,3,4]. The μTAS concept is to shrink an entire analytical procedure, such as cell sorting, single-cell capture, captured-cell transport, cell lysis, and intracellular analysis, into a miniaturized multifunctional chip [5][6][7][5,6,7], and nowadays its well-known synonym is called lab-on-a-chip (LOC) [3][4][3,4]. This growing field has garnered considerable attention since scaled-down biochemical analysis has several key advantages over both conventional and current laboratory benchtop methods [3][5][3,5]. These advantages are consistently demonstrated in clinical medicine, engineering, biology, and life science, etc., for example, to expedite the experimental process by embracing automation and parallelization [1][8][9][1,8,9]; to lower the cost by reducing the volume of expensive reagents [1][5][10][11][1,5,10,11]; to yield better interpretation of experimental results by gleaning vital information at cellular even molecular levels [12][13][14][12,13,14].
Interest in device miniaturization [15][16][17][15,16,17], combined with advances in bio-microfabrication and enabling materials [18], is motivating various microfluidic methods in which microchips can be mass-manufactured at extremely low cost via polymers (e.g., polydimethylsiloxane, PDMS) and soft lithography for microfabrication [5][19][5,19]. Microfluidics is the science of microscale devices that process and manipulate extremely low (10−9 to 10−18 L) amounts of fluids in microchannels with dimensions of tens of micrometers [10]. Conventional macroscale experimental technologies meet difficulties to deal with such low amounts of fluids, impeding their development in various fields. Conversely, microfluidic technologies begin to address numerous tough challenges, because fluid phenomena at the microscale are dramatically different from those at the macroscale [3]. For instance, capillary forces and surface tension are more dominant than gravitational forces [3], allowing for passively pumping fluids in opposition to gravity [20]. Flows at the microscale are laminar instead of turbulent, resulting in more predictable liquid handling and diffusion kinetics [5]. Based on the different phenomena behaving at the microscale, microfluidic technologies offer a sensitive, predictable, and controllable avenue for bioanalysis [21].
Despite all the attractive capacities of LOC/microfluidics devices that have enabled the widespread implementation of microchip-based systems in biology and life science [3][4][5][22][3,4,5,22], microfluidic technologies often only improve the performance of existing macroscale assays or provide equivalent alternatives [13]. Conversely, they have not reached their full potential due to the lack of essentially new capacities [3]. In recent years, however, LOC/microfluidics technologies begin to address some problems that have not yet been solved by current laboratory benchtop methods. An excellent example can be found in wearable/ambulatory healthcare monitoring and sports analytics harnessing skin-interfaced wearable biosensors [15][23][15,23]. Although this field is still in its infancy, the fundamentals of it are exceptionally strong: in the past decade, the wearable LOC devices gradually integrated with well-established techniques, including biocompatible materials [24][25][24,25], flexible electronics [26][27][28][29][30][26,27,28,29,30], optical/electrochemical sensors [14][26][31][32][14,26,31,32], microfluidics [21][33][34][35][21,33,34,35], near-field communications (NFC) [36], pain-free microneedles [37][38][39][40][37,38,39,40], as well as big data and cloud computing [14][41][42][14,41,42].
These above-mentioned enabling techniques establish the foundations for a new generation of wearable biosensors that directly interfaced with the human epidermis instead of rigid packages embedded in wrist straps or bands [23][43][44][45][23,43,44,45]. The distinguishing characteristics of the emerging wearable biosensors, lightweight, flexibility, and portability [31][36][46][31,36,46], have made them especially suitable for point-of-care testing (POCT). Therefore, brand-new wearable biosensors capable of real-time physiological monitoring quickly emerge, as shown in Figure 1. However, these wearable biosensors are mainly designed for health monitoring [15][34][41][45][47][48][15,34,41,45,47,48], especially, some of them are only developed to measure the physical strain/stress bending change [25][49][50][25,49,50]. Although many wearable devices have been deployed in sports, they are used to monitoring biophysical markers [23], such as movement [51] and cardiovascular information (e.g., blood oxygenation) [26][52][53][26,52,53].
