Phenolic compounds are secondary metabolites widely spread throughout the plant kingdom that can be categorized as flavonoids and non-flavonoids.
- antioxidant activity,bioavailability,flavonoids
Thanks so much for your check. We sincerely hope you may create this entry, since you are the expert in this academic research. You can click the “submit” button to upload it and revise it. We will help you layout after you submit it. Moreover, we will link your article at the entry, and more scholars and students can look through it.
1. Introduction
Phenolic compounds are secondary metabolites widely spread throughout the plant kingdom with around 8000 different phenolic structures [1][1]. They are involved in adaptation processes in plants during stress conditions such as wounding, infection or exposure to UV radiation [2][2].
In relation to their chemical structure, these compounds contain at least one phenol group [3][3]. This phenol is composed of an aromatic ring with one or more hydroxyl groups. Although phenolic compounds can be present in their free form in plants, they are generally present bound to sugars or proteins [4][4].
The interest of phenolic compounds has increased during the last decade due to their antioxidant power. Their free radical-scavenging properties help to the prevention of chronic and oxidative stress-related disorders such as cancer, cardiovascular and neurodegenerative diseases [5][5].
Phenolic compounds can be classified in different ways. In relation to their carbon chain, they can be divided into 16 classes (for a detailed review, see [4][4]). On the other hand, according to the most important classes found in the human diet, phenolic compounds are organized in phenolic acids, flavonoids and tannins.
2. Classification
The classification used in this entry is based on the number of phenol rings and on the structural elements linking such rings, so that phenolic compounds are categorized as flavonoids and non-flavonoids [6][6].
2.1 Flavonoids
Flavonoids constitute the major group of phenolic compounds. They are responsible, along with carotenoids and chlorophylls, for the blue, purple, yellow, orange and red colors in plants [7][7]. They have a C6-C3-C6 skeleton with two aromatic rings connected by a three-carbon link (Figure 1) [8][8]. Their antioxidant activity depends on the presence, number and position of hydroxyl groups in the chemical structure of these compounds [4][4]. Flavonoids can be divided into six subclasses: flavones, isoflavones, flavonols, anthocyanins, flavanols and flavanones. The differences between them are due to variations in the number and positions of the hydroxyl groups as well as in their range of alkylation and glycosylation (Figure 1) [6][6].
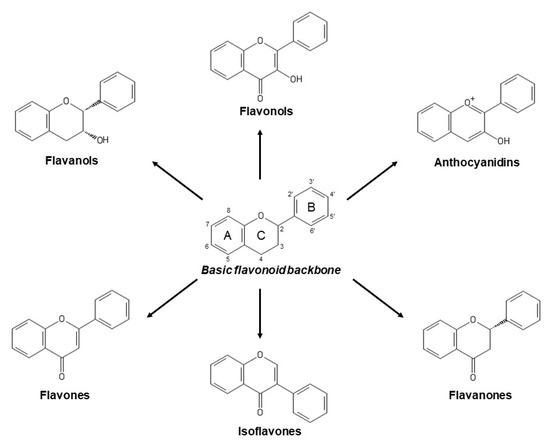
Figure 1. Basic flavonoid structure and main types of flavonoids.
Flavones, which contain a keto group at C4, a double bond between C2 and C3, and a B ring linked to C2 (Figure 1), represent the most basic flavonoid structures. Apigenin, luteolin and their glycosides are the most abundant flavones in fruit and vegetables [9][9].
Isoflavones are flavones with the B ring connected to C3 (Figure 1). They are phytoestrogenic compounds found in leguminous plants. Genistein and daidzein are their principal members [1][1].
Flavonols are flavones hydroxylated at C3 (Figure 1) and they represent the most numerous flavonoids in fruit and vegetables. The main compounds in this subclass are myricetin, quercetin and kaempferol [9][9]. Cherries, grapes, apricots, red wine, chocolate and various types of tea are all rich in flavonols [5][5].
Anthocyanins are water-soluble pigments that provide blue, purple and red colors to vegetables. The most common are cyanidin, delphinidin, malvidin, pelargonidin, petunidin, and peonidin [8][8].
Flavanols comprise a complex subclass and their predominant compounds are catechin, epicatechin, gallocatechin, epigallocatechin, their 3-O-gallates, polymers and oligomers. Proanthocyanidins are oligomeric flavanols but their polymeric form are called condensed tannins [9][9]. Flavanols contribute to astringency, bitterness, sourness, salivary viscosity, aroma and color formation in food [1][1].
Flavanones compose the smallest subclass because of their limited presence, mostly, in citrus. Hesperidin is responsible of sour taste in orange juice while naringin possesses a sweeter taste [1][1].
Chalcones and dihydrochalcones can be also considered flavonoids because they are intermediate products in their biosynthesis. The most frequent compounds within this group are phloretin and its glucoside phloridzin, which are found in apples [4][4].
2.2. Non-flavonoids
Compounds with smaller and simpler chemical structures than flavonoids belong to this class. However, there are also non-flavonoids with complex structures and high molar mass [9][9]. Phenolic acids, coumarins, stilbenes and lignans constitute mainly this group.
Phenolic acids participate in color stability, aroma profile and antioxidant activity, which depends on the number of hydroxyl groups included in the molecule [4,5][4][5]. They are classified in hydroxybenzoic and hydroxycinnamic acids [4,5][4][5]. Hydroxybenzoic acids have a C6-C1 skeleton and are the simplest phenolic acids found in nature [1,4][1][4]. They are frequently glycosylated, joined to small organic acids or linked to structural compounds of plant cells [9][9]. The content of these compounds in edible plants is low, except for certain red fruits and onions [6][6]. Some of the most common hydroxybenzoic acids are gallic, protocatechuic, vanillic, syringic and salicylic acids [4][4]. On the other hand, hydroxycinnamic acids are composed by a C6-C3 skeleton and they are more common than hydroxybenzoic acids [6]. The principal dietary sources of these compounds are fruits such as apples, cherries, peaches, and citrus fruits. Some examples of hydroxybenzoic acids are coumaric, caffeic, ferulic and rosmarinic acids, p-coumaric and caffeic acid being the most abundant in fruits [6,8][6][8].
Coumarins are a group of phenolic compounds biosynthesized via the shikimic acid pathway, being structural derivatives of o-cumaric acid [10][10]. They are present in plants in either their free forms or as coumarin glycosides, and they absorb UV light which results in their characteristic blue fluorescence [11][11]. Coumarins are mostly found in olive oil, oats and spices, and their main representatives are coumarin, umbelliferone, esculetin and scopoletin [1,4][1][4].
Stilbenes have a C6-C2-C6 structure and are derivatives of the same biosynthetic route as flavonoids since the first part of the synthetic pathway is common to both compounds [12][12]. Stilbenes help to protect plant tissues from the attack of fungi, insects, and other organisms. Moreover, the synthesis of antifungal stilbenes can be induced by infections or by abiotic stimulus such as UV light [10][10]. Resveratrol is the most important and studied stilbene due to its antitumoral effect and can be found in grapes and wines [1][1].
Lignans are phytoestrogens and are synthetized by union of two cinnamic acid residues or their biogenic relatives [1]. They are present in all the organs of many vascular plant families but exhibit a low concentration in cereals, fruits, nuts, and vegetables [1,9][1][9]. An example of a plant lignan is secoisolariciresinol diglucoside. When ingested, this compound is first converted into enterodiol and eventually transformed into enterolactone by microbial enzymes in the colon [13][13].
3. Biological Effects of Phenolic Compounds
Phenolic compounds have been recently widely studied due to their biological effects, which could be beneficial for human health [32][14]. These benefits are mainly related to both their direct and indirect antioxidant actions. In fact, polyphenols are able to donate electrons to oxidant species, scavenge free radicals and chelate metal ions [29][15], but can also indirectly attenuate production of reactive oxygen species (ROS) by either improving antioxidant enzymes’ activity or inhibiting enzymes that induce pro-oxidant effects [33][16]. For example, kaempferol depicts a high antioxidant capacity because of its tendency to donate electrons [34][17]. Similarly, fisetin shows protective effects against cell death, ROS scavenging actions and stimulation of glutathione antioxidant capacity, thus significantly reducing oxidative damage in lipids, DNA and proteins in Chinese hamster lung fibroblasts [35][18]. The same effect was observed with a grape seed extract rich in catechins, proanthocyanidins and anthocyanidins in human keratinocyte cell line HaCaT. The antioxidant power of this extract protected keratinocytes against ROS formation, thereby reducing oxidative stress, DNA damage and apoptosis, while increasing cell survival [36][19]. Antioxidant activity, whether direct or indirect, has also been demonstrated in other phenolic compounds such as phenolic acids and tyrosol, the former prevents metal catalysis and free radicals formation and the latter inhibits damage caused by ROS in human umbilical cord vein endothelial cells [37,38][20][21]. On the other hand, phenolic compounds also possess other biological effects related to their antioxidant capacity such as antimicrobial, anti-inflammatory, anticancer and cardioprotective activities (Figure 2). Additionally, it is important to note that phytochemical mixtures can influence the expected biological effect caused by the individual compounds. For example, it has been observed that the combination of extracts from Potentilla fruticose L. leaves and green tea polyphenols showed enhanced radical scavenging properties mainly due to the synergistic activities of the phenolic compound hyperoside (abundant in P. fruticose L. leaves) with epicatechin gallate (ECG; present in green tea) [39][22]. Likewise, it has been shown that the combination of chlorogenic acid and isoquercitrin improved their superoxide anion scavenging actions [40][23].
