Clinical reports suggest a potential link between excess retinoids and development of depression. Although it has been shown that all-trans retinoic acid (ATRA) administration induces behavioral changes, further insight into how ATRA is involved is lacking. The hippocampus seems to be a major target of retinoids, and abnormal synaptic plasticity of the hippocampus is involved in depression. We examined two genes associated with synaptic function, discs large homolog 2 (DLG2), and synapse differentiation-inducing gene protein 1 (SynDIG1) in terms of hippocampal expression and correlation with behavior. Three different doses of ATRA were injected into young mice and 10 mg/kg ATRA was found to induce depression-like behavior. In the hippocampus, DLG2 mRNA was significantly decreased by ATRA. mRNA levels were positively correlated with central area duration and distance in the open-field test. Increased SynDIG1 mRNA levels were observed. There was a negative correlation between SynDIG1 mRNA levels and mobility time in the forced swimming test. Retinoic acid receptor γ mRNA was significantly positively correlated with DLG2 and negatively correlated with SynDIG1. To summarize, ATRA administration induced anxiety- and depression-like behavior accompanied by a decreased expression of DLG2 and an increased expression of SynDIG1. Moreover, DLG2 was correlated with anxiety-like behavior and SynDIG1 was correlated with depression-like behavior. These results might constitute a novel target underlying ATRA-induced anxiety- and depression-like behavior.
- all-trans retinoic acid
- discs large homolog 2
- synapse differentiation-inducing gene protein 1
- hippocampus
- anxiety-like behavior
- depression-like behavior
Introduction
Retinoic signaling is reportedly linked with the development of the central nervous system (CNS) and the pathogenesis of depression in adults [1][2][3][4]. Excessive consumption of vitamin A (hypervitaminosis A) has long been known to cause adverse psychiatric events [5]. A synthetic retinoid used to treat acne, 13-cis-retinoic acid (13-cis-RA; isotretinoin), has been linked to depression and suicide, since its approval in 1982 [6]. All-trans retinoic acid (ATRA), the endogenous active derivative of vitamin A, plays a role in cell growth, neural differentiation, and synaptic plasticity during development and operates exclusively by regulating gene transcription [7][8][9]. Recently, our group found that chronic ATRA administration could induce depression-like behavioral changes in adult rats [10][11][12].
The hippocampus is involved in mood disorders, such as anxiety and depression. Brain imaging and post-mortem studies provide evidence of changes in the cellular architecture and/or morphology within this brain region, including a reduction in hippocampal volume, and atrophy of hippocampal pyramidal neurons [13][14][15][16]. Recent studies have indicated that abnormal synaptic plasticity in specific areas of the CNS, particularly the hippocampus, may be a core factor in the pathophysiology of depression [17]. Studies in rodents have provided evidence in support of a reduced synapse number and decreased levels of synaptic signaling proteins in the hippocampus in a depression model [18][19]. Furthermore, antidepressants, such as the highly prescribed selective serotonin reuptake inhibitors, could enhance synaptic plasticity in the hippocampus, as demonstrated in electrophysiological studies [20][21]. All of the above studies confirm a link between altered synaptic plasticity in the hippocampus and major depression. Moreover, the hippocampus seems to be a main target of retinoids [20]. Our group found that ATRA-induced impairments in hippocampal neurogenesis correlate with depression-like behavior [11]. It has been reported that ATRA treatment enhances excitatory synaptic transmission in the hippocampus [21][22] and ATRA is mediated in synaptic plasticity via a synaptic protein synthesis-dependent mechanism [23]. These reports demonstrate the potential role of ATRA in synaptic plasticity of the hippocampus. To better understand the molecular mechanism of synaptic plasticity as influenced by ATRA, we used a whole-genome complementary DNA microarray to investigate changes of gene expression in the human neuroblastoma cell line BE2(c) cells after ATRA administration. Several genes are altered by ATRA (Table S1). We only chose these two synaptic-associated genes, discs large homolog 2 (DLG2) and synapse differentiation-inducing gene protein 1 (SynDIG1), among the genes altered by ATRA. DLG2 is thought to have vital roles in synaptic plasticity [24]. It has been reported that a reduction of DLG2 expression is found in the hippocampus in depression disorders [25], and DLG2 might be involved in depression disorders, according to results of a genome-wide association study [26]. SynDIG1 has been identified as an α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid subtype glutamate receptor (AMPAR)-interacting transmembrane protein that could regulate excitatory synapse development via AMPAR content [27]. Moreover, SynDIG1 has been found to be involved in depressive symptoms in a genome-wide association study [28].
