Glaucoma is a neurodegenerative disease and a worldwide leading cause of irreversible vision loss. In the last decades, high efforts have been made to develop novel treatments effective in inducing protection and/or recovery of neural function in glaucoma, including neurotrophic factors (NTFs). These approaches have shown encouraging data in preclinical setting; however, the challenge of sustained, targeted delivery to the retina and optic nerve still prevents the clinical translation.
- glaucoma
- neurotrophic factors (NTFs)
- neurotrophins (NTs)
- neuroprotection
- drug delivery systems
- microspheres
- gene therapy
- polymers
- nanoparticles
- implants
- glaucoma,neurotrophic factors (NTFs),neurotrophins (NTs),neuroprotection,drug delivery systems,microspheres,gene therapy,polymers,nanoparticles,implants
1. Introduction
Glaucoma is a chronic and progressive optic neuropathy, characterized by degeneration of retinal ganglion cells (RGCs) and axons. It affects more than 60 million people worldwide, causing legal blindness in more than 10% of cases [1]. The only available treatments for glaucoma are effective in lowering intraocular pressure (IOP) and therefore in halting or slowing the disease progression [2]. Currently, treatments able to recover retinal and neural function are not available for clinical use. In the last decades, encouraging perspectives in glaucoma treatment have emerged, and ongoing research is focusing on the study of novel molecules with neuroprotective and/or neuroregenerative activity [2]. To date, only glutamate N-methyl-D-aspartate (NMDA) receptor antagonist memantine and α
2- agonist brimonidine have reached large scale randomized controlled trials (RCTs), although results on their potential neuroprotective effects have not proven decisive [3][4].
- agonist brimonidine have reached large scale randomized controlled trials (RCTs), although results on their potential neuroprotective effects have not proven decisive [3,4].
Several preclinical studies have demonstrated that topical or intravitreal neurotrophic factors (NTFs) are able to prevent, slow, or reverse RGC death in animal models of experimental glaucoma [5][6][7][8][9]. In fact, it has been demonstrated that the deprivation of intrinsic growth factors promotes apoptotic ganglion cell death in chronic course, and that the administration of exogenous neurotrophic agents has the potential to establish survival permissive conditions [10][11]. Neurotrophins (NTs) are key modulators of multiple signaling pathways essential for neuronal survival and differentiation of central and peripheral nervous systems, as well as for synaptic plasticity and axonal regeneration [12][13][14]. The family of NTs consists of nerve growth factor (NGF), brain-derived neurotrophic factor (BNDF), neurotrophin 3 (NT3), and neurotrophin 4/5 (NT4/5). The glial cell-derived neurotrophic factor (GDNF) family and the neuropoietic cytokines have also been shown to regulate development and maintenance of neuronal cells [15].
Several preclinical studies have demonstrated that topical or intravitreal neurotrophic factors (NTFs) are able to prevent, slow, or reverse RGC death in animal models of experimental glaucoma [5,6,7,8,9]. In fact, it has been demonstrated that the deprivation of intrinsic growth factors promotes apoptotic ganglion cell death in chronic course, and that the administration of exogenous neurotrophic agents has the potential to establish survival permissive conditions [10,11]. Neurotrophins (NTs) are key modulators of multiple signaling pathways essential for neuronal survival and differentiation of central and peripheral nervous systems, as well as for synaptic plasticity and axonal regeneration [12,13,14]. The family of NTs consists of nerve growth factor (NGF), brain-derived neurotrophic factor (BNDF), neurotrophin 3 (NT3), and neurotrophin 4/5 (NT4/5). The glial cell-derived neurotrophic factor (GDNF) family and the neuropoietic cytokines have also been shown to regulate development and maintenance of neuronal cells [15].
Currently, clinical use of NTFs in glaucoma has been hindered by the difficulty to provide enough evidence of a safe, steady, controlled in vivo delivery to RGCs. Therefore, the potential of neuronal survivals might be further enhanced from advancement in intraocular delivery devices, enabling sustained drug release to the retina and optic nerve and improved safety profile.
2. Neuroprotection: Insights on Biochemical Pathways and Treatment Opportunities
Neuroprotection for glaucoma refers to any intervention aiming at preserving retinal ganglion cells and related signal transduction pathways. Mean increase of IOP is still considered as the most common risk factor for glaucoma progression, and it is well known that its reduction allows slowing or halting the disease progression in most cases [16]. However, up to 20% of patients with glaucoma show disease progression despite optimal IOP control [17]. In addition, 30–90% of patients showing glaucomatous optic disc damage and visual field loss have normal values of IOP, suggesting that multiple factors may contribute to the pathogenesis of RGCs death in glaucoma [16][18][19][20][21]. Several studies demonstrated that neuronal death represents the ultimate process in the pathophysiology of glaucoma damage, and, in this scenario, neuroprotective therapeutical approaches appear as crucial to prolong RGC life and possibly improve visual function [22][23].