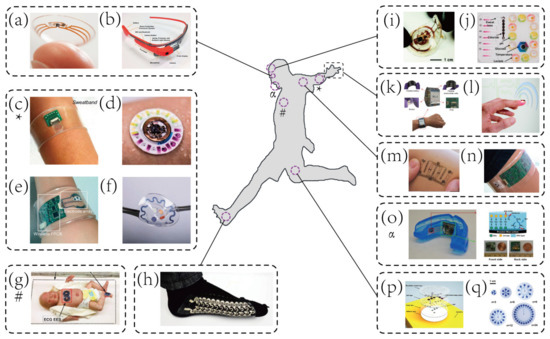
Figure 1.
a
b) Google glass for immunochromatographic diagnostic test analysis [54]. Copyright 2014, American Chemical Society. (
c
d) A hybrid sensor for simultaneous electrochemical, colorimetric, and volumetric analysis of sweat [55]. Copyright 2019, American Association for Advancement of Science. (
e) A wearable sensor for autonomous sweat extraction and analysis [56]. Copyright 2017, National Academy of Sciences of United States of America (NAS). (
f
g) Binodal, wireless epidermal electronic systems with in-sensor analytics for neonatal intensive care [57]. Copyright 2019, American Association for Advancement of Science. (
h) Wearable textile-based self-powered sensors [58]. Copyright 2016, Royal Society of Chemistry. (
i
j) A microfluidic system for colorimetric analysis of sweat biomarkers and temperature [59]. Copyright 2019, American Chemical Society. (
k) A smartwatch for continuous sweat glucose monitoring [60]. Copyright 2019, American Chemical Society. (
l
m) An epidermal stimulation and sensing platform for sensorimotor prosthetic control, management of lower back exertion, and electrical muscle activation [61]. Copyright 2016, Wiley. (
n
2+ and pH [32]. Copyright 2016, American Chemical Society. (
o) A wearable salivary uric acid mouthguard sensor [62]. Copyright 2015, Elsevier. (
p) A microfluidic system for time-sequenced discrete sampling and chloride analysis [63]. Copyright 2018, Wiley. (
q) Skin-mounted microfluidic networks for chrono-sampling of sweat [64]. Copyright 2017, Wiley.
2. Key Analytes and Wearable Biosensing Platforms
2.1. Key Analytes in Wearable Biosensing
Biofluids are largely unexplored resources that contain a rich milieu of important biomarkers, from electrolytes [14][65][66], metabolites [21][34], heavy metals [31], cytokines [67][68], hormones [69][70][71] and amino acids [72] to exogenous drugs [73][74], each of which provides valuable insights into physiological status, health conditions, pharmacokinetics, and sports performance. Sweat and ISF are two representative biofluids that offer great potential for home-based healthcare monitoring or on-field sports performance assessment due to ease of non/minimally-invasive collection and analysis by using wearable biosensors [15][41][75]. Although traditional wearable platforms have demonstrated the effectiveness of measuring a few biophysical markers [25][49][50], skin-interfaced biosensors mainly exploit valuable resources—sweat and ISF biochemical markers for healthcare and sports monitoring.