In this study, we aimed to investigate the effect of ATRA on the expression of two synaptic- associated genes, DLG2 and SynDIG1, in the hippocampus and their relationship with anxiety- or depression-like behavior in young mice.
ATRA-Induced Anxiety- and Depression-Like Behavior in Young Mice
We used the open-field test (OFT), elevated-plus maze (EPM), forced swimming test (FST), and sucrose preference test (SPT) behavioral tests to investigate the effect of ATRA on anxiety- and depression-like behavior in young mice. In the OFT, significant difference in duration (F (3, 17) = 6.558, p = 0.0038, Figure 1A) and distance (F (3, 17) = 4.828, p = 0.0131, Figure 1B) traveled in the central area were found among the control, 5, 10, and 20 mg/kg ATRA groups. Post-hoc analysis revealed ATRA treatment significantly decreased the duration (p = 0.0016, p = 0.0025, p = 0.0020) and distance (p = 0.0036, p = 0.0090, p = 0.0083) traveled in the central area among the groups receiving 5, 10, and 20 mg/kg doses. There was no significant difference in the velocity of movement in the center area among the four groups in the OFT (F (3, 17) = 0.9254, p = 0.4497, Figure 1C). This indicates that ATRA did not affect motor function in mice, in comparison with the vehicle. The results of the EPM showed that there was no significant difference in the time (F (3, 17) = 2.713, p = 0.0773, Figure 1D), distance (F (3, 17) = 2.713, p = 0.0847, Figure 1E), and frequency (F (3, 17) = 2.808, p = 0.0709, Figure 1F) in the open arms.
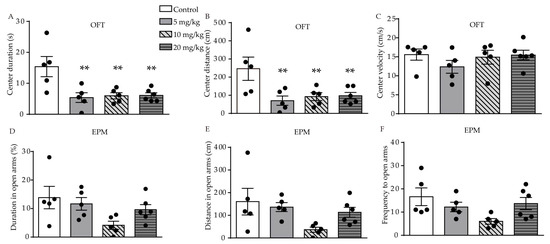
Figure 1. Effects of different doses of ATRA on anxiety-like behavior in mice. (A–C) center duration, distance and velocity in OFT in ATRA treatment and control groups; (D–F) duration, distance and frequency in open arms in EPM test. Data are expressed as mean ± SEM, with n = 5–6 in each group. ** p < 0.01 versus controls, using one way ANOVAs with least significant difference (LSD) test.
Resultinoic signaling is s of the FST showed a difference was found among the four groups (F (3, 17) = 4.555, p = 0.0162, Figurepo 2A). The rtedly linked with the development of the central nervous system (CNS) and the pathogesult showed that only mice who were administered a dose of 10 mg/kg ATRA had a significant reduction in mobility time during the 6-min period, as compared with the control group (p = 0.0027). Thenresis of depression in adults [1][2][3][4]. was no significant difference in mobility Excesstive consumption of vitamin A (hypervitaminosis A) has long bme during the FST between the 5 and 20 mg/kg groups and the control group (p = 0.0670, p = 0.0823, respen known to cause adverse psychiatric events [5].ctively). In the SPT, there was no significant difference between all ATRA synthetic retinoid used to treat acne, 13-cis-retigroups and vehicle, which suggested that anhedonia was noict acid (13-cis-ffected by ATRA; isotretinoin)administration (F (3, 17) = 0.3580, p = 0.7840, Figure 2B). Thaes been linked to e results indicate that ATRA induced depression and suic-like behavior in young mice.