Neuroprotection for glaucoma refers to any intervention aiming at preserving retinal ganglion cells and related signal transduction pathways. Mean increase of IOP is still considered as the most common risk factor for glaucoma progression, and it is well known that its reduction allows slowing or halting the disease progression in most cases [16]. However, up to 20% of patients with glaucoma show disease progression despite optimal IOP control [17]. In addition, 30–90% of patients showing glaucomatous optic disc damage and visual field loss have normal values of IOP, suggesting that multiple factors may contribute to the pathogenesis of RGCs death in glaucoma [16,18,19,20,21]. Several studies demonstrated that neuronal death represents the ultimate process in the pathophysiology of glaucoma damage, and, in this scenario, neuroprotective therapeutical approaches appear as crucial to prolong RGC life and possibly improve visual function [22,23].
The rationale for neuroprotectants is to act against the main pathways involved in the apoptotic ganglion cell death process, including: (i) the deprivation of NTs resulting from the blockage of the retrograde axonal transport from the lateral geniculate nucleus of the thalamus; (ii) the abnormal increase of excitatory neurotransmitters and reactive oxygen species; (iii) the deregulation of ion channel activities; and (iv) the loss of intracellular self-repair processes. All of these different cellular mechanisms invariably lead to RCG loss and glial cells activation.
Several classes of neuroprotective agents have been studied in glaucoma, most notably NTFs, glutamate N-methyl-D-aspartate (NMDA) receptor antagonists, α
2
-adrenergic agonists, antioxidant and free radical scavengers, and calcium channel blockers. Among them, only memantine, a non-competitive NMDA receptor antagonist, and brimonidine, a topical α
2
- agonist, were evaluated in large randomized controlled trials (RCTs). Specifically, the efficacy of memantine in reducing glaucoma progression was evaluated through two long-term, phase III RCTs conducted by Allergan (Irvine, CA). The results of these studies show that daily treatment with 10 or 20 mg of memantine for 48 months was not proven to significantly delay glaucomatous damage progression, when compared with placebo [3]. Neuroprotective properties of brimonidine were investigated in a multi-center, phase II RCT and compared with topical timolol in patients with low-pressure glaucoma (Low Pressure Glaucoma Treatment Study, LoGTS) [4]. The potential mechanisms of neuroprotective effects of brimonidine include upregulation of brain-derived neurotrophic factor (BDNF) and ciliary neurotrophic factor (CNTF) in RGCs, activation of cell survival pathways and antiapoptotic genes, and modulation of NMDA receptor.
In LoGTS, twice-daily treatment with topical 0.2% brimonidine tartrate for four years appeared to have beneficial effect on visual function independently of IOP lowering when compared with 0.5% timolol maleate eye drops. Nevertheless, problems related with the study design, patient adherence to treatment, the occurrence of adverse events leading to significant drop-out, the different profiles and diurnal effects of used drugs, and the missing data on visual acuity and vertical cup-disc ratio questioned the reliability of the study. A recent Cochrane systematic review concluded that results were not decisive, and that additional clinical trials are strongly recommended to demonstrate whether neuroprotective agents, including brimonidine eye drops, may be beneficial for patients with glaucoma [24].
2.1. Neurotrophic Factors Rationale for Use in Glaucoma Treatment and Preliminary Results
NTFs are secreted peptides regulating neuronal survival and function, which include the family of NTs, the glial cell-derived neurotrophic factor (GDNF) family, and the neuropoietic cytokine family.
NTs belong to a small family of pleiotropic molecules that are indispensable for regulating neuronal development, survival, and differentiation and promoting synaptic plasticity and axonal regeneration [12]. Currently, four NTs have been isolated: nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4/5 (NT-4/5). NTs and their receptors modulate multiple signaling pathways through the activation of two types of transmembrane glycoproteins: the tropomyosin receptor kinases (TrkA, TrkB, and TrkC) and the low-affinity neurotrophin receptor p75
NTR
. Specifically, NGF and BDNF bind with high affinity to TrkA and TrkB, respectively. NT-3 preferentially binds to TrkC, but it may bind with low affinity to both TrkA and TrkB depending on cellular context. NT-4/5 predominantly acts through TrkB. The Trk activation induces signaling cascades including the Ras/ERK (extracellular signal-regulated kinase) protein kinase pathway with stimulation of mitogen-activated protein (MAP) kinases, the phosphatidylinositol-3-kinase (PI-3 kinase)/Akt kinase pathway, and phospholipase C (PLC)-γ1. On the other hand, immature precursor forms of NTs (proNTs) are able to bind and activate p75
NTR
with different functional outcomes in terms of apoptosis or cell survival in dependence on the concurrent expression of Trk receptors. Therefore, a delicate balance between the relative percentage of pro- and mature NTs and/or the interaction between Trk and p75
NTR receptor availability determines cellular homeostasis in the nervous system [13][14].
receptor availability determines cellular homeostasis in the nervous system [13,14].
