High-throughput screening is a potent technique to accelerate the discovery and development of new materials. By performing massive synthesis and characterization processes in parallel, it can rapidly discover materials with desired components, structures and functions. Among the various approaches for high-throughput screening, microfluidic platforms have attracted increasing attention. Compared with many current strategies that are generally based on robotic dispensers and automatic microplates, microfluidic platforms can significantly increase the throughput and reduce the consumption of reagents by several orders of magnitude.
- Microfluidic High-Throughput Platforms,High-throughput screening,nanomaterials
1. Introduction
Compared with traditional microplate-based HTPs that require samples of at least several microliters in each well, microfluidic platforms consume much less reagents with the scale of nanoliters to picoliters, which significantly reduces the cost and is beneficial to save rare samples. Microarray is one of the major microfluidic platforms, which integrates a large quantity of isolated reactors on one substrate. Additionally, each reactor is microscaled with volumes ranging from nanoliters to picoliters. It allows multiple parameters to be tested in parallel by simultaneously performing tens to thousands of experiments per batch. For example, Zhang and his coworkers developed a hydrogel microarray (Figure 1a), in which 2000 individual microgels with varying bioactivities were regularly patterned on a standard microscope slide, providing a high-throughput platform to rapidly screen desired polymers with thermal-responsive properties [1][34]. Perera et al. developed an automatic synthetic platform for drug discovery, which integrated commercially available components into a highly integrated module unit to perform both nanomole-scale reactions and micromole-scale syntheses [2][35]. This setup allows screenings of more than 1500 homogeneous reactions within 24 h under different temperature, pressure, and solvent, which has the advantages of real-time analysis, sufficient mixing, and avoidance of solvent evaporation. Due to the application of microarray-based HTPs, reactions were performed in parallel under a broad range of experimental parameters so that appropriate conditions for generating nanostructures with specific morphologies can be rapidly identified. Moreover, Duffy et al. described a hydrogel microarray that integrated 80 unique holes on a single microscope slide using three-dimensional (3D) printing [3][36]. By filling the holes with double network hydrogels, the novel platform offered a powerful tool to screen hydrogels with desired compressive and tensile properties, which could be further optimized for drug delivery, cell encapsulation, and tissue engineering. Microarrays have also been widely applied in a wide range of biomedical applications, such as pharmaceutical discovery, small molecule and protein screening, toxicity tests, etc. [4][5][6][7][8][9][37,38,39,40,41,42]. For example, Hay et