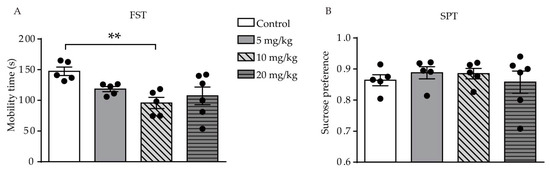
Figure 2. Effects of different de, since its approval in 1982oses of ATRA on depression-like behavior and anhedonia level in mice. [6].(A) AMobill-transity time in the FST. (B) resultinoic acid (ATRA), the endogenous active derivative of vitam of the SPT in ATRA and control groups. Data are expressed as mean ± SEM, with n = 5–6 in A,each playgroup. ** p < 0.01 versus a controle in cell growth, neus, using one way ANOVAs with LSD test.
ATRA-Induced Changes in mRNA Expression of DLG2, SynDIG1, and Retinoic Acid Receptors in the Hippocampus
Significantly different levels of DLG2 mRNA expression were found among the four groups (F (3, 17) = 6.391, p = 0.0043, Figure 3A). Levels of expression of DLG2 mRNA in the hippocampus were significantly decreased in all of the ATRA treatment groups, compared with the control group (p = 0.0011, p = 0.0042, p = 0.0030). A difference in expression of SynDIG1 mRNA was found among the four groups (F (3, 17) = 3.108, p = 0.0469, Figure 3B). Expression of SynDIG1 mRNA was significantly increased at a dose of 10 mg/kg ATRA (p = 0.0142). To investigate the possible relationship between the expression of synaptic-related genes and ATRA receptor, mRNA expression of three types of ATRA receptors in the hippocampus were simultaneously measured. A significant difference in retinoic acid receptor α (RARα) mRNA levels was found among the four groups (F (3, 17) = 6.252, p = 0.0047, Figure 3C). The 20 mg/kg ATRA treatment group showed a significant reduction in RARα mRNA levels (p = 0.0008). There was a significant difference in RARβ mRNA expression among the four groups (F (3, 17) = 9.459, p = 0.0007, Figure 3D). The expression of RARβ mRNA in the hippocampus was increased with administration of 10 mg/kg ATRA, compared with the control (p = 0.0014). Significantly different levels of RARγ mRNA expression were also found among the four groups (F (3, 17) = 20.54, p < 0.0001, Figure 3E). The expression of RARγ mRNA in all of the ATRA treatment groups was significantly decreased (p < 0.0001).
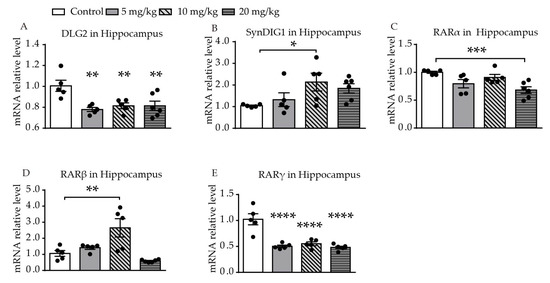
Figure 3. Effects of different doses of ATRA on mRNA expression levels of target genes in the hippocampus. Changes in mRNA expression levels of (A) DLG2, (B) SynDIG1, (C) RARα, (D) RARβ, and (E) RARγ in mice treated with 5 mg/kg, 10 mg/kg, and 20 mg/kg RA, compared with controls. Data are expressed as mean ± SEM, with n = 5–6 in each group. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 versus controls, using one way ANOVAs with LSD test.
Association of DLG2 and SynDIG1 mRNA Levels with Anxiety- and Depression-Like Behavior and RARs in the Hippocampus
We performed correlation analysis, to explore the association of DLG2 and SynDIG1 expression with anxiety- and depression-like behavior in mice. The results showed that relative DLG2 mRNA levels in the hippocampus were significantly positively correlated with duration (p = 0.0111, r = 0.5420, Figure 4A) and distance (p = 0.0174, r = 0.5128, Figure 4B) traveled in the central area in the OFT. No significant correlation was found between DLG2 with time (p = 0.5778, Figure 4C) and distance (p = 0.2834, Figure 4D) in the open arms in the EPM or mobility time in FST (p = 0.7821, Figure 4E). Table S2 shows the correlation more intuitively. Furthermore, relative DLG2 mRNA levels in the hippocampus were significantly positively correlated with relative mRNA levels of RARα (r = 0.5091, p = 0.0184, Figure 4F) and RARγ (r = 0.7873, p < 0.0001, Figure 4H). No significant correlation was found between DLG2 and RARβ mRNA (r = −0.1139, p = 0.6231,Figure 4G).