Electrolytes, the most abundant analytes in sweat, are widely tested in wearable biosensing [76]. Significant interest in Na
+
+
4+
2+ stems from their applications in disease diagnostics and sports analytics. For example, the breakdown of proteins can result in the presence of ammonium in blood before the liver converts them to urea [77]. There, high levels of NH
4+ in excreted sweat can be used as indicators of hepatic diseases, such as hepatitis, cirrhosis, and related disease—hepatic encephalopathy (HE) [35][78]. Kim et al. [35] reported a wearable microfluidic biosensor with integrated enzymatic reactions for colorimetric sensing of the concentration of ammonia and ethanol in sweat. This sweat biosensor could serve as a diagnostic tool of HE. Besides, ionized calcium is another important marker of homeostasis. Excessive variations of Ca
2+ levels in biofluids are detrimental to the human body, causing myeloma, renal failure, kidney stones, and hyperparathyroidism [32]. Moreover, electrolytes, especially Na
+
+
Metabolites are rich solutes in sweat which are highly attractive for diverse applications in wearable disease diagnostics and sports analytics. For instance, glucose and lactate attract most research efforts [45]. Real-time tracking of glucose concentration in biofluids not only has utility in the diagnosis of diabetes mellitus [79], but also can reflect energy availability and consumption for athletic monitoring [43]. Likewise, sweat lactate represents a key indicator of muscle fatigue, tissue hypoxia, and exercise intensity [21][80]. Thus, many wearable biosensors capable of simultaneously monitoring sweat glucose and lactate have been successfully developed [14][21][55][59][80], because of the importance of continuously gleaning metabolic information from sweat. Additionally, another three sweat metabolites, including urea, uric acid, and creatinine, create opportunities for diagnostics of kidney disorders. The levels of sweat urea and creatinine are typically higher in patients with kidney disorders than in healthy individuals, since the failure of glomerular function [81][82]. As described previously, Zhang et al. [34] reported a microfluidic biosensor for colorimetric analysis of urea and creatinine for POCT of kidney diseases. Similarly, newborns usually undergo sweat chloride tests for the diagnosis of cystic fibrosis (CF) [56][83].
2.2. Wearable Sweat Biosensing Platforms for Healthcare and Sports Monitoring
The rich composition of sweat analytes makes the wearable sweat biosensing platform applicable to healthcare and sports monitoring. Over the past decade, an increasingly large number of wearable biosensors have been developed. However, the potential impact of wearable sweat biosensing technologies on sports analytics has not yet been fully recognized. To broaden the scope of wearable sweat sensing (like other analytes, integrated biosensors), several wearable platforms will be briefly introduced herein.
In one example, Reeder et al. [33] developed a waterproof microfluidic platform for sweat volumetry, biomarker analysis, and thermography in aquatic settings (
Figure 2a). For biomarker analysis, colorimetric sensing was introduced: the reagent resided in the adjacent chamber reacted with sweat to create a colorimetric response corresponding to the concentration of chloride. A printed color reference dial at the center facilitated visual inspection. Besides, two representative biosensors exploited electrochemical sensing for in situ monitoring of heavy metals—Zn, Cd, Pb, Cu, and Hg [31] (
Figure 2b), and an exogenous drug—caffeine [84] (
Figure 2c). These systems based on electrochemical biosensing are characterized by high selectivity, sensitivity, and reproducibility. Tai et al. [84] reported a wearable biosensor with the capability of methylxanthine drug monitoring by electrochemical DPV, which is an important step to complement the traditional method of drug dosage tracking—blood sampling and analysis. Sports medicine personnel could be specifically interested in this work due to their ability to monitor the drug dosage during exercise intervention of the diseased. Another example of using wearable biosensors for drug monitoring was combined with microneedles technologies.
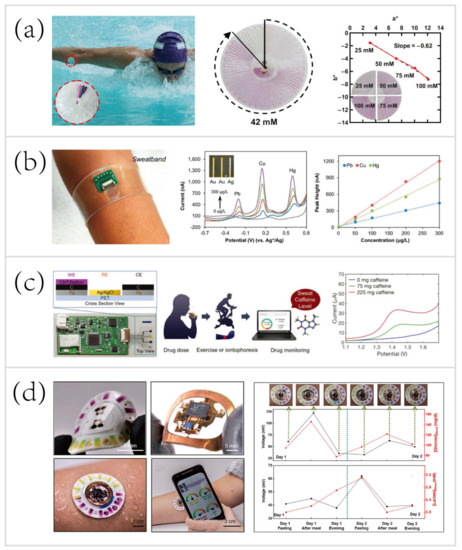
Figure 210.
a
b
c) A wearable biosensor for methylxanthine drug (caffeine) monitoring [84]. Copyright 2018, Wiley. (
d) A skin-mounted hybrid sensor for analysis of sweat glucose, lactate, pH, and chloride; correlations of data acquired from sweat biosensors (black line) with that acquired from blood glucose and lactate meters (red line), respectively [55].Copyright 2019, American Association for the Advancement of Science.