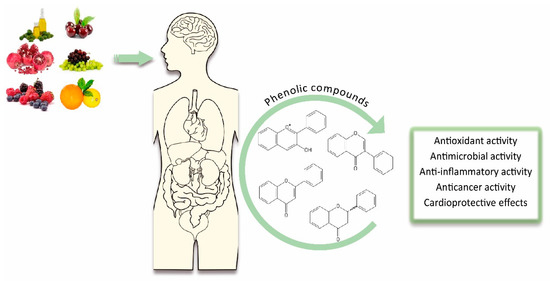
Figure 2. An overview on the biological effects of dietary phenolic compounds.
3.1. Antimicrobial Activity
The antimicrobial properties of phenolic compounds are due to the ability of their hydroxyl groups to bind the active sites of key enzymes and modify the metabolism of microorganisms [41][24]. Antimicrobial activity depends on the position of the hydroxyl substitution in the aromatic ring, as well as on the length of the saturated side-chain [42][25]. For example, it has been demonstrated that caffeic acid possesses higher antimicrobial activity than p-coumaric acid because the first one has more hydroxyl groups substituted in the phenolic ring [43][26].
Some of the investigations that examine antibacterial and antifungal activity of different phenolic compounds are highlighted in the next few lines. For instance, catechin inhibits the growth of Helicobacter pylori and Escherichia coli [44,45][27][28]. Likewise, resveratrol displays antibacterial capacity against Enterococcus faecalis, Campylobacter spp., Arcobacter butzleri and Candida albicans [46,47,48][29][30][31]. Moreover, the pulp of two varieties of Portuguese red grape shows an effective growth inhibition against Klebsiella pneumoniae, Staphylococcus epidermis, Listeria monocytogenes and Staphylococcus aureus due to their content in anthocyanins and tannins [49][32]. Finally, essential oils from different thyme species that are rich in phenolic compounds act against microorganisms like Pseudomonas aeruginosa, Cronobacter sakazakii, Listeria innocua, Streptococcus pyogenes, Candida albicans, Saccharomyces cerevisiae, Staphylococcus aureus and Salmonella enterica [50,51][33][34].
Synergistic interactions of phenolic compounds with antibiotics have been also investigated with the aim of counteracting antibiotic resistance. Thus, licoarylcoumarin, glycycoumarin and gancaonin, which are found in licorice, were reported to evoke antibacterial effect on vancomycin-resistant strains of Enterococcus faecium and Enterococcus faecalis [52][35]. Furthermore, polyphenols present in Cabernet Sauvignon grape pomace were shown to potentiate the effect of different classes of antibiotics against Staphylococcus aureus and Escherichia coli, especially against multi-drug resistant clinical isolates [53][36].
As for in vitro antiviral effect, various studies have reported the potential of phenolic compounds. For example, it has been observed that gallic acid presented a potent effect on Herpes simplex virus type 1 (HSV-1) and parainfluenza type 3 [54][37]. Besides, one flavanone, naringenin, has been shown to inhibit Dengue virus replication in infected primary human monocytes [55][38]. Additionally, epigallocatechin gallate (EGCG), particularly abundant in green tea, was demonstrated to block Hepatitis B virus entry into immortalized human primary hepatocytes [56][39]. In this line, EGCG alongside delphinidin were proven to reduce the infectivity of Dengue virus, Zika virus and West Nile virus by affecting the attachment and entry steps of the viruses life cycle [57][40]. Both compounds have also demonstrated to impede hepatitis C virus entry in primary human hepatocytes by alteration of the viral particle structure, which hinders its attachment to the cell surface [58][41].
3.2. Anti-Inflammatory Activity
The inflammation process exacerbates ROS and reactive nitrogen species (RNS) production, thereby increasing the activity of proinflammatory agents [6][6]. Anti-inflammatory activity of phenolic compounds interrupts ROS-dependent inflammation cycle[42] [59] and acts against pro-inflammatory mediators like tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, -6 and -8, inducible nitric oxide synthase (iNOS), COX, and leukotrienes. Besides, phenolic compounds also regulate expression of transcription factors such as nuclear factor kappa-light-chain-enhancer of activated B cells (NK-κB) [26].
Among phenolic compounds, stilbenes are one of the most thoroughly studied compounds. In this line, various studies have shown the capacity of resveratrol to inhibit COX activity, inactivate peroxisome proliferator-activated receptor gamma (PPARγ) and induce endothelial nitric oxide synthase (eNOS) in murine and rat macrophages. Besides, a resveratrol analog, RVSA40, was proven to inhibit TNF-α and IL-6 production in RAW 264.47 (murine macrophages cell line) [59][42]. Likewise, a study with 25 different stilbenes revealed that piceatannol and pinostilbene demonstrated a comparable activity to the anti-inflammatory drugs zileuton and ibuprofen in inhibiting COX-1, COX-2 and 5-lipoxygenase (5-LO) activity [60][43]. In addition, in the same study, it was shown that the majority of those 25 compounds managed to reduce the activity of NF-κB/activator protein-1 (AP-1) and attenuate the expression of TNF-α in human monocytic leukemia cell line THP-1 [60][43]. Similarly, in another study, it was observed the inhibition of COX-2 by pinostilbene and of COX-1 by pinostilbene and oxyresveratrol. In relation to 5-LO, the effective inhibitors were shown to be pterolstilbene alongside oxyresveratrol [61][44].
Anti-inflammatory effects were also found in different metabolites of phenolic compounds. For example, 4’-O-methyl-gallic acid is a metabolite generated after fruit juice consumption that reduces the release of pro-inflammatory cytokines and inhibits the expression of COX-2 and iNOS genes by the suppression of NF-κB activation in macrophages [26]. Likewise, quercetin-3’-O-glucuronide proved to decrease the transcription of genes implicated in inflammation, such as pro-inflammatory interleukins and enzymes involved in oxidative stress responses [62][45]. Other metabolite, apigenin-7’-O-glucuronide, was reported to suppress the release of nitric oxide (NO) and TNF-α in lipopolysaccharide-stimulated RAW 264.47 macrophages, and also prevented lipopolysaccharide-induced mRNA expression of iNOS, COX-2 and TNF-α [63,64][46][47].
3.3. Anticancer Activity
Sustained oxidative stress and increased ROS levels are typical features of cancer. Phenolic compounds can interrupt or reverse carcinogenesis by both modulating intracellular signaling molecules involved in cancer initiation and/or promotion, and blocking the progression of cancer [15][48]. For instance, gingerol, a ginger-derived phenolic compound, and its derivative 6-shogaol show anticancer activity in brain, lung and breast cancer [65,66][49][50]. Such an anticancer ability was also demonstrated for hesperetin, which reduced cell viability and induced apoptosis in human cervical cancer SiHa cells [67][51].
Numerous studies have shown the important role of phenolic compounds in different types of cancer. Specifically, flavonoids were shown to hinder cell proliferation and stimulate DNA repair by reducing oxidative stress, thus preventing cancer initiation and promotion. Furthermore, in the progression stage, flavonoids were also reported to inhibit proangiogenic factors, regulate metastasis-related proteins and induce apoptosis [33][16]. On the other hand, EGCG was described to decrease the number and size of tumors, improve oxidative stress markers and inhibit expression of proangiogenic factor such as CD44, VEGF, Ki-67 and MMP-2 in rats bearing mammary cancer [68][52]. Likewise, it has been demonstrated that the combination of EGCG and curcumin produced a synergistic effect that enhanced inhibitory actions of EGCG on cell proliferation of PC3 prostate cancer cell line [69][53].
Phenolic compounds found in olive oil have been also widely studied in relation to the prevention of cancer risk in different tissues. For example, oleocanthal was shown to inhibit the growth of three breast cancer cell lines, namely BT-474, MCF-7 and T-47D, in mitogen-free media by decreasing nuclear expression of estrogen receptor-α. Moreover, combined treatment with oleocanthal and tamoxifen produced a synergic inhibition of cell proliferation in these cell lines, thereby suggesting that oleocanthal improves sensibility to tamoxifen treatment [70][54]. Likewise, hydroxytyrosol, a metabolite of oleuropein, as well as phenylacetic and hydroxyphenylpropionic acids were informed to arrest cell cycle and induce apoptosis in Caco-2 and HT-29 cell lines [71][55]. On the other hand, high doses of hydroxytyrosol also caused apoptosis in papillary (TPC-1 y FB-2) and follicular (WRO) thyroid cancer cell lines via mitochondrial apoptotic mechanism involving the release of cytochrome c and the up-regulation of p53 and BAD [72][56]. Finally, combination of oleuropein and doxorubicin produced stronger cytotoxic and apoptotic effects than individual treatment with high doses of doxorubicin in MDA-MB-231 breast cancer xenografts [73][57].
Regarding human trials, it has been demonstrated that consumption of freeze-dried strawberries powder for 6 months reduced the histologic grade of dysplastic premalignant lesions in 80.6% of patients with esophageal dysplastic lesions in a high-risk area for esophageal cancer. Besides, this strawberry powder also decreased expression levels of the pro-inflammatory mediator iNOS and did not cause any toxic effect [74][58]. In other study, the supplementation of pomegranate extract in patients with colorectal cancer had a significant down-regulating effect on the expression of colorectal cancer-related genes such as CD44, CTNNB1, CDKN1A, and EGFR in surgical colon samples [75][59].