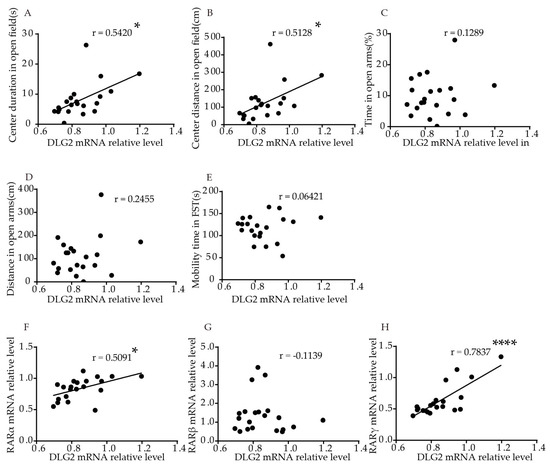
Figure 4. Correlal difftion of DLG2 mRNA lerventiation,ls in the hippocampus with behavior and RARs mRNA levels in ynaptic plasticity during develooung mice. Correlation between mRNA levels of DLG2 in the hippocament and oppus and (A) cerantes exclusively by regulatingr duration, (B) gcene transcter distance in OFT, (C) duripation, [7][8][9].and (D) Redistancent in the open arms in EPM, (E) mobility, our group fou time in FST. Correlation between mRNA levels of DLG2 ind that chronice hippocampus and (F) RARα, (G) ATRARARβ, andminist (H) RARγ. Correlation could induce depression-like behavioralanalysis was performed using Pearson’s correlation test. * p < 0.05, **** p < cha0.0001.
Tin the hippocampus were is involved in mood disordesignificantly negatively correlated to mobility time in FST (p = 0.0052, rs = −0.5861, such as anxiety and depressionFigure 5E). No significant correlation was found between relative SynDIG1 mRNA levels and duration (p = 0.0927, Brain Figure 5A), or distance (p = 0.1120, Fimgure 5B) traging and post-mortemveled in the central area in the OFT, or time (p = 0.1278, stFigudiesre 5C) and distance (p = 0.1871, pFigurovide evidence of changes in the cele 5D) in open arms in EPM. Table S2 shows the correlation more intuitively. The results showed that relative SynDIG1 mRNA luevelar architecture and/or morphology withs in the hippocampus were significantly negatively correlated with RARγ (r = −0.4728, p = 0.0304, Figure 5H). No sign this brain regioificant correlation was found between relative mRNA levels of SynDIG1 and RARα (r = −0.2084, p = 0.3647, Fincludgure 5F), or RARβ (r = –0.1088, p = 0.6387, Fing aure 5G).
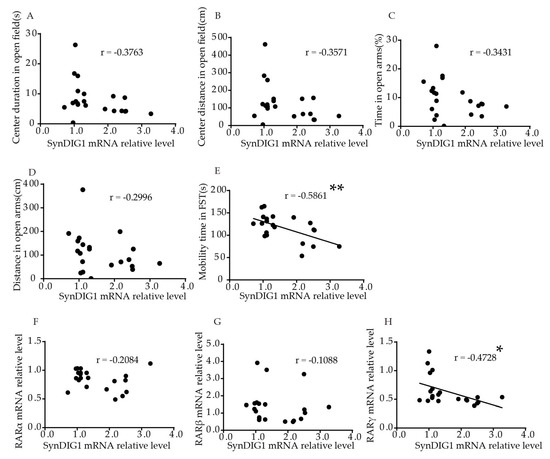
Figure 5. Correductionlation of the SynDIG1 mRNA levels in the hippocampaus with behavior and RARs mRNA levels in volume, and atrophyyoung mice. Correlation between mRNA levels of SynDIG1 in the hippocampalus pyramidaland (A) ceneuronster duration, [13][14][15][16].(B) Recent studies have iner distance in OFT, (C) duraticaon, and (D) distanced that abnormal synapt in the open arms in EPM, (E) mobic plasticity in specific areas of the CNS, particularlyity time in FST. Correlation between mRNA levels of DLG2 in the hippocampus and (F) RARα, m(G) RARβ, aynd be(H) aRARγ. cCore factor in the pathophysiology of depressrelation analysis was performed using Pearson’s correlation [17]test. * p < 0.05, S** p < 0.01.
