Naringenin, a natural flavanone, was first identified from extracts of the dormant peach (Prunus persica) flower buds, with the chemical name of 5,7,4′-trihydroxyflavanone.
- COPD,naringenin,pathogenesis,mechanism
1. Chronic Obstructive Pulmonary Disease
Chronic obstructive pulmonary disease (COPD), one of the most common chronic respiratory disease, is characterized by progressive and irreversible airflow limitation resulting from the emphysematous destruction of the alveolar structure and the remodeling and narrowing of small airways [1,2][1][2]. COPD is considered as a multifactor disease, and cigarette smoking is demonstrated as the dominant driving force for the development of the disease [3][3]. Since its high prevalence, morbidity, and mortality, COPD induces substantial economic and social burden worldwide. It is predicted that COPD will become the third-ranked leading disease of death worldwide in 2030, and there may be over 5.4 million deaths annually from it in 2060 due to the increasing numbers of smokers and aging populations [4,5][4][5].
Clinical phenotypes of COPD vary among patients due to the differences in the age of onset, the rate of progression, the frequency of exacerbations, and the association with comorbidities, with some patients predominantly suffering from small airway disease, while others mainly suffer from pulmonary diseases such as emphysema [6][6]. Although several treatments of COPD, including inhaled corticosteroids, long-acting muscarinic antagonists, and long-acting β2-agonists have already demonstrated to have a certain degree of clinical efficacy, it seems that the side effects of these currently available therapies are unavoidable and time- or dose-dependent [7][7]. In addition, the precise mechanisms of COPD pathogenesis have not been clarified at present. Therefore, it is critical to elucidate the molecular mechanisms underlying COPD and identify an alternative ingredient that can treat COPD with fewer side effects.
2. Naringenin and its Glycoside Naringin
Naringenin, a natural flavanone, was first identified from extracts of the dormant peach (Prunus persica) flower buds, with the chemical name of 5,7,4′-trihydroxyflavanone [8] [8] (Figure 1). As a common dietary constituent consumed by humans, naringenin is abundantly present in citrus fruits and vegetables such as grapefruit, lemon, oranges, and tomatoes. Naringin is a flavanone glycoside composed of naringenin and neohesperidose attached at C-7, which is partly absorbed by gastrointestinal tracts and is mostly metabolized by gastrointestinal bacteria into naringenin after oral ingestion [9][9]. Thus, naringin is mainly introduced into the body as a form of naringenin [10][10]. In recent years, accumulating studies have reported on the potential pharmacological activities of naringenin, including beneficial effects in chronic airway disease, lung diseases, liver diseases, cardiovascular diseases, and cancer [11,12,13,14,15][11][12][13][14][15]. Evidence suggests that it had antioxidative, anti-inflammatory, antifibrogenic, antiatherogenic, and antiproliferative bioactivities [16,17,18][16][17][18]. Even though its therapeutic effects in the treatment of COPD are seldom reported, these findings still indicate that naringenin and its glycoside naringin appear to be full of potential therapeutic value in COPD.
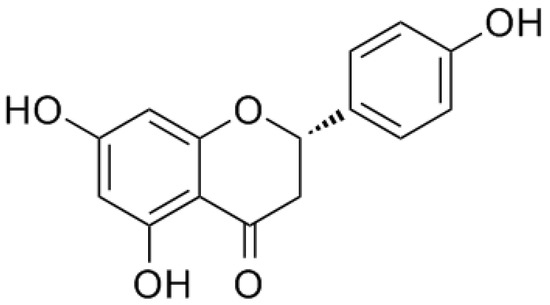
Figure 1. Chemical structure of naringenin.
The traditional concept of “one drug for one target for one disease” was the predominant paradigm in drug discovery in the past. However, advances in systems biology suggest that complex diseases may not be effectively treatable by interventions at single targets [19][19]. As a classic method of bioinformatics, network pharmacology can save cost and time compared with conventional experiments, and, more importantly, the core of network pharmacology is consistent with the holistic philosophy, which contributes to overcoming complex diseases such as COPD, in a systematic manner [20,21][20][21]. Consequently, in this study, we combined literature review with network pharmacology analysis to evaluate the possible therapeutic effect of naringenin on COPD and its underlying mechanisms, expecting to provide a promising treatment option for COPD.