3.4. Cardioprotective Actions
Cardiovascular diseases are mostly caused by oxidative stress and behavioral risk factors such as tobacco use, alcohol abuse, sedentary lifestyles and high-fat diets. Different studies have claimed that the consumption of phenolic-rich food reduces the risk of suffering such diseases [33][16]. In fact, phenolic compounds can alter lipid metabolism, prevent oxidation of low-density lipoproteins (LDL), increase levels of high-density lipoproteins (HDL) and manifest vasodilatory properties, hence decreasing the risk of coronary issues, ischemia and cardiomyopathies. These compounds also promote antiplatelet aggregation, improve endothelial function and diminish the expression of cell adhesion molecules [76][60].
Atherosclerosis is a multifactorial disease characterized by an endothelial dysfunction that involves ROS-dependent LDL oxidation (ox-LDL) [33][16]. In this regard, quercetin has been demonstrated to inhibit ox-LDL-induced adhesion of endothelial leukocytes by attenuating the Toll-like receptor(TLR)-NF-κB signaling pathway and decreasing the atherosclerotic inflammatory process in rats fed a hypercholesterolemic diet [77][61]. This flavonoid is also capable of regulating expression of NADPH oxidase subunits, which are the main source of ROS in phagocytic and vascular cells, and reducing the atherosclerotic plaque area in mouse fed a high-fat diet [78][62]. On the other hand, extra virgin olive oil has been proven to modulate expression of microRNAs (miRNAs), which are small non-coding RNA molecules involved in post-translational regulation of gene expression that has been found to be dysregulated in different diseases such as atherosclerosis or cancer. Specifically, consumption of extra virgin olive oil, which is rich in phenolic compounds, diminished the overexpression of miR-146b-5p (characteristic of atherosclerotic plaques), miR-769-5p and miR-192-5p (associated with the alteration of glucose metabolism, fatty liver and acute myocardial infarction) [79][63]. Besides, an olive extract rich in secoiridoids has shown its capacity to weaken initial steps of atherosclerosis due to significant reduction of endothelial dysfunction biomarkers such as E-selectin, VCAM-1, MCP-1, ICAM-1 and F4/80 in ApoE−/− mice, an atherosclerosis-prone mouse model [80][64]. Moreover, epicatechin was shown to attenuate pro-atherogenic inflammatory processes in female ApoE*3-Leiden transgenic mice, a well-established model of hyperlipidemia and atherosclerosis induced by diet [81][65].
The cardioprotective effect of phenolic compounds has been shown in different other studies. For instance, it was observed that characteristics of metabolic syndrome induced with a high-fat, high-fructose diet in Wistar rats were attenuated by supplementation with a grape pomace that was rich in 26 different phenolic compounds [82][66]. Additionally, Wistar rats fed for 14 months with an extract rich in malvidin, delphinidin, rutin, quercetin, catechin, coumaric acid, kaempferol and trans-cinnamic acid prevented hypertrophy, inflammation, fibrosis and cardiomyocytes apoptosis [83][67]. Finally, vasorelaxant activity of a ferulic acid metabolite, ferulic acid-4-O-sulfate, was also demonstrated in both isolated mouse arteries and anesthetized mice [84][68].
As for human studies, two trials investigated the effect of green tea catechins on LDL oxidation. In both studies, total antioxidant capacity of plasma was increased and LDL oxidizability was reduced. These findings suggest the importance of green tea catechins intake with regards to the reduction of atherosclerosis risk [85][69]. Likewise, another study explored the effect of consuming phenolics-rich extra virgin olive oil in 18 healthy subjects and noticed that its consumption decreased systolic pressure, apparently, through the modulation of ACE and NR1H2 gene expression, which are related to the renin-angiotensin-aldosterone system [86][70].
3.5. Other Health Benefits
There are other beneficial effects, such as antidiabetic effects, that have been attributed to different polyphenols. Type II diabetes mellitus causes chronic oxidative stress as a consequence of hyperglycemia, insulin resistance, inflammation and dyslipidemia, and may result in defective expression of insulin gene and impaired insulin secretion [33][16]. Two flavanones, naringin and naringenin, have been reported to produce antidiabetic actions by enhancing expression of insulin receptor, glucose transporter GLUT4 and adiponectin in type II diabetic rats, thereby improving insulin resistance [87][71]. Similar results were observed with the procyanidin cinnamtannin A2, which prevented hyperglycemia and enhanced glucose tolerance by promoting GLUT4 translocation and glucose uptake [88][72]. In addition, other flavanone, hesperidin, has been demonstrated to improve glycemic control and reduce DNA oxidative damage and lipid peroxidation associated to hyperglycemia in type II diabetes patients [89][73].
The health benefits of phenolic compounds have been also studied in neurodegenerative diseases such as Alzheimer’s and Parkinson’s. In Alzheimer’s disease, which is characterized by accumulation of amyloid-β (Aβ) and tau aggregates, EGCG was shown to be an effective inhibitor of tau aggregation and toxicity, which could be beneficial to hinder Alzheimer’s progression [90][74]. Previous research in mice has also shown that quercetin administration reduced histopathological characteristics of the disease such as extracellular β-amyloidosis, tauopathy, astrogliosis and microgliosis in the hippocampus and the amygdala, while improving cognitive and emotional dysfunction[75] [91]. Importantly, in a clinical study, it has been demonstrated that daily consumption of anthocyanin-rich cherry juice improves verbal fluency, short-term memory, and long-term memory in older adults with Alzheimer’s. Besides, both systolic and diastolic blood pressure was lower in these patients [92][76].
As for Parkinson’s, which is characterized by dopaminergic neuronal loss in substantia nigra associated to oxidative stress, neuroinflammation and apoptosis, some phenolic compounds have been proven potentially effective as therapeutic agents. Thus, the flavone apigenin was reported to decrease both α-synuclein accumulation, a protein associated with sporadic and hereditary cases of the disease, and motor deficits in a rotenone-induced rat model of Parkinson’s. Furthermore, apigenin also protected against neuronal apoptosis via regulation of tyrosine hydroxylase, and increased dopamine biosynthesis as well as the expression of dopamine D2 receptor [93][77]. Finally, rutin was informed to suppress the expression of genes related to dopaminergic neuronal cell death such as Park5, Park7 and Casp3, while isoquercitrin inhibited Park5 and Park7 expression in rat pheochromocytoma PC12 cells [94][78].
3.6. Safety Profile of Phenolic Compounds
Although phenolic compounds reportedly possess a plethora of biological activities, it is necessary to address the issue of their safe dosage. In fact, phenolic compounds generally exhibit bimodal pharmacological effects as they can exert therapeutic actions at low doses while producing toxicity at high doses. However, current studies on the harmful effects of phenolic compounds are mostly based on cell experiments and animal models, with few studies in humans. For instance, safety studies on EGCG have established a no-observed adverse effect level (NOAEL) of 500 mg EGCG/kg/day in both rats and dogs [95][79]. In this line, a NOAEL of 600 mg EGCG/day and an acceptable daily intake for 70-kg adult humans of 322 mg EGCG/day were reported for humans [96][80]. As for resveratrol, it is quite well tolerated by experimental models, with oral doses of 200 mg/kg/day in rats and 600 mg/kg/day in dogs showing no apparent side effects [97][81]. Likewise, a dose of 450 mg resveratrol/day was described to be safe for a 60-kg person [98][82]. In the case of proanthocyanidins, toxicity tests indicated that Oligopin® and Enzogenol®, two pine bark extracts rich in proanthocyanidins, were well tolerated following repeated oral administration to rats, with a NOAEL of 1000 mg/kg/day and 2500 mg/kg/day, respectively [99,100][83][84]. Moreover, consumption of 960 mg/day for 5 weeks in human studies suggested lack of toxicity of Enzogenol® [100][85]. It has been also described that protocatechuic acid is well tolerated in animal models, the lethal dose 50 of protocatechuic acid being 800 mg/kg for intraperitoneal injection, 3.5 g/kg for intravenous injection, and 500 mg/kg for oral administration [101][86]. Therefore, it seems obvious that toxic doses of phenolic compounds are much higher than those delivered by daily food consumption; however, dosage of phenolic compounds in the context of dietary supplements may require further research in animal models and clinical trials before a definite recommendation is done.
Reference (we'll rearrange the references after you submitted it)
- Carocho, M.; Ferreira, I. The role of phenolic compounds in the fight against cancer—A review. Anticancer Agents Med. Chem. 2013, 13, 1236–1258.
- Ansari, M.; Anurag, A.; Fatima, Z.; Hameed, S. Natural phenolic compounds: A potential antifungal agent. In Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2013; pp. 1189–1195.
- Peñarrieta, J.M.; Tejeda, L.; Mollinedo, P.; Vila, J.L.; Bravo, J.A. Phenolic compounds in food. Boliv. Chem. J. 2014, 31, 68–81.
- Reis Giada, M.L. Food phenolic compounds: Main classes, sources and their antioxidant power. In Oxidative Stress and Chronic Degenerative Diseases—A Role for Antioxidants; Morales-Gonzalez, J.A., Ed.; IntechOpen: London, UK, 2013; pp. 87–112.
- Bhuyan, D.J.; Basu, A. Utilisation of bioactive compounds derived from waste in the food industry. In Utilisation of Bioactive Compounds from Agricultural and Food Production Waste; Vuong, Q.V., Ed.; CRC Press: Boca Ratón, FL, USA, 2017; pp. 342–357.
- Caleja, C.; Ribeiro, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Phenolic compounds as nutraceuticals or functional food ingredients. Curr. Pharm. Des. 2017, 23, 2787–2806.
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for analysis of plant phenolic compounds. Molecules 2013, 18, 2328–2375.