The role of NTs in the maintenance and survival of retinal cells during several degenerative diseases including glaucoma has been clearly demonstrated [23][24][25][26]. Specifically, various evidence showed that the blockage of axoplasmic flow at the lamina cribrosa in glaucoma would markedly compromise axonal long-range retrograde transportation via endosome neurotrophic signaling from the lateral geniculate nucleus in the CNS to ganglion cell bodies [10][11]. The decrease in neuronal trophic support, in turn, triggers programmed cell death in RGCs, as shown after experimental axotomy of the optic nerve in animals [27]. Furthermore, as a result of degenerative changes in the RGCs and optic nerve head, local production of NTs in the retina is markedly reduced, contributing to disease progression (
The role of NTs in the maintenance and survival of retinal cells during several degenerative diseases including glaucoma has been clearly demonstrated [23,24,25,26]. Specifically, various evidence showed that the blockage of axoplasmic flow at the lamina cribrosa in glaucoma would markedly compromise axonal long-range retrograde transportation via endosome neurotrophic signaling from the lateral geniculate nucleus in the CNS to ganglion cell bodies [10,11]. The decrease in neuronal trophic support, in turn, triggers programmed cell death in RGCs, as shown after experimental axotomy of the optic nerve in animals [27]. Furthermore, as a result of degenerative changes in the RGCs and optic nerve head, local production of NTs in the retina is markedly reduced, contributing to disease progression (
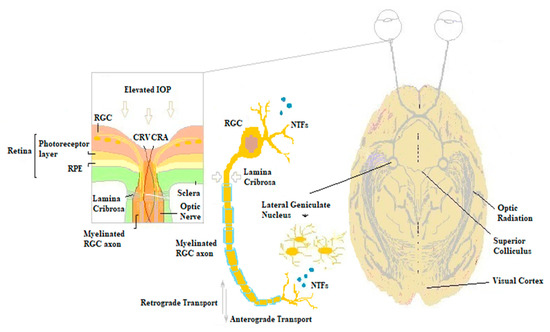
Figure 1.
Biochemical mechanisms of glaucomatous damage. The elevated intraocular pressure (IOP) at the lamina cribrosa is responsible for the blockage of the retrograde axonal transport of NTs from the lateral geniculate nucleus of the thalamus to retinal ganglion cells. The deprivation of neuronal trophism in turn triggers apoptotic ganglion cells death, resulting in decreased local NTFs processing and impaired anterograde transportation along RGCs axons. RGC, retinal ganglion cell; CRV, central retinal vein; CRA, central retinal artery; NTFs, neurotrophic factors; RPE, retinal pigment epithelium.
3. Conclusions
Glaucoma is a primary optic neuropathy characterized by irreversible retinal ganglion cells loss. Neuroprotective treatments act directly on the pathogenetic mechanism of glaucomatous damage and have the potential to reverse the progressive degeneration of RGCs.
The deprivation of NTFs has been shown to play a crucial role in the loss of RGCs and related axonal damage in glaucoma, and numerous pre-clinical studies have demonstrated that exogenous topical or intravitreal NTFs efficiently promote RGC recovery.
Although NTFs represent an innovative therapeutical approach in clinical management of glaucoma, the medical need of a continuous delivery system to the retina and optic nerve is still open.
In recent decades, considerable progress has been made in drug and gene delivery technology for neurotrophic active substances. Delivery systems allow for steady release of factors at the required physiological site of action, prolonged interval between treatments, fewer adverse events, and improved patient adherence.
The results emerging from clinical trials in several neurodegenerative diseases support the possible effectiveness of NTFs treatment in glaucoma. Various evidence shows that different delivery strategies for the use of NTFs in the treatment of glaucoma are proving effective for achieving long-term RGC survival and functional improvements, associated with a good safety profile. Specifically, the most promising technologies include cell-mediated gene therapy, currently being evaluated in phase II clinical trials for the treatment of glaucoma, and gene delivery approaches via viral and non-viral vectors, which showed encouraging preclinical results in glaucoma models and need prompt clinical investigation.