Reference (we'll rearrange the references after you submitted it)
- Hikichi, M.; Mizumura, K.; Maruoka, S.; Gon, Y. Pathogenesis of chronic obstructive pulmonary disease (COPD) induced by cigarette smoke. J. Thorac. Dis. 2019, 11, S2129–S2140.
- Goncalves, I.; Guimaraes, M.J.; van Zeller, M.; Menezes, F.; Moita, J.; Simao, P. Clinical and molecular markers in COPD. Pulmonology 2018, 24, 250–259.
- Zhang, Y.; Xu, C.B. The roles of endothelin and its receptors in cigarette smoke-associated pulmonary hypertension with chronic lung disease. Pathol. Res. Pract. 2020, 216, 153083.
- Singh, D.; Agusti, A.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Criner, G.J.; Frith, P.; Halpin, D.; Han, M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: The GOLD science committee report 2019. Eur. Respir. J. 2019, 53, 1900164.
- Belchamber, K.; Donnelly, L.E. Targeting defective pulmonary innate immunity—A new therapeutic option? Pharmacol. Ther. 2020, 209, 107500.
- Castaldi, P.J.; Dy, J.; Ross, J.; Chang, Y.; Washko, G.R.; Curran-Everett, D.; Williams, A.; Lynch, D.A.; Make, B.J.; Crapo, J.D.; et al. Cluster analysis in the COPDGene study identifies subtypes of smokers with distinct patterns of airway disease and emphysema. Thorax 2014, 69, 415–422.
- Cazzola, M.; Rogliani, P.; Stolz, D.; Matera, M.G. Pharmacological treatment and current controversies in COPD. F1000Res. 2019, 8, 1533.
- Hendershott, C.H.; Walker, D.R. Identification of a growth inhibitor from extracts of dormant peach flower buds. Science 1959, 130, 798–800.
- Zeng, X.; Su, W.; Liu, B.; Chai, L.; Shi, R.; Yao, H. A Review on the pharmacokinetic properties of naringin and its therapeutic efficacies in respiratory diseases. Mini-Rev. Med. Chem. 2020, 20, 286–293.
- Bai, Y.; Peng, W.; Yang, C.; Zou, W.; Liu, M.; Wu, H.; Fan, L.; Li, P.; Zeng, X.; Su, W. Pharmacokinetics and metabolism of naringin and active metabolite naringenin in rats, dogs, humans, and the differences between species. Front. Pharmacol. 2020, 11, 364.
- Chin, L.H.; Hon, C.M.; Chellappan, D.K.; Chellian, J.; Madheswaran, T.; Zeeshan, F.; Awasthi, R.; Aljabali, A.A.; Tambuwala, M.M.; Dureja, H.; et al. Molecular mechanisms of action of naringenin in chronic airway diseases. Eur. J. Pharmacol. 2020, 879, 173139.
- Fouad, A.A.; Albuali, W.H.; Jresat, I. Protective effect of naringenin against lipopolysaccharide-induced acute lung injury in rats. Pharmacology 2016, 97, 224–232.
- Hernández-Aquino, E.; Muriel, P. Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World J. Gastroenterol. 2018, 24, 1679–1707.
- Testai, L.; Calderone, V. Nutraceutical value of citrus flavanones and their implications in cardiovascular disease. Nutrients 2017, 9, 502.
- Salehi, B.; Fokou, P.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of naringenin: A review of clinical trials. Pharmaceuticals. 2019, 12, 11.
- Zeng, W.; Jin, L.; Zhang, F.; Zhang, C.; Liang, W. Naringenin as a potential immunomodulator in therapeutics. Pharmacol. Res. 2018, 135, 122–126.
- Patel, K.; Singh, G.K.; Patel, D.K. A review on pharmacological and analytical aspects of naringenin. Chin. J. Integr. Med. 2018, 24, 551–560.
- Zaidun, N.H.; Thent, Z.C.; Latiff, A.A. Combating oxidative stress disorders with citrus flavonoid: Naringenin. Life Sci. 2018, 208, 111–122.
- Hopkins, A.L. Network pharmacology. Nat. Biotechnol. 2007, 25, 1110–1111.
- Boezio, B.; Audouze, K.; Ducrot, P.; Taboureau, O. Network-based approaches in pharmacology. Mol. Inform. 2017, 36, 36.
- Li, S.; Zhang, B. Traditional Chinese medicine network pharmacology: Theory, methodology, and application. Chin. J. Nat. Med. 2013, 11, 110–120.