- Vuolo, M.M.; Lima, V.S.; Maróstica-Junior, M.R. Phenolic compounds: Structure, classification, and antioxidant power. In Bioactive Compounds: Health Benefits and Potential Applications; Segura-Campos, M.R., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 33–50.
- de la Rosa, L.A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Phenolic compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E., Carrillo-López, A., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 253–271.
- Matos, M.J.; Santana, L.; Uriarte, E.; Abreu, O.A.; Molina, E.; Yordi, E.G. Coumarins—An important class of phytochemicals. In Phytochemicals—Isolation, Characterisation and Role in Human Health; InTech: London, UK, 2015; pp. 113–140.
- Stringlis, I.A.; De Jonge, R.; Pieterse, C.M.J. The age of coumarins in plant-microbe interactions. Plant Cell Physiol. 2019, 60, 1405–1419.
- Lattanzio, V. Phenolic compounds: Introduction. In Natural Products: Phytochemistry, Botany and Metabolism of Akaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1543–1580.
- de Souza, J.E.; Casanova, L.M.; Costa, S.S. Biodisponibilidade de compostos fenólicos: Um importante desafio para o desenvolvimento de fármacos? Rev. Fitos 2015, 9, 55–67.
- Quiñones, M.; Miguel, M.; Aleixandre, A. Los polifenoles, compuestos de origen natural con efectos saludables sobre el sistema cardiovascular. Nutr. Hosp. 2012, 27, 76–89.
- Zeng, X.; Su, W.; Zheng, Y.; Liu, H.; Li, P.; Zhang, W.; Liang, Y.; Bai, Y.; Peng, W.; Yao, H. UFLC-Q-TOF-MS/MS-based screening and identification of flavonoids and derived metabolites in human urine after oral administration of exocarpium citri grandis extract. Molecules 2018, 23, 895.
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2011, 16, 251–280.
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897.
- Ballard, C.R.; Maróstica, M.R. Health benefits of flavonoids. In Bioactive Compounds: Health Benefits and Potential Applications; Segura-Campos, M.R., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 185–201.
- Dar, R.A.; Brahman, P.K.; Khurana, N.; Wagay, J.A.; Lone, Z.A.; Ganaie, M.A.; Pitre, K.S. Evaluation of antioxidant activity of crocin, podophyllotoxin and kaempferol by chemical, biochemical and electrochemical assays. Arab. J. Chem. 2017, 10, 1119–1128.
- Kang, K.A.; Piao, M.J.; Kim, K.C.; Cha, J.W.; Zheng, J.; Yao, C.W.; Chae, S.; Hyun, J.W. Fisetin attenuates hydrogen peroxide-induced cell damage by scavenging reactive oxygen species and activating protective functions of cellular glutathione system. In Vitro Cell. Dev. Biol.-Anim. 2014, 50, 66–74.
- Perde-Schrepler, M.; Chereches, G.; Brie, I.; Tatomir, C.; Postescu, I.D.; Soran, L.; Filip, A. Grape seed extract as photochemopreventive agent against UVB-induced skin cancer. J. Photochem. Photobiol. B Biol. 2013, 118, 16–21.
- Rashmi, H.B.; Negi, P.S. Phenolic acids from vegetables: A review on processing stability and health benefits. Food Res. Int. 2020, 136, 109298.
- Venturi, F.; Sanmartin, C.; Taglieri, I.; Nari, A.; Andrich, G.; Terzuoli, E.; Donnini, S.; Nicolella, C.; Zinnai, A. Development of phenol-enriched olive oil with phenolic compounds extracted from wastewater produced by physical refining. Nutrients 2017, 9, 916.
- Liu, Z.; Luo, Z.; Jia, C.; Wang, D.; Li, D. Synergistic effects of Potentilla fruticosa L. leaves combined with green tea polyphenols in a variety of oxidation systems. J. Food Sci. 2016, 81, C1091–C1101.
- Rutkowska, M.; Olszewska, M.A.; Kolodziejczyk-Czepas, J.; Nowak, P.; Owczarek, A. Sorbus domestica Leaf Extracts and Their Activity Markers: Antioxidant Potential and Synergy Effects in Scavenging Assays of Multiple Oxidants. Molecules 2019, 24, 2289.
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429.
- Cueva, C.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Rivas, C.L.; Requena, T.; Rodríguez, J.M.; Bartolomé, B. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 2010, 161, 372–382.
- Stojković, D.; Petrović, J.; Soković, M.; Glamočlija, J.; Kukić-Marković, J.; Petrović, S. In situ antioxidant and antimicrobial activities of naturally occurring caffeic acid, p-coumaric acid and rutin, using food systems. J. Sci. Food Agric. 2013, 93, 3205–3208.
- Díaz-Gómez, R.; López-Solís, R.; Obreque-Slier, E.; Toledo-Araya, H. Comparative antibacterial effect of gallic acid and catechin against Helicobacter pylori. LWT-Food Sci. Technol. 2013, 54, 331–335.
- Díaz-Gómez, R.; Toledo-Araya, H.; López-Solís, R.; Obreque-Slier, E. Combined effect of gallic acid and catechin against Escherichia coli. LWT-Food Sci. Technol. 2014, 59, 896–900.
- del Valle, P.; García-Armesto, M.R.; de Arriaga, D.; González-Donquiles, C.; Rodríquez-Fernández, P.; Rúa, J. Antimicrobial activity of kaempferol and resveratrol in binary combinations with parabens or propyl gallate against Enterococcus faecalis. Food Control 2016, 61, 213–220.
- Duarte, A.; Alves, A.C.; Ferreira, S.; Silva, F.; Domingues, F.C. Resveratrol inclusion complexes: Antibacterial and anti-biofilm activity against Campylobacter spp. and Arcobacter butzleri. Food Res. Int. 2015, 77, 244–250.
- Li, X.Z.; Wei, X.; Zhang, C.J.; Jin, X.L.; Tang, J.J.; Fan, G.J.; Zhou, B. Hypohalous acid-mediated halogenation of resveratrol and its role in antioxidant and antimicrobial activities. Food Chem. 2012, 135, 1239–1244.
- Silva, V.; Igrejas, G.; Falco, V.; Santos, T.P.; Torres, C.; Oliveira, A.M.P.; Pereira, J.E.; Amaral, J.S.; Poeta, P. Chemical composition, antioxidant and antimicrobial activity of phenolic compounds extracted from wine industry by-products. Food Control 2018, 92, 516–522.
- Varga, E.; Bardocz, A.; Belák, A.; Maráz, A.; Boros, B.; Felinger, A.; Böszörményi, A.; Horváth, G. Antimicrobial activity and chemical composition of thyme essential oils and the polyphenolic content of different Thymus extracts. Farmacia 2015, 6, 357–361.
- Delgado-Adámez, J.; Garrido, M.; Bote, M.E.; Fuentes-Pérez, M.C.; Espino, J.; Martín-Vertedor, D. Chemical composition and bioactivity of essential oils from flower and fruit of Thymbra capitata and Thymus species. J. Food Sci. Technol. 2017, 54, 1857–1865.
- Eerdunbayaer, M.A.; Aoyama, H.; Kuroda, T.; Hatano, T. Structures of two new flavonoids and effects of licorice phenolics on vancomycin-resistant enterococcus species. Molecules 2014, 19, 3883–3897.
- Sanhueza, L.; Melo, R.; Montero, R.; Maisey, K.; Mendoza, L.; Wilkens, M. Synergistic interactions between phenolic compounds identified in grape pomace extract with antibiotics of different classes against Staphylococcus aureus and Escherichia coli. PLoS ONE 2017, 12, e0172273.
- Özçelik, B.; Kartal, M.; Orhan, I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2011, 49, 396–402.
- Frabasile, S.; Koishi, A.C.; Kuczera, D.; Ferreira-Silveira, G.; Verri, W.A.; Duarte-dos Santos, C.N.; Bordignon, J. The citrus flavanone naringenin impairs dengue virus replication in human cells. Sci. Rep. 2017, 7, 41864.
- Huang, H.C.; Tao, M.H.; Hung, T.M.; Chen, J.C.; Lin, Z.J.; Huang, C. (-)-Epigallocatechin-3-gallate inhibits entry of hepatitis B virus into hepatocytes. Antiviral Res. 2014, 111, 100–111.
- Vázquez-Calvo, A.; Jiménez-de Oya, N.; Martín-Acebes, M.A.; Garcia-Moruno, E.; Saiz, J.C. Antiviral properties of the natural polyphenols delphinidin and epigallocatechin gallate against the flaviviruses West Nile Virus, Zika Virus, and Dengue Virus. Front. Microbiol. 2017, 8, 1314.
- Calland, N.; Sahuc, M.E.; Belouzard, S.; Pène, V.; Bonnafous, P.; Mesalam, A.A.; Deloison, G.; Descamps, V.; Sahpaz, S.; Wychowski, C.; et al. Polyphenols inhibit hepatitis C virus entry by a new mechanism of action. J. Virol. 2015, 89, 10053–10063.
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618.
- Leláková, V.; Šmejkal, K.; Jakubczyk, K.; Veselý, O.; Landa, P.; Václavík, J.; Bobáľ, P.; Pížová, H.; Temml, V.; Steinacher, T.; et al. Parallel in vitro and in silico investigations into anti-inflammatory effects of non-prenylated stilbenoids. Food Chem. 2019, 285, 431–440.