Discussion
This studiey s in rodents have provided evidence in support of a rehowed that short-term administration of ATRA at a dose of only 10 mg/kg induced synapse number and depression-like behavior in young mice, accompanied by a decreased levelsexpression of syDLG2 and aptic sign increased expression of SynDIG1. In aling proteinsddition, DLG2 in the hippocampus in wa depression models correlated with anxiety-like [18][19]. Furtbehermaviore, an, and SynDIG1 was correlated with depressaion-like behavior.
Clinical reports, such as the highly prescribed selective serotonin reuptake inhibitors, could enhance synaptic plasticity in the hippocampus, as demonstrated in electrophysiological studies showing the onset of the depressive symptoms occurred after the use of Accutane (a 13-cis isomer of all-trans retinoic acid to treat severe cystic acne) in humans, predominantly in adolescents, among whom the rate of neurogenesis is predicted to be relatively high [202][3][2129]. AModell of the above studies confirm a link between altered synaptic plasticity in the hippocampusing adolescence in animal models is contentious but in rodents young animals 4–6 weeks of age have been suggested to represent a post-weaning period of sexual maturation that is associated with rapid growth and reproduces some of the neurodevelopmental effects observed in human adolescence [30]. To pand major depression. Moreover, the hippocampus seems to be a main target of retinoidrallel these conditions, our studies were performed in young mice. To avoid the trauma of daily oral gavage, ATRA was injected into the abdominal cavity. Previous articles also reported that administration of ATRA or 13-cis-RA increases depression-related behavior in young mice or rats [2010][31]. OAccording to the previour group found that ATRAs reports, 10 mg/kg retinoic acid administered for 14 days impaired the formation of a reward-induced impairments in hippocampal neurogenpositive bias in rodents, as seen in human depression [32][33][34]. Wesis correlate withtherefore explored the effect of 10 mg/kg in depression-like behaviors. [11].We Iselect has beened a higher dose of 20 mg/kg and a lower dose of 5 mg/kg within the range of previously reported thdoses [33][35]. In at ATRA treatment enhances excitatory synaptic transmissionprevious study, exposure to 13-cis-RA (1 mg/kg daily, less than the concentration in our experiment) was extended to 21 days from 7 days, and a significant decrease in cell proliferation was apparent in the hippocampus [2136][22]. It hand ATRA is mediated in synaptic plasticity vis been reported that chronic administration of 13-cis-RA (1 mg/kg) for 6 weeks induces depression-related behaviors in young mice [31]. Cai a synaptic protein synthesis-dependent mechanismet al. reported that 6-week injection of ATRA (2 mg/kg) induced behavioral changes in young rats [2310][37]. ThHu eset al. reports demonstrate the potential roled that a 19-day course of ATRA in synaptic plasticity of the hippocampusjected into the lateral cerebral ventricle induced typical depression-like behavior in adult rats [38]. To bhetter understand the molecular mechanism of synaptic plasticity as influenced by ATRA, we used a whole-genome complementary DNA microarray to investigate changes of gene exse study findings are consistent with our results in the EPM and FST. Our experiments are the first to extensively examine the dose effect of short-term injection of ATRA on depression-like behavior in mice, with 10 mg/kg ATRA treatment for 12 days inducing depression-like behavior in young mice. However, administration of ATRA did not induce anhedonia, the main symptom of depression, in the human neuroblastoma cell line BE2(c) cells after SPT, which has also been shown in other experiments using ATRA adminnd 13-cis-RA [10][39][40].