al. used the polymer microarray with high content screening system and Pathfinder software to screen and discover new extracellular substrates, which can promote hepatic endoderm, drug-inducible metabolism and toxicology [5][38]. Additionally, Khan et al. proposed a microarray platform combined with a high-throughput screening approach to screen and analyze the biological functionality of 135 polymer blends, leading to the identification of cell-compatible biopolymers permissive for human skeletal stem cell growth in both in vitro and in vivo applications [7][40].
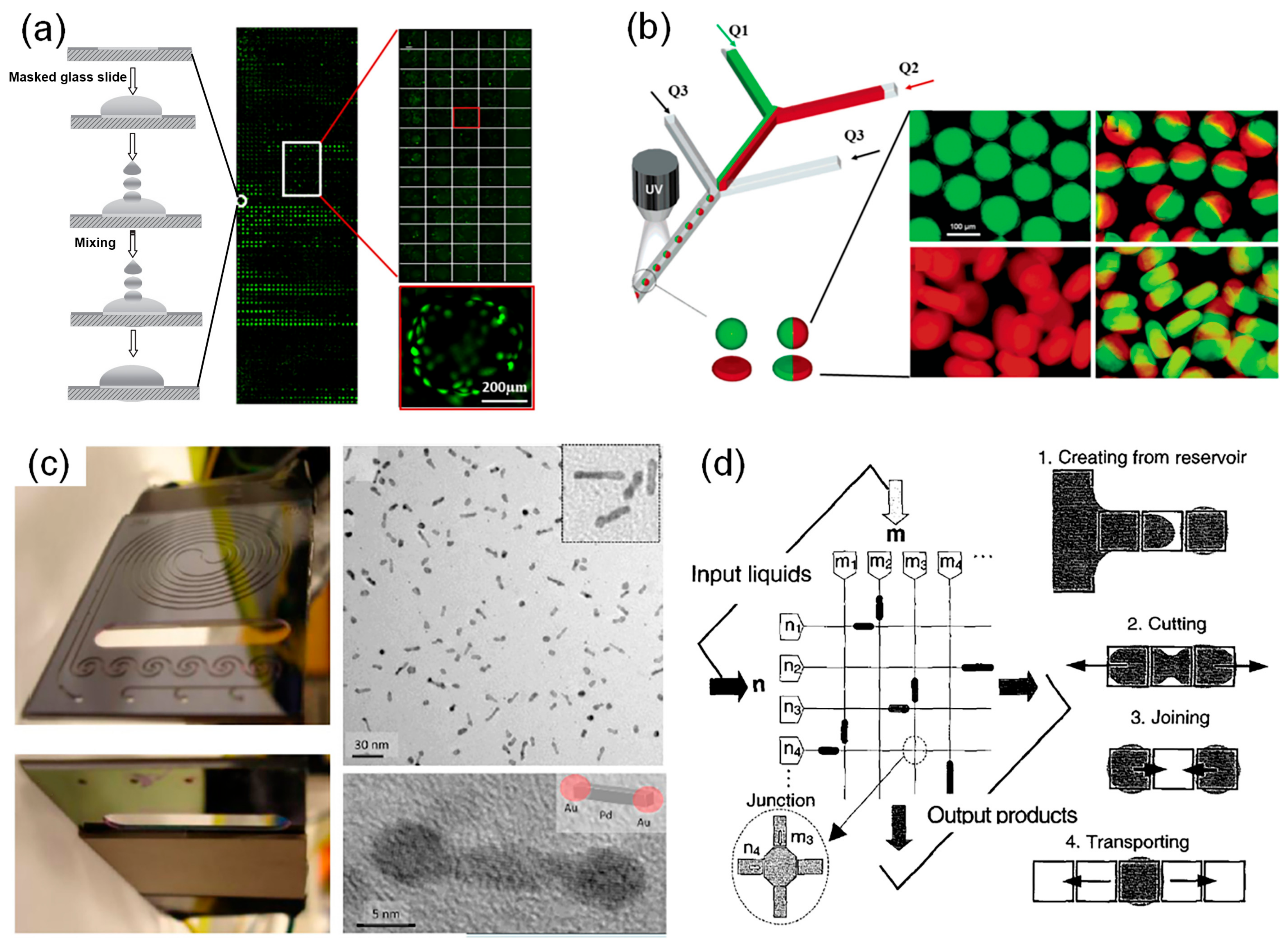
Despite the improvement in throughput, microarray-based HTPs are still limited in many cases that required higher screening efficiency. To address the issue, microdroplet technology has drawn increasing attention and been developed for high-throughput screenings [13][46]. Microfluidic droplet chips can be divided into continuous microfluidic chips (Figure 1b,c) [14][15][16][17][18][47,48,49,50,51] and digital microfluidic chips (Figure 1d) [19][20][21][52,53,54]. Shepherd’s group provided a continuous microfluidic device (Figure 1b) to generate monodisperse colloid-filled hydrogel particles with different shapes and compositions [10][43]. Additionally, Jensen et al. described a new device for the production of Au-Pd dumbbell-like nanostructure with high electrocatalytic activity [11][44]. This device was integrated with a sequential-addition microfluidic reactor and an ultrasonic to control the growth of Au onto the both sides of Pd nanorods (Figure 1c). As the key platform of microdroplet technology, continuous microfluidic chip can generate monodisperse droplets (usually at nano- or picoliters) at very high frequencies (from tens to thousands of droplets per second) [22][23][55,56]. Additionally, each microdroplet serves as an independent microreactor, in which synthesis of materials can be carried out without interference under certain conditions. Digital microfluidics employed electrowetting to control and discretize the continuous flow into individual droplets. Sung et al. fully reported the functional digital microfluidic circuits and the four fundamental droplet operations mechanisms [12][45]. It provides a promising experimental platform with advantages of a fast response, high precision, and digital readouts. Microdroplet-based HTPs has many advantages [24][25][26][57,58,59]. Firstly, it consumes much less reactants since the working volume of a plate well (e.g., 10 μL for each well of a 384-well plate) is ten million times that of a single droplet (1.0 pL) [27][60]. Secondly, the high surface-to-volume ratio of microdroplets and short diffusion distance in microdroplets result in pronounced acceleration of reactions and thus can significantly shorten the screening time. Thirdly, it provides chemical and physical confinement to avoid cross-contamination. Using this technique, a large quantity of independent experiments can be easily performed within a very short period and only a small amount of reagents are consumed.