- Kutil, Z.; Kvasnicova, M.; Temml, V.; Schuster, D.; Marsik, P.; Fernández-Cusimamani, E.; Lou, J.D.; Vanek, T.; Landa, P. Effect of dietary stilbenes on 5-lipoxygenase and cyclooxygenases activities in vitro. Int. J. Food Prop. 2015, 18, 1471–1477.
- Derlindati, E.; Dall’Asta, M.; Ardigò, D.; Brighenti, F.; Zavaroni, I.; Crozier, A.; Del Rio, D. Quercetin-3-O-glucuronide affects the gene expression profile of M1 and M2a human macrophages exhibiting anti-inflammatory effects. Food Funct. 2012, 3, 1144–1152.
- Hu, W.; Wang, X.; Wu, L.; Shen, T.; Ji, L.; Zhao, X.; Si, C.L.; Jiang, Y.; Wang, G. Apigenin-7-O-β-d-glucuronide inhibits LPS-induced inflammation through the inactivation of AP-1 and MAPK signaling pathways in RAW 264.7 macrophages and protects mice against endotoxin shock. Food Funct. 2016, 7, 1002–1013.
- Kamalakararao, K.; Gopalakrishnan, V.K.; Hagos, Z.; Rao, G.D.; Padal, S.B.; Karri, K.C. Anti-inflammatory activity of bioactive flavonoid apigenin- 7-O-ß-D- glucuronide methyl ester from ethyl acetate leaf extract of Manilkara zapota on lipopolysaccharideinduced pro-inflammatory mediators nitric oxide (NO), prostaglandin E2(PGE2) in Raw 264. Drug Invent. Today 2018, 10, 531–535.
- Lee, D.H.; Kim, D.W.; Jung, C.H.; Lee, Y.J.; Park, D. Gingerol sensitizes TRAIL-induced apoptotic cell death of glioblastoma cells. Toxicol. Appl. Pharmacol. 2014, 279, 253–265.
- Hsu, Y.L.; Hung, J.Y.; Tsai, Y.M.; Tsai, E.M.; Huang, M.S.; Hou, M.F.; Kuo, P.L. 6-shogaol, an active constituent of dietary ginger, impairs cancer development and lung metastasis by inhibiting the secretion of CC-chemokine ligand 2 (CCL2) in tumor-associated dendritic cells. J. Agric. Food Chem. 2015, 63, 1730–1738.
- Alshatwi, A.A.; Ramesh, E.; Periasamy, V.S.; Subash-Babu, P. The apoptotic effect of hesperetin on human cervical cancer cells is mediated through cell cycle arrest, death receptor, and mitochondrial pathways. Fundam. Clin. Pharmacol. 2013, 27, 581–592.
- Abd El-Rahman, S.S.; Shehab, G.; Nashaat, H. Epigallocatechin-3-Gallate: The prospective targeting of cancer stem cells and preventing metastasis of chemically-induced mammary cancer in rats. Am. J. Med. Sci. 2017, 354, 54–63.
- Eom, D.W.; Lee, J.H.; Kim, Y.J.; Hwang, G.S.; Kim, S.N.; Kwak, J.H.; Cheon, G.J.; Kim, K.H.; Jang, H.J.; Ham, J.; et al. Synergistic effect of curcumin on epigallocatechin gallate-induced anticancer action in PC3 prostate cancer cells. BMB Rep. 2015, 48, 461–466.
- Ayoub, N.M.; Siddique, A.B.; Ebrahim, H.Y.; Mohyeldin, M.M.; El Sayed, K.A. The olive oil phenolic (-)-oleocanthal modulates estrogen receptor expression in luminal breast cancer in vitro and in vivo and synergizes with tamoxifen treatment. Eur. J. Pharmacol. 2017, 810, 100–111.
- López De Las Hazas, M.C.; Piñol, C.; Macià, A.; Motilva, M.J. Hydroxytyrosol and the colonic metabolites derived from virgin olive oil intake induce cell cycle arrest and apoptosis in colon cancer cells. J. Agric. Food Chem. 2017, 65, 6467–6476.
- Toteda, G.; Lupinacci, S.; Vizza, D.; Bonofiglio, R.; Perri, E.; Bonofiglio, M.; Lofaro, D.; La Russa, A.; Leone, F.; Gigliotti, P.; et al. High doses of hydroxytyrosol induce apoptosis in papillary and follicular thyroid cancer cells. J. Endocrinol. Investig. 2017, 40, 153–162.
- Elamin, M.H.; Elmahi, A.B.; Daghestani, M.H.; Al-Olayan, E.M.; Al-Ajmi, R.A.; Alkhuriji, A.F.; Hamed, S.S.; Elkhadragy, M.F. Synergistic anti-breast-cancer effects of combined treatment with oleuropein and doxorubicin in vivo. Altern. Ther. Health Med. 2019, 25, 17–24.
- Chen, T.; Yan, F.; Qian, J.; Guo, M.; Zhang, H.; Tang, X.; Chen, F.; Stoner, G.D.; Wang, X. Randomized phase II trial of lyophilized strawberries in patients with dysplastic precancerous lesions of the esophagus. Cancer Prev. Res. 2012, 5, 41–50.
- Nuñez-Sánchez, M.A.; González-Sarrías, A.; García-Villalba, R.; Monedero-Saiz, T.; García-Talavera, N.V.; Gómez-Sánchez, M.B.; Sánchez-Álvarez, C.; García-Albert, A.M.; Rodríguez-Gil, F.J.; Ruiz-Marín, M.; et al. Gene expression changes in colon tissues from colorectal cancer patients following the intake of an ellagitannin-containing pomegranate extract: A randomized clinical trial. J. Nutr. Biochem. 2017, 42, 126–133.
- Gonçalves, A.C.; Bento, C.; Jesus, F.; Alves, G.; Silva, L.R. Sweet cherry phenolic compounds: Identification, characterization, and health benefits. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier Science: Amsterdam, The Netherlands, 2018; Volume 59, pp. 31–78.
- Bhaskar, S.; Sudhakaran, P.R.; Helen, A. Quercetin attenuates atherosclerotic inflammation and adhesion molecule expression by modulating TLR-NF-κB signaling pathway. Cell. Immunol. 2016, 310, 131–140.
- Xiao, L.; Liu, L.; Guo, X.; Zhang, S.; Wang, J.; Zhou, F.; Tang, Y.; Yao, P. Quercetin attenuates high fat diet-induced atherosclerosis in apolipoprotein E knockout mice: A critical role of NADPH oxidase. Food Chem. Toxicol. 2017, 105, 22–33.
- D’Amore, S.; Vacca, M.; Cariello, M.; Graziano, G.; D’Orazio, A.; Salvia, R.; Sasso, R.C.; Sabbà, C.; Palasciano, G.; Moschetta, A. Genes and miRNA expression signatures in peripheral blood mononuclear cells in healthy subjects and patients with metabolic syndrome after acute intake of extra virgin olive oil. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2016, 1861, 1671–1680.
- Catalán, U.; López de las Hazas, M.C.; Piñol, C.; Rubió, L.; Motilva, M.J.; Fernandez-Castillejo, S.; Solà, R. Hydroxytyrosol and its main plasma circulating metabolites attenuate the initial steps of atherosclerosis through inhibition of the MAPK pathway. J. Funct. Foods 2018, 40, 280–291.
- Morrison, M.; van der Heijden, R.; Heeringa, P.; Kaijzel, E.; Verschuren, L.; Blomhoff, R.; Kooistra, T.; Kleemann, R. Epicatechin attenuates atherosclerosis and exerts anti-inflammatory effects on diet-induced human-CRP and NFκB invivo. Atherosclerosis 2014, 233, 149–156.
- Perdicaro, D.J.; Rodriguez-Lanzi, C.; Fontana, A.R.; Antoniolli, A.; Piccoli, P.; Miatello, R.M.; Diez, E.R.; Vazquez Prieto, M.A. Grape pomace reduced reperfusion arrhythmias in rats with a high-fat-fructose diet. Food Funct. 2017, 8, 3501–3509.
- Chacar, S.; Hajal, J.; Saliba, Y.; Bois, P.; Louka, N.; Maroun, R.G.; Faivre, J.F.; Fares, N. Long-term intake of phenolic compounds attenuates age-related cardiac remodeling. Aging Cell 2019, 18, e12894.
- Van Rymenant, E.; Van Camp, J.; Pauwels, B.; Boydens, C.; Vanden Daele, L.; Beerens, K.; Brouckaert, P.; Smagghe, G.; Kerimi, A.; Williamson, G.; et al. Ferulic acid-4-O-sulfate rather than ferulic acid relaxes arteries and lowers blood pressure in mice. J. Nutr. Biochem. 2017, 44, 44–51.
- Suzuki-Sugihara, N.; Kishimoto, Y.; Saita, E.; Taguchi, C.; Kobayashi, M.; Ichitani, M.; Ukawa, Y.; Sagesaka, Y.M.; Suzuki, E.; Kondo, K. Green tea catechins prevent low-density lipoprotein oxidation via their accumulation in low-density lipoprotein particles in humans. Nutr. Res. 2016, 36, 16–23.
- Martín-Peláez, S.; Castañer, O.; Konstantinidou, V.; Subirana, I.; Muñoz-Aguayo, D.; Blanchart, G.; Gaixas, S.; de la Torre, R.; Farré, M.; Sáez, G.T.; et al. Effect of olive oil phenolic compounds on the expression of blood pressure-related genes in healthy individuals. Eur. J. Nutr. 2017, 56, 663–670.