Recent progress has led to the advent of hybrid biosensors, which can monitor multiple parameters by simultaneously performing electrochemical, colorimetric, and volumetric sensing. To yield a comprehensive evaluation of the physiology and sports performance of end-users, Bandodkar et al. [55] developed a skin-mounted microfluidic/electronic biosensor for simultaneous monitoring of chloride, pH, lactate, glucose, and sweat loss (
Figure 2d). The hybrid biosensor contained a reusable, thin NFC subsystem and a soft, disposable microfluidic subsystem. The NFC subsystem allowed for electrochemical sensing in a mode that targeted glucose and lactate, and spontaneously generated electrical signals proportional to their concentrations. The microfluidic subsystem embedded colorimetric reagents for tracking the pH and concentration of chloride, with an additional ratcheted microchannel for quantification of sweat rate and total sweat loss. As shown in
Figure 2d (right), a field study aimed to suggest the potential for acquiring semi-quantitative data by analyzing sweat biochemistry. This study focused on comparing temporal variations in glucose and lactate levels in blood and sweat due to exercise engagement or food intake. The results showed a comparison between the data acquired from hybrid biosensors with the data acquired from blood glucose and lactate meters, respectively. Over two days, the blood glucose and lactate levels after each session followed trends that qualitatively similar to those of data measured in sweat. These correlations were confirmed by literature [56][79][85][86][87].
2.3. Microneedles Platforms
Several decades ago, microneedles (MNs) were first introduced to perform transdermal drug delivery [88][179]. MNs with small length (usually less than 1000 μm [89][90][180,181]) penetrate the stratum corneum and open windows for the ISF biosensing or form transient microchannels for drug delivery while preventing the nerves and blood vessels from stimulation and impairment. Currently, MNs have been utilized for applications in the following areas: ISF sampling [89][91][180,182], disease diagnostics [79][92][93][153,183,184], drug delivery [88][94][95][179,185,186], cosmetics [90][96][97][98][181,187,188,189], etc.
MNs have wide applications and show tremendous promise for applying biomedical advances into wearable healthcare and sports monitoring due to the three following intrinsic advantages.
-
Enhanced compliance. The patients and athletes will not suffer from pain, discomfort, and needle-phobia [88][89][99][100][179,180,190,191].
-
Easy-to-use. MNs are user-friendly biosensing and drug delivery devices that can be directly applied to the skin. Conventional methods, hypodermic injections, conversely, demand professional personnel who undergo rigorous medical training [88][179].
-
Real-time in situ biosensing and controllable/long-term drug delivery. MNs realize pain-free, in situ diagnostics even combine with feedback-based long-term drug delivery [101][160].
Recent advances in wearable MN patches capable of minimally-invasive, transdermal sensing biofluids [79][101][88][102][103] [153,160,179,192,193] and pain-free drug delivery [184,194,195] [93][104][105] provide an opportunity to perform sports monitoring, especially beneficial to sports nutrition and sports medicine [106][196]. These techniques combined with electrochemical/microfluidic biosensing modalities are expected to lead to several breakthroughs in wearable biosensing.
2.3.1. Microneedles for Transdermal Biosensing
In recent years, although the majority of MNs are still focused on painless drug delivery, MNs for transdermal biosensing are promising and receive considerable attention [99]. ISF contains rich physiological/pathological information than ever envisaged [107], in the meantime, the MN-based biosensor can serve as a portal for the pain-free acquisition of valuable information. Besides, recent research efforts have led to the emergence of advanced microfabrication and biophotonics that facilitate the manufacture of MNs [37][39][108]. More complicated MN platforms that integrate electrochemical biosensors [109][110][90] or biofuel cells [37] (harvesting energy from biofluids, such as sweat and ISF, to power wearable platforms) have been manufactured. These integrated biosensors demonstrate great promise for monitoring physiological status and athletic performance in field tests, as well as detecting doping in sports competitions.