References
- Hikichi, M.; Mizumura, K.; Maruoka, S.; Gon, Y. Pathogenesis of chronic obstructive pulmonary disease (COPD) induced by cigarette smoke. J. Thorac. Dis. 2019, 11, S2129–S2140.
- Goncalves, I.; Guimaraes, M.J.; van Zeller, M.; Menezes, F.; Moita, J.; Simao, P. Clinical and molecular markers in COPD. Pulmonology 2018, 24, 250–259.
- Zhang, Y.; Xu, C.B. The roles of endothelin and its receptors in cigarette smoke-associated pulmonary hypertension with chronic lung disease. Pathol. Res. Pract. 2020, 216, 153083.
- Singh, D.; Agusti, A.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Criner, G.J.; Frith, P.; Halpin, D.; Han, M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: The GOLD science committee report 2019. Eur. Respir. J. 2019, 53, 1900164.
- Belchamber, K.; Donnelly, L.E. Targeting defective pulmonary innate immunity—A new therapeutic option? Pharmacol. Ther. 2020, 209, 107500.
- Castaldi, P.J.; Dy, J.; Ross, J.; Chang, Y.; Washko, G.R.; Curran-Everett, D.; Williams, A.; Lynch, D.A.; Make, B.J.; Crapo, J.D.; et al. Cluster analysis in the COPDGene study identifies subtypes of smokers with distinct patterns of airway disease and emphysema. Thorax 2014, 69, 415–422.
- Cazzola, M.; Rogliani, P.; Stolz, D.; Matera, M.G. Pharmacological treatment and current controversies in COPD. F1000Res. 2019, 8, 1533.
- Hendershott, C.H.; Walker, D.R. Identification of a growth inhibitor from extracts of dormant peach flower buds. Science 1959, 130, 798–800.
- Zeng, X.; Su, W.; Liu, B.; Chai, L.; Shi, R.; Yao, H. A Review on the pharmacokinetic properties of naringin and its therapeutic efficacies in respiratory diseases. Mini-Rev. Med. Chem. 2020, 20, 286–293.
- Bai, Y.; Peng, W.; Yang, C.; Zou, W.; Liu, M.; Wu, H.; Fan, L.; Li, P.; Zeng, X.; Su, W. Pharmacokinetics and metabolism of naringin and active metabolite naringenin in rats, dogs, humans, and the differences between species. Front. Pharmacol. 2020, 11, 364.
- Chin, L.H.; Hon, C.M.; Chellappan, D.K.; Chellian, J.; Madheswaran, T.; Zeeshan, F.; Awasthi, R.; Aljabali, A.A.; Tambuwala, M.M.; Dureja, H.; et al. Molecular mechanisms of action of naringenin in chronic airway diseases. Eur. J. Pharmacol. 2020, 879, 173139.
- Fouad, A.A.; Albuali, W.H.; Jresat, I. Protective effect of naringenin against lipopolysaccharide-induced acute lung injury in rats. Pharmacology 2016, 97, 224–232.
- Hernández-Aquino, E.; Muriel, P. Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World J. Gastroenterol. 2018, 24, 1679–1707.
- Testai, L.; Calderone, V. Nutraceutical value of citrus flavanones and their implications in cardiovascular disease. Nutrients 2017, 9, 502.
- Salehi, B.; Fokou, P.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of naringenin: A review of clinical trials. Pharmaceuticals. 2019, 12, 11.
- Zeng, W.; Jin, L.; Zhang, F.; Zhang, C.; Liang, W. Naringenin as a potential immunomodulator in therapeutics. Pharmacol. Res. 2018, 135, 122–126.
- Patel, K.; Singh, G.K.; Patel, D.K. A review on pharmacological and analytical aspects of naringenin. Chin. J. Integr. Med. 2018, 24, 551–560.
- Zaidun, N.H.; Thent, Z.C.; Latiff, A.A. Combating oxidative stress disorders with citrus flavonoid: Naringenin. Life Sci. 2018, 208, 111–122.
- Hopkins, A.L. Network pharmacology. Nat. Biotechnol. 2007, 25, 1110–1111.
- Boezio, B.; Audouze, K.; Ducrot, P.; Taboureau, O. Network-based approaches in pharmacology. Mol. Inform. 2017, 36, 36.
- Li, S.; Zhang, B. Traditional Chinese medicine network pharmacology: Theory, methodology, and application. Chin. J. Nat. Med. 2013, 11, 110–120.