- Ahmed, O.M.; Hassan, M.A.; Abdel-Twab, S.M.; Abdel Azeem, M.N. Navel orange peel hydroethanolic extract, naringin and naringenin have anti-diabetic potentials in type 2 diabetic rats. Biomed. Pharmacother. 2017, 94, 197–205.
- Yamashita, Y.; Wang, L.; Nanba, F.; Ito, C.; Toda, T.; Ashida, H. Procyanidin promotes translocation of glucose transporter 4 in muscle of mice through activation of insulin and AMPK signaling pathways. PLoS ONE 2016, 11, e0161704.
- Homayouni, F.; Haidari, F.; Hedayati, M.; Zakerkish, M.; Ahmadi, K. Hesperidin supplementation alleviates oxidative DNA damage and lipid peroxidation in type 2 diabetes: A randomized double-blind placebo-controlled clinical trial. Phyther. Res. 2017, 31, 1539–1545.
- Wobst, H.J.; Sharma, A.; Diamond, M.I.; Wanker, E.E.; Bieschke, J. The green tea polyphenol (-)-epigallocatechin gallate prevents the aggregation of tau protein into toxic oligomers at substoichiometric ratios. FEBS Lett. 2015, 589, 77–83.
- Sabogal-Guáqueta, A.M.; Muñoz-Manco, J.I.; Ramírez-Pineda, J.R.; Lamprea-Rodriguez, M.; Osorio, E.; Cardona-Gómez, G.P. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology 2015, 93, 134–145.
- Kent, K.; Charlton, K.; Roodenrys, S.; Batterham, M.; Potter, J.; Traynor, V.; Gilbert, H.; Morgan, O.; Richards, R. Consumption of anthocyanin-rich cherry juice for 12 weeks improves memory and cognition in older adults with mild-to-moderate dementia. Eur. J. Nutr. 2017, 56, 333–341.
- Anusha, C.; Sumathi, T.; Joseph, L.D. Protective role of apigenin on rotenone induced rat model of Parkinson’s disease: Suppression of neuroinflammation and oxidative stress mediated apoptosis. Chem. Biol. Interact. 2017, 269, 67–79.
- Magalingam, K.B.; Radhakrishnan, A.; Ramdas, P.; Haleagrahara, N. Quercetin glycosides induced neuroprotection by changes in the gene expression in a cellular model of Parkinson’s disease. J. Mol. Neurosci. 2014, 55, 609–617.
- Isbrucker, R.A.; Edwards, J.A.; Wolz, E.; Davidovich, A.; Bausch, J. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 2: Dermal, acute and short-term toxicity studies. Food Chem. Toxicol. 2006, 44, 636–650.
- Yates, A.A.; Erdman, J.W.; Shao, A.; Dolan, L.C.; Griffiths, J.C. Bioactive nutrients—Time for tolerable upper intake levels to address safety. Regul. Toxicol. Pharmacol. 2017, 84, 94–101.
- Johnson, W.D.; Morrissey, R.L.; Usborne, A.L.; Kapetanovic, I.; Crowell, J.A.; Muzzio, M.; McCormick, D.L. Subchronic oral toxicity and cardiovascular safety pharmacology studies of resveratrol, a naturally occurring polyphenol with cancer preventive activity. Food Chem. Toxicol. 2011, 49, 3319–3327.
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential adverse effects of resveratrol: A literature review. Int. J. Mol. Sci. 2020, 21, 2084.
- Segal, L.; Penman, M.G.; Piriou, Y. Evaluation of the systemic toxicity and mutagenicity of OLIGOPIN®, procyanidolic oligomers (OPC) extracted from French Maritime Pine Bark extract. Toxicol. Rep. 2018, 5, 531–541.
- Frevel, M.A.E.; Pipingas, A.; Grigsby, W.J.; Frampton, C.M.; Gilchrist, N.L. Production, composition and toxicology studies of Enzogenol® Pinus radiata bark extract. Food Chem. Toxicol. 2012, 50, 4316–4324.
- Kakkar, S.; Bais, S. A Review on Protocatechuic Acid and Its Pharmacological Potential. ISRN Pharmacol. 2014, 2014, 952943.
References
- Carocho, M.; Ferreira, I. The role of phenolic compounds in the fight against cancer—A review. Anticancer Agents Med. Chem. 2013, 13, 1236–1258.
- Ansari, M.; Anurag, A.; Fatima, Z.; Hameed, S. Natural phenolic compounds: A potential antifungal agent. In Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2013; pp. 1189–1195.
- Peñarrieta, J.M.; Tejeda, L.; Mollinedo, P.; Vila, J.L.; Bravo, J.A. Phenolic compounds in food. Boliv. Chem. J. 2014, 31, 68–81.
- Reis Giada, M.L. Food phenolic compounds: Main classes, sources and their antioxidant power. In Oxidative Stress and Chronic Degenerative Diseases—A Role for Antioxidants; Morales-Gonzalez, J.A., Ed.; IntechOpen: London, UK, 2013; pp. 87–112.
- Bhuyan, D.J.; Basu, A. Utilisation of bioactive compounds derived from waste in the food industry. In Utilisation of Bioactive Compounds from Agricultural and Food Production Waste; Vuong, Q.V., Ed.; CRC Press: Boca Ratón, FL, USA, 2017; pp. 342–357.
- Caleja, C.; Ribeiro, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Phenolic compounds as nutraceuticals or functional food ingredients. Curr. Pharm. Des. 2017, 23, 2787–2806.
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for analysis of plant phenolic compounds. Molecules 2013, 18, 2328–2375.
- Vuolo, M.M.; Lima, V.S.; Maróstica-Junior, M.R. Phenolic compounds: Structure, classification, and antioxidant power. In Bioactive Compounds: Health Benefits and Potential Applications; Segura-Campos, M.R., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 33–50.
- de la Rosa, L.A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Phenolic compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E., Carrillo-López, A., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 253–271.
- Matos, M.J.; Santana, L.; Uriarte, E.; Abreu, O.A.; Molina, E.; Yordi, E.G. Coumarins—An important class of phytochemicals. In Phytochemicals—Isolation, Characterisation and Role in Human Health; InTech: London, UK, 2015; pp. 113–140.
- Stringlis, I.A.; De Jonge, R.; Pieterse, C.M.J. The age of coumarins in plant-microbe interactions. Plant Cell Physiol. 2019, 60, 1405–1419.
- Lattanzio, V. Phenolic compounds: Introduction. In Natural Products: Phytochemistry, Botany and Metabolism of Akaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1543–1580.
- de Souza, J.E.; Casanova, L.M.; Costa, S.S. Biodisponibilidade de compostos fenólicos: Um importante desafio para o desenvolvimento de fármacos? Rev. Fitos 2015, 9, 55–67.
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897.
- Quiñones, M.; Miguel, M.; Aleixandre, A. Los polifenoles, compuestos de origen natural con efectos saludables sobre el sistema cardiovascular. Nutr. Hosp. 2012, 27, 76–89.
- Ballard, C.R.; Maróstica, M.R. Health benefits of flavonoids. In Bioactive Compounds: Health Benefits and Potential Applications; Segura-Campos, M.R., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 185–201.
- Dar, R.A.; Brahman, P.K.; Khurana, N.; Wagay, J.A.; Lone, Z.A.; Ganaie, M.A.; Pitre, K.S. Evaluation of antioxidant activity of crocin, podophyllotoxin and kaempferol by chemical, biochemical and electrochemical assays. Arab. J. Chem. 2017, 10, 1119–1128.
- Kang, K.A.; Piao, M.J.; Kim, K.C.; Cha, J.W.; Zheng, J.; Yao, C.W.; Chae, S.; Hyun, J.W. Fisetin attenuates hydrogen peroxide-induced cell damage by scavenging reactive oxygen species and activating protective functions of cellular glutathione system. In Vitro Cell. Dev. Biol.-Anim. 2014, 50, 66–74.
- Perde-Schrepler, M.; Chereches, G.; Brie, I.; Tatomir, C.; Postescu, I.D.; Soran, L.; Filip, A. Grape seed extract as photochemopreventive agent against UVB-induced skin cancer. J. Photochem. Photobiol. B Biol. 2013, 118, 16–21.
- Rashmi, H.B.; Negi, P.S. Phenolic acids from vegetables: A review on processing stability and health benefits. Food Res. Int. 2020, 136, 109298.
- Venturi, F.; Sanmartin, C.; Taglieri, I.; Nari, A.; Andrich, G.; Terzuoli, E.; Donnini, S.; Nicolella, C.; Zinnai, A. Development of phenol-enriched olive oil with phenolic compounds extracted from wastewater produced by physical refining. Nutrients 2017, 9, 916.
- Liu, Z.; Luo, Z.; Jia, C.; Wang, D.; Li, D. Synergistic effects of Potentilla fruticosa L. leaves combined with green tea polyphenols in a variety of oxidation systems. J. Food Sci. 2016, 81, C1091–C1101.
- Rutkowska, M.; Olszewska, M.A.; Kolodziejczyk-Czepas, J.; Nowak, P.; Owczarek, A. Sorbus domestica Leaf Extracts and Their Activity Markers: Antioxidant Potential and Synergy Effects in Scavenging Assays of Multiple Oxidants. Molecules 2019, 24, 2289.
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429.
- Cueva, C.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Rivas, C.L.; Requena, T.; Rodríguez, J.M.; Bartolomé, B. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 2010, 161, 372–382.