MNs only penetrate the stratum corneum of the skin, therefore do not reach the capillary to sample blood for biosensing. Instead of hypodermic needles that sample blood for medical testing, the transdermal MN-based biosensors adopt a minimally-invasive way to sample the ISF for therapeutic drug monitoring (TDM). Using the TDM to optimize the drug dosage is important for improving therapeutic efficacy and predicting any adverse outcome, such as resistance and toxicity [111]. For example, Gowers et al. [74] developed a TDM system (
Figure 3a), an MN-based biosensor for real-time continuous monitoring levels of β-lactam antibiotics in vivo. The MNs were coated with multiple layers, including a gold working electrode layer, a pH-sensitive iridium oxide layer, a β-lactamase hydrogel layer, and a poly(ethylenimine) layer. The pH-sensitive layer detected changes in local pH as a result of β-lactam hydrolysis with the presence of β-lactamase. Afterwards, the potentiometric modality was applied for establishing the relationship between variations in local pH and the potential measured. Such a biosensor is capable of real-time tracking penicillin concentrations, paving the way for personalized drug dosage that is a large step towards precision medicine. But, these MN-based biosensors are still at an early stage of development. A daunting challenge still exists, namely the need for a continuous, reliable power supply.
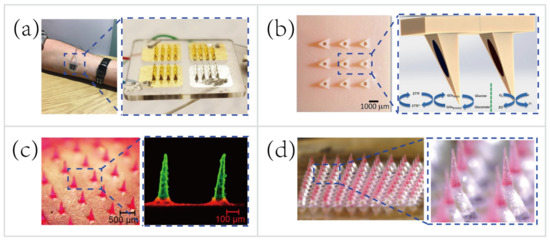
Figure 311.
a) MN-based biosensor for continuous monitoring of β-lactam antibiotic concentration in vivo [74]. Copyright 2019, American Chemical Society. (
b
c
d
A majority of wearable biosensing devices rely on the external power supply [14][26][57][61][79], which causes numerous technical difficulties in the field tests. Thus, researchers begin to seek new methods to obviate the need for an external power supply. Bandodkar et al. [112] made a major thrust of biofuel-cell (BFC) wearable devices that scavenged energy from human sweat. The wearable energy harvester they developed converted readily available sweat lactate into electricity. Lightweight, ultrathin, stretchable, high power density were the attractive features of this wearable harvester. Nowadays most MN-based sensing patches are still relying on the external power supply [74][79][113]. To overcome this major drawback, Valdés-Ramírez et al. [37] developed an MN-based self-powered BFC glucose biosensor that harvested biochemical energy from the wearer’s ISF (
Figure 3b). The self-powered biosensor harnessed glucose as the fuel to eliminate the need for an external power supply, and also provided power signals proportional to the glucose concentration. Besides, this self-powered MN-based sensor displayed high selectivity and stability that showed considerable promise for battery-free transdermal glucose monitoring. In short, the combination of energy harvester, electrochemical sensor, and MN technology will address some key challenges, such as integrating monitoring multiple analytes in ISF and automatic feedback-based transdermal drug delivery using MN-based self-powered BFC biosensors.
2.3.2. Microneedles for Pain-free Drug Delivery
Microneedles for transdermal drug delivery reduce the systemic side effects and improve dosage efficacy and patient compliance [89] [180] compared to hypodermic needles [38][89][114][38,180,202]. Typically, external small molecules (e.g., drug contents) can be minimally-invasive delivered percutaneously harnessing passive mass transfer. To facilitate the establishment of closed-loop diagnostic and therapeutic systems, MNs for drug delivery are also attractive, especially for controlled or long-term non-irritating drug release. In the past few years, great efforts have been made to find a way for controllable/long-term drug delivery using MNs, such as thermo-responsive MNs [79][153], stretch-triggered MNs [106][196], multilayered MNs [38], and effervescent MNs [40].