2. Basic Principles
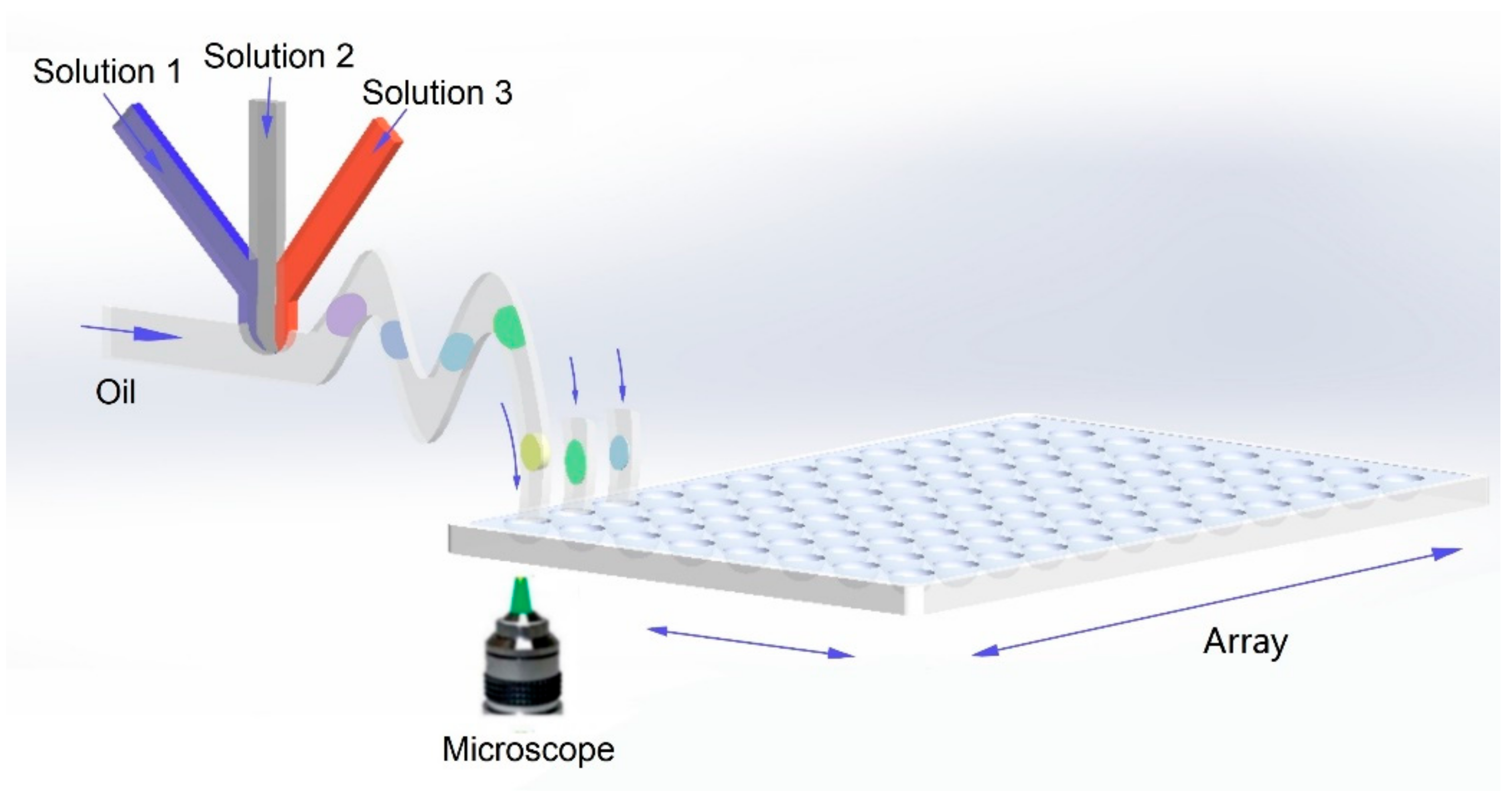
Both microarrays- and microdroplets-based HTPs can significantly increase the screening throughput and accelerate development of material science. For microarray-based HTPs, the synthetic parameters of each reaction can be precisely encoded by spatial coordinates, but the throughput is limited by device area and density of reaction sites. For microdroplet-based HTPs, the throughput greatly increases owing to the continuous and rapid generation of microdroplets. However, it remains challenging to accurately encode the synthetic parameters of each microdroplet, thus limiting the further increasement of its throughput. Recently, a novel high-throughput method called “droplet library”, which combines a microfluidic droplet generator with microarrays, are proposed [28][29][75,113]. The basic principles are shown in Figure 2. Firstly, droplets containing small compounds are prepared by parallel microfluidic devices and subsequently transported to microarray plates. Then the following droplets with different compounds could be gathered in one tube as a droplet library. The droplet library was then reinjected into another device to mix with a target for screening the compounds with optimal antimicrobial activities. This integrated platform takes significantly less time than conventional microdroplet-based HTPs. Although mainly applied for biological experiments, such as investigations of antimicrobial activities, pharmacological screening, drug-resistance analysis, etc., the novel integrated approach shows great potentials in screening materials with ultra-high throughput, providing a promising approach towards the development of next-generation HTPs.
Moreover, to achieve truly high-throughput screening, it is necessary to establish highly integrated HTPs with multiple functions of material synthesis, characterization and data analysis. Zhou et al. [30][114] have proposed a high-throughput screening system. It combined a microfluidic reactor to generate hydrogel droplets with different crystals of drugs, a camera to capture the optical images of the droplets, and deep learning to analyze and classify the obtained images. Additionally, the microfluidic chip was fabricated with a flow-focusing geometry to produce droplets. Their system offered a new high-throughput platform and could be applied to quickly synthesize the massive materials and accurately analyze the data. With massive materials informatics and databases, it offers a potent platform to accelerate the development of the new materials. Despite the great advancements in material synthesis, the performance of current HTPs in high-throughput characterization is still far from satisfactory. Therefore, developing compatible high-throughput characterization techniques to combine with synthetic modules is one of the important trends of future HTPs. Additionally, as HTPs usually produce massive data, approaches for high-throughput data processing are also in great demand. Machine learning is a powerful tool to process and analyze massive information, which shows promising applications in future HTPs. Since the application of HTPs has gradually played a critical role in new material preparation, it will show significant impact on the development of material science, biological science, biomedical engineering and military science in the future.