- Stojković, D.; Petrović, J.; Soković, M.; Glamočlija, J.; Kukić-Marković, J.; Petrović, S. In situ antioxidant and antimicrobial activities of naturally occurring caffeic acid, p-coumaric acid and rutin, using food systems. J. Sci. Food Agric. 2013, 93, 3205–3208.
- Díaz-Gómez, R.; López-Solís, R.; Obreque-Slier, E.; Toledo-Araya, H. Comparative antibacterial effect of gallic acid and catechin against Helicobacter pylori. LWT-Food Sci. Technol. 2013, 54, 331–335.
- Díaz-Gómez, R.; Toledo-Araya, H.; López-Solís, R.; Obreque-Slier, E. Combined effect of gallic acid and catechin against Escherichia coli. LWT-Food Sci. Technol. 2014, 59, 896–900.
- del Valle, P.; García-Armesto, M.R.; de Arriaga, D.; González-Donquiles, C.; Rodríquez-Fernández, P.; Rúa, J. Antimicrobial activity of kaempferol and resveratrol in binary combinations with parabens or propyl gallate against Enterococcus faecalis. Food Control 2016, 61, 213–220.
- Duarte, A.; Alves, A.C.; Ferreira, S.; Silva, F.; Domingues, F.C. Resveratrol inclusion complexes: Antibacterial and anti-biofilm activity against Campylobacter spp. and Arcobacter butzleri. Food Res. Int. 2015, 77, 244–250.
- Li, X.Z.; Wei, X.; Zhang, C.J.; Jin, X.L.; Tang, J.J.; Fan, G.J.; Zhou, B. Hypohalous acid-mediated halogenation of resveratrol and its role in antioxidant and antimicrobial activities. Food Chem. 2012, 135, 1239–1244.
- Silva, V.; Igrejas, G.; Falco, V.; Santos, T.P.; Torres, C.; Oliveira, A.M.P.; Pereira, J.E.; Amaral, J.S.; Poeta, P. Chemical composition, antioxidant and antimicrobial activity of phenolic compounds extracted from wine industry by-products. Food Control 2018, 92, 516–522.
- Varga, E.; Bardocz, A.; Belák, A.; Maráz, A.; Boros, B.; Felinger, A.; Böszörményi, A.; Horváth, G. Antimicrobial activity and chemical composition of thyme essential oils and the polyphenolic content of different Thymus extracts. Farmacia 2015, 6, 357–361.
- Delgado-Adámez, J.; Garrido, M.; Bote, M.E.; Fuentes-Pérez, M.C.; Espino, J.; Martín-Vertedor, D. Chemical composition and bioactivity of essential oils from flower and fruit of Thymbra capitata and Thymus species. J. Food Sci. Technol. 2017, 54, 1857–1865.
- Eerdunbayaer, M.A.; Aoyama, H.; Kuroda, T.; Hatano, T. Structures of two new flavonoids and effects of licorice phenolics on vancomycin-resistant enterococcus species. Molecules 2014, 19, 3883–3897.
- Sanhueza, L.; Melo, R.; Montero, R.; Maisey, K.; Mendoza, L.; Wilkens, M. Synergistic interactions between phenolic compounds identified in grape pomace extract with antibiotics of different classes against Staphylococcus aureus and Escherichia coli. PLoS ONE 2017, 12, e0172273.
- Özçelik, B.; Kartal, M.; Orhan, I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2011, 49, 396–402.
- Frabasile, S.; Koishi, A.C.; Kuczera, D.; Ferreira-Silveira, G.; Verri, W.A.; Duarte-dos Santos, C.N.; Bordignon, J. The citrus flavanone naringenin impairs dengue virus replication in human cells. Sci. Rep. 2017, 7, 41864.
- Huang, H.C.; Tao, M.H.; Hung, T.M.; Chen, J.C.; Lin, Z.J.; Huang, C. (-)-Epigallocatechin-3-gallate inhibits entry of hepatitis B virus into hepatocytes. Antiviral Res. 2014, 111, 100–111.
- Vázquez-Calvo, A.; Jiménez-de Oya, N.; Martín-Acebes, M.A.; Garcia-Moruno, E.; Saiz, J.C. Antiviral properties of the natural polyphenols delphinidin and epigallocatechin gallate against the flaviviruses West Nile Virus, Zika Virus, and Dengue Virus. Front. Microbiol. 2017, 8, 1314.
- Calland, N.; Sahuc, M.E.; Belouzard, S.; Pène, V.; Bonnafous, P.; Mesalam, A.A.; Deloison, G.; Descamps, V.; Sahpaz, S.; Wychowski, C.; et al. Polyphenols inhibit hepatitis C virus entry by a new mechanism of action. J. Virol. 2015, 89, 10053–10063.
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618.
- Leláková, V.; Šmejkal, K.; Jakubczyk, K.; Veselý, O.; Landa, P.; Václavík, J.; Bobáľ, P.; Pížová, H.; Temml, V.; Steinacher, T.; et al. Parallel in vitro and in silico investigations into anti-inflammatory effects of non-prenylated stilbenoids. Food Chem. 2019, 285, 431–440.
- Kutil, Z.; Kvasnicova, M.; Temml, V.; Schuster, D.; Marsik, P.; Fernández-Cusimamani, E.; Lou, J.D.; Vanek, T.; Landa, P. Effect of dietary stilbenes on 5-lipoxygenase and cyclooxygenases activities in vitro. Int. J. Food Prop. 2015, 18, 1471–1477.
- Derlindati, E.; Dall’Asta, M.; Ardigò, D.; Brighenti, F.; Zavaroni, I.; Crozier, A.; Del Rio, D. Quercetin-3-O-glucuronide affects the gene expression profile of M1 and M2a human macrophages exhibiting anti-inflammatory effects. Food Funct. 2012, 3, 1144–1152.
- Hu, W.; Wang, X.; Wu, L.; Shen, T.; Ji, L.; Zhao, X.; Si, C.L.; Jiang, Y.; Wang, G. Apigenin-7-O-β-d-glucuronide inhibits LPS-induced inflammation through the inactivation of AP-1 and MAPK signaling pathways in RAW 264.7 macrophages and protects mice against endotoxin shock. Food Funct. 2016, 7, 1002–1013.
- Kamalakararao, K.; Gopalakrishnan, V.K.; Hagos, Z.; Rao, G.D.; Padal, S.B.; Karri, K.C. Anti-inflammatory activity of bioactive flavonoid apigenin- 7-O-ß-D- glucuronide methyl ester from ethyl acetate leaf extract of Manilkara zapota on lipopolysaccharideinduced pro-inflammatory mediators nitric oxide (NO), prostaglandin E2(PGE2) in Raw 264. Drug Invent. Today 2018, 10, 531–535.
- Gioxari, A.; Kogiannou, D.A.A.; Kalogeropoulos, N.; Kaliora, A.C. Phenolic compounds: Bioavailability and health effects. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Elsevier: Cambridge, MA, USA, 2016; pp. 339–345.
- Lee, D.H.; Kim, D.W.; Jung, C.H.; Lee, Y.J.; Park, D. Gingerol sensitizes TRAIL-induced apoptotic cell death of glioblastoma cells. Toxicol. Appl. Pharmacol. 2014, 279, 253–265.
- Hsu, Y.L.; Hung, J.Y.; Tsai, Y.M.; Tsai, E.M.; Huang, M.S.; Hou, M.F.; Kuo, P.L. 6-shogaol, an active constituent of dietary ginger, impairs cancer development and lung metastasis by inhibiting the secretion of CC-chemokine ligand 2 (CCL2) in tumor-associated dendritic cells. J. Agric. Food Chem. 2015, 63, 1730–1738.
- Alshatwi, A.A.; Ramesh, E.; Periasamy, V.S.; Subash-Babu, P. The apoptotic effect of hesperetin on human cervical cancer cells is mediated through cell cycle arrest, death receptor, and mitochondrial pathways. Fundam. Clin. Pharmacol. 2013, 27, 581–592.
- Ballard, C.R.; Maróstica, M.R. Health benefits of flavonoids. In Bioactive Compounds: Health Benefits and Potential Applications; Segura-Campos, M.R., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 185–201.
- Abd El-Rahman, S.S.; Shehab, G.; Nashaat, H. Epigallocatechin-3-Gallate: The prospective targeting of cancer stem cells and preventing metastasis of chemically-induced mammary cancer in rats. Am. J. Med. Sci. 2017, 354, 54–63.
- Eom, D.W.; Lee, J.H.; Kim, Y.J.; Hwang, G.S.; Kim, S.N.; Kwak, J.H.; Cheon, G.J.; Kim, K.H.; Jang, H.J.; Ham, J.; et al. Synergistic effect of curcumin on epigallocatechin gallate-induced anticancer action in PC3 prostate cancer cells. BMB Rep. 2015, 48, 461–466.
- Ayoub, N.M.; Siddique, A.B.; Ebrahim, H.Y.; Mohyeldin, M.M.; El Sayed, K.A. The olive oil phenolic (-)-oleocanthal modulates estrogen receptor expression in luminal breast cancer in vitro and in vivo and synergizes with tamoxifen treatment. Eur. J. Pharmacol. 2017, 810, 100–111.
- López De Las Hazas, M.C.; Piñol, C.; Macià, A.; Motilva, M.J. Hydroxytyrosol and the colonic metabolites derived from virgin olive oil intake induce cell cycle arrest and apoptosis in colon cancer cells. J. Agric. Food Chem. 2017, 65, 6467–6476.