To deliver the drug contents in a controllable manner, Lau et al. [38] [38] developed a multilayered pyramidal dissolving MN patch with flexible pedestals (Figure 311c). The drug release time and rate were regulated by the dissolution rate of diverse biomaterials in different layers of the MNs. Hence, the MNs were applicable to control the release rate of the anti-inflammatory drug to facilitate personalized sports medicine. For instance, the rapid dissolution of the tip’s outermost layer of the multilayered pyramidal MNs can quickly control inflammation and continuously treat chronic inflammation via sustained dissolution of the inner layer.
Another group demonstrated the pursuit of long-term personalized drug delivery. Wei Li et al. [40] [40] developed an effervescent system to increase access to long-acting contraception (Figure 311d). The MNs consisted of drug-loaded tips and effervescent backings containing polyvinylpyrrolidone (PVP), sodium bicarbonate, and citric acid. Once inserted in the skin, the ISF solubilized the effervescent backing and the CO2 generated from the reaction of citric acid with sodium bicarbonate facilitated the separation of the interfaces between the MNs’ tips and patch backings (detachment efficiency of 91.7 ± 2.4%). Long-acting contraception was achieved in vivo by the dissolution of MNs’ tips to release levonorgestrel (LNG) for 2 months and maintained the LNG concentrations above the human contraceptive threshold level for >1 month (after rats being administered MNs).
In sum, MNs are not only appealing avenues to interrogate the ISF for biosensing, to carry out controllable/long-term transdermal drug delivery [88][94][95][179,185,186], but also can facilely incorporate with electrochemical biosensors [109][110][90] [157,169,181] to realize closed-loop personalized diagnostics and therapeutics [79][103][115][153,193,203]. Moreover, recent advances in biofuel cells [58][112][116][117][118] [89,200,204,205,206] could further address the key issue, lack of thin and reliable wearable power sources. These eminent features would enable a broad range of important applications in the sports field where real-time athletic performance monitoring and personalized sports injury treatments are urgently demanded [106][196]. We envisage that the advancement of MNs for biosensing and drug delivery will encourage the emergence of new generations of wearable athletic biosensors, that would revolutionize sports monitoring and sports medicine in the near future.
3. Conclusions and Perspectives
Recent advances in stretchable materials, microfluidics, optical/electrochemical sensors, image processing and analysis, NFC and wireless power supply, microneedles, as well as big data and cloud computing provide a robust foundation for wearable biosensing, especially the field of microfluidics for sweat and ISF biosensing. The emerging sampling and sensing modalities for obtaining sweat biochemical information offer profound insights into human physiology, metabolism, and sports performance, which complement traditional biophysical sensing modalities. Representative examples of wearable biosensors capable of in situ monitoring sweat biomarkers, from electrolytes, metabolites, heavy metals, cytokines [67][146], hormones [69][70][71][80,148,149], amino acids [72][150] to exogenous drugs [74][84][83,156], provide a window on the valuable biochemical resources that have a substantial impact on disease diagnostics, precision medicine, drug delivery, and sports analytics. However, the majority of advanced wearable sweat biosensors still focus on healthcare monitoring to improve home-based disease diagnosis. Although wearable biosensors for sports analytics are underdeveloped, the transition from focusing on healthcare monitoring to combining with sports analytics is an important step in a trend towards better preventive healthcare and sports & health science. It offers revolutionary capacities for future wearable technologies. It also in its infancy, and a great deal of work still needs to be done, such as integration of well-developed technologies (e.g., ultrasonics, implants, optofluidics, optoelectronics, microneedles, etc.), for building up a picture of future biosensing technologies. Despite the remarkable progress that several research groups achieved over the past five years, daunting challenges remain in the power supply, data interpretation, the cost of mass-fabrication, as well as in-depth interdisciplinary collaboration. With these challenges overcome, the commercialization and widespread adoption of wearable biosensors in sport-related fields will be fully realized, and state-of-the-art athletic monitoring and sports analytics will be transformed fundamentally.