- Toteda, G.; Lupinacci, S.; Vizza, D.; Bonofiglio, R.; Perri, E.; Bonofiglio, M.; Lofaro, D.; La Russa, A.; Leone, F.; Gigliotti, P.; et al. High doses of hydroxytyrosol induce apoptosis in papillary and follicular thyroid cancer cells. J. Endocrinol. Investig. 2017, 40, 153–162.
- Elamin, M.H.; Elmahi, A.B.; Daghestani, M.H.; Al-Olayan, E.M.; Al-Ajmi, R.A.; Alkhuriji, A.F.; Hamed, S.S.; Elkhadragy, M.F. Synergistic anti-breast-cancer effects of combined treatment with oleuropein and doxorubicin in vivo. Altern. Ther. Health Med. 2019, 25, 17–24.
- Chen, T.; Yan, F.; Qian, J.; Guo, M.; Zhang, H.; Tang, X.; Chen, F.; Stoner, G.D.; Wang, X. Randomized phase II trial of lyophilized strawberries in patients with dysplastic precancerous lesions of the esophagus. Cancer Prev. Res. 2012, 5, 41–50.
- Nuñez-Sánchez, M.A.; González-Sarrías, A.; García-Villalba, R.; Monedero-Saiz, T.; García-Talavera, N.V.; Gómez-Sánchez, M.B.; Sánchez-Álvarez, C.; García-Albert, A.M.; Rodríguez-Gil, F.J.; Ruiz-Marín, M.; et al. Gene expression changes in colon tissues from colorectal cancer patients following the intake of an ellagitannin-containing pomegranate extract: A randomized clinical trial. J. Nutr. Biochem. 2017, 42, 126–133.
- Gonçalves, A.C.; Bento, C.; Jesus, F.; Alves, G.; Silva, L.R. Sweet cherry phenolic compounds: Identification, characterization, and health benefits. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier Science: Amsterdam, The Netherlands, 2018; Volume 59, pp. 31–78.
- Bhaskar, S.; Sudhakaran, P.R.; Helen, A. Quercetin attenuates atherosclerotic inflammation and adhesion molecule expression by modulating TLR-NF-κB signaling pathway. Cell. Immunol. 2016, 310, 131–140.
- Xiao, L.; Liu, L.; Guo, X.; Zhang, S.; Wang, J.; Zhou, F.; Tang, Y.; Yao, P. Quercetin attenuates high fat diet-induced atherosclerosis in apolipoprotein E knockout mice: A critical role of NADPH oxidase. Food Chem. Toxicol. 2017, 105, 22–33.
- D’Amore, S.; Vacca, M.; Cariello, M.; Graziano, G.; D’Orazio, A.; Salvia, R.; Sasso, R.C.; Sabbà, C.; Palasciano, G.; Moschetta, A. Genes and miRNA expression signatures in peripheral blood mononuclear cells in healthy subjects and patients with metabolic syndrome after acute intake of extra virgin olive oil. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2016, 1861, 1671–1680.
- Catalán, U.; López de las Hazas, M.C.; Piñol, C.; Rubió, L.; Motilva, M.J.; Fernandez-Castillejo, S.; Solà, R. Hydroxytyrosol and its main plasma circulating metabolites attenuate the initial steps of atherosclerosis through inhibition of the MAPK pathway. J. Funct. Foods 2018, 40, 280–291.
- Morrison, M.; van der Heijden, R.; Heeringa, P.; Kaijzel, E.; Verschuren, L.; Blomhoff, R.; Kooistra, T.; Kleemann, R. Epicatechin attenuates atherosclerosis and exerts anti-inflammatory effects on diet-induced human-CRP and NFκB invivo. Atherosclerosis 2014, 233, 149–156.
- Perdicaro, D.J.; Rodriguez-Lanzi, C.; Fontana, A.R.; Antoniolli, A.; Piccoli, P.; Miatello, R.M.; Diez, E.R.; Vazquez Prieto, M.A. Grape pomace reduced reperfusion arrhythmias in rats with a high-fat-fructose diet. Food Funct. 2017, 8, 3501–3509.
- Chacar, S.; Hajal, J.; Saliba, Y.; Bois, P.; Louka, N.; Maroun, R.G.; Faivre, J.F.; Fares, N. Long-term intake of phenolic compounds attenuates age-related cardiac remodeling. Aging Cell 2019, 18, e12894.
- Van Rymenant, E.; Van Camp, J.; Pauwels, B.; Boydens, C.; Vanden Daele, L.; Beerens, K.; Brouckaert, P.; Smagghe, G.; Kerimi, A.; Williamson, G.; et al. Ferulic acid-4-O-sulfate rather than ferulic acid relaxes arteries and lowers blood pressure in mice. J. Nutr. Biochem. 2017, 44, 44–51.
- Suzuki-Sugihara, N.; Kishimoto, Y.; Saita, E.; Taguchi, C.; Kobayashi, M.; Ichitani, M.; Ukawa, Y.; Sagesaka, Y.M.; Suzuki, E.; Kondo, K. Green tea catechins prevent low-density lipoprotein oxidation via their accumulation in low-density lipoprotein particles in humans. Nutr. Res. 2016, 36, 16–23.
- Martín-Peláez, S.; Castañer, O.; Konstantinidou, V.; Subirana, I.; Muñoz-Aguayo, D.; Blanchart, G.; Gaixas, S.; de la Torre, R.; Farré, M.; Sáez, G.T.; et al. Effect of olive oil phenolic compounds on the expression of blood pressure-related genes in healthy individuals. Eur. J. Nutr. 2017, 56, 663–670.
- Ahmed, O.M.; Hassan, M.A.; Abdel-Twab, S.M.; Abdel Azeem, M.N. Navel orange peel hydroethanolic extract, naringin and naringenin have anti-diabetic potentials in type 2 diabetic rats. Biomed. Pharmacother. 2017, 94, 197–205.
- Yamashita, Y.; Wang, L.; Nanba, F.; Ito, C.; Toda, T.; Ashida, H. Procyanidin promotes translocation of glucose transporter 4 in muscle of mice through activation of insulin and AMPK signaling pathways. PLoS ONE 2016, 11, e0161704.
- Homayouni, F.; Haidari, F.; Hedayati, M.; Zakerkish, M.; Ahmadi, K. Hesperidin supplementation alleviates oxidative DNA damage and lipid peroxidation in type 2 diabetes: A randomized double-blind placebo-controlled clinical trial. Phyther. Res. 2017, 31, 1539–1545.
- Wobst, H.J.; Sharma, A.; Diamond, M.I.; Wanker, E.E.; Bieschke, J. The green tea polyphenol (-)-epigallocatechin gallate prevents the aggregation of tau protein into toxic oligomers at substoichiometric ratios. FEBS Lett. 2015, 589, 77–83.
- Sabogal-Guáqueta, A.M.; Muñoz-Manco, J.I.; Ramírez-Pineda, J.R.; Lamprea-Rodriguez, M.; Osorio, E.; Cardona-Gómez, G.P. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology 2015, 93, 134–145.
- Kent, K.; Charlton, K.; Roodenrys, S.; Batterham, M.; Potter, J.; Traynor, V.; Gilbert, H.; Morgan, O.; Richards, R. Consumption of anthocyanin-rich cherry juice for 12 weeks improves memory and cognition in older adults with mild-to-moderate dementia. Eur. J. Nutr. 2017, 56, 333–341.
- Anusha, C.; Sumathi, T.; Joseph, L.D. Protective role of apigenin on rotenone induced rat model of Parkinson’s disease: Suppression of neuroinflammation and oxidative stress mediated apoptosis. Chem. Biol. Interact. 2017, 269, 67–79.
- Magalingam, K.B.; Radhakrishnan, A.; Ramdas, P.; Haleagrahara, N. Quercetin glycosides induced neuroprotection by changes in the gene expression in a cellular model of Parkinson’s disease. J. Mol. Neurosci. 2014, 55, 609–617.
- Isbrucker, R.A.; Edwards, J.A.; Wolz, E.; Davidovich, A.; Bausch, J. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 2: Dermal, acute and short-term toxicity studies. Food Chem. Toxicol. 2006, 44, 636–650.
- Yates, A.A.; Erdman, J.W.; Shao, A.; Dolan, L.C.; Griffiths, J.C. Bioactive nutrients—Time for tolerable upper intake levels to address safety. Regul. Toxicol. Pharmacol. 2017, 84, 94–101.
- Johnson, W.D.; Morrissey, R.L.; Usborne, A.L.; Kapetanovic, I.; Crowell, J.A.; Muzzio, M.; McCormick, D.L. Subchronic oral toxicity and cardiovascular safety pharmacology studies of resveratrol, a naturally occurring polyphenol with cancer preventive activity. Food Chem. Toxicol. 2011, 49, 3319–3327.
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential adverse effects of resveratrol: A literature review. Int. J. Mol. Sci. 2020, 21, 2084.
- Segal, L.; Penman, M.G.; Piriou, Y. Evaluation of the systemic toxicity and mutagenicity of OLIGOPIN®, procyanidolic oligomers (OPC) extracted from French Maritime Pine Bark extract. Toxicol. Rep. 2018, 5, 531–541.
- Frevel, M.A.E.; Pipingas, A.; Grigsby, W.J.; Frampton, C.M.; Gilchrist, N.L. Production, composition and toxicology studies of Enzogenol® Pinus radiata bark extract. Food Chem. Toxicol. 2012, 50, 4316–4324.
- Kakkar, S.; Bais, S. A Review on Protocatechuic Acid and Its Pharmacological Potential. ISRN Pharmacol. 2014, 2014, 952943.