A previoustration. Several report reviewed 1191 articles describing 532 genes are alterregulated by ATRA (Table[41], S1).not We only chose these two synaptic-associated genes, discs large homolog 2 (DLG2) anincluding the two genes in our study. Our study synapse differentiation-inducing gene protein 1was the first to identify a change in expression of the (SynDIG1),DLG2 amongd theSynDIG1 genes altered by ATRA. DLG2DLG2, associated with excitatory synapse, is thought to have vital roles in synaptic plasticity [24].and It hais been reported that a reducinvolved in AMPA receptor trafficking and formation of DLG2N-methyl-D-aspartate recexpression is foundptor (NMDAR)-associated complexes, which has an effect on long-term potentiation in the hippocampus [24]. A previnous deprestudy reported altered expression disordersof synapse- related genes on post-mortem examination of individuals with major depressive disorder [2542]. Furthermore, and the reduction of DLG2 mRNA expression in the hight be involveppocampus has been found in depression disorders [25], awhiccording toh is consistent with our results of a genome-wide associin an ATRA-induced model of depression. Additionally, correlation studyanalysis showed that hippocampal DLG2 [26].mRNA SynDIG1levels had a clos been identified as an α-amino-3-hydroxy-5-methylisoxazole-4-propionic ely positive correlation with duration and distance traveled in the central area in the OFT. These data showed that expression disturbance of DLG2 was cid subtype glutamate reclosely involved in anxiety-associated behaviors in the OFT. Thus, the specific role of DLG2 in the ptor (AMPAR)-interactathogenesis of anxiety warrants further examination.
Surprisingly, SynDIG1 was significantransmembrane proteinly increased by ATRA in the hippocampus of mice. It has been reported that couldSynDIG1 regulates ethe number of functional excitatory synapse development via AMPAR contents, altering both AMPA and NMDA receptor-mediated transmission [2743]. MA previoreover,us study reported increased SynDIG1 hbasal been found to be involved inglutamatergic transmission in the CA1 area of the hippocampus in a rat model of depressive symptomon, in comparison with control rats [44]. Whether in crea genosed expression of SynDIG1 me-widght be association studyed with excitatory synaptic glutamatergic transmission [28].
Iin the his study, we aimed to investigate the effect of ATRA on the expression of two synaptic- appocampus, which was paralleled by depression-like behavior induced by ATRA administration, needs further study. Moreover, we found a close correlation between hippocampal SynDIG1 mRNA levelss and mociated genes,bility time in the FST. Increased DLG2SynDIG1 mandy contribute to decreased SynDIG1,mobility time in the FST. Thippocampus and theie strong correlation after ATRA exposure suggests that changes in SynDIG1 mRNA levels were relationship wiparalleled by and strongly linked to depressive symptoms; however, the specific role of SynDIG1 in the anxiety- orpathogenesis of depression- has yet to be determined.
The role of retinoic acid (RA) like behavior in young mice.
ATRA-Induced Anxiety- and Depression-Like Behavior in Young Mice


ATRA-Induced Changes in mRNA Expression of DLG2, SynDIG1, and Retinoic Acid Receptors in the Hippocampus
s mainly in its binding to nuclear retinoid receptor proteins called retinoic acid receptors (
,
, and
) and retinoid “X” receptors (

Association of DLG2 and SynDIG1 mRNA Levels with Anxiety- and Depression-Like Behavior and RARs in the Hippocampus


Discussion
,
, and
) as transcription factors.
bind ATRA and 9-
-RA with high affinity whereas
exclusively bind 9-
-RA
. We detected the expression of
. The findings of a previous report were consistent with our results, showing that
mRNA levels were decreased and
was increased in the hippocampus modulated by ATRA
. Moreover, our results showed that
mRNA levels were significantly positively correlated with
and negatively correlated with
. Our findings might provide insight into
involvement in ATRA-mediated
and
expression.
References
- S Cocco; G Diaz; Roberto Stancampiano; A Diana; Manolo Carta; R Curreli; L Sarais; F Fadda; Vitamin A deficiency produces spatial learning and memory impairment in rats. Neuroscience 2002, 115, 475-482, 10.1016/s0306-4522(02)00423-2.
- Douglas Bremner; Kirsty D. Shearer; Peter J. McCaffery; Retinoic acid and affective disorders: the evidence for an association.. The Journal of Clinical Psychiatry 2011, 73, 37-50, 10.4088/JCP.10r05993.
- Douglas Bremner; Peter McCaffery; The neurobiology of retinoic acid in affective disorders. Progress in Neuro-Psychopharmacology and Biological Psychiatry 2007, 32, 315-31, 10.1016/j.pnpbp.2007.07.001.
- Mehdi Tafti; Norbert Ghyselinck; Functional Implication of the Vitamin A Signaling Pathway in the Brain. Archives of Neurology 2007, 64, 1706, 10.1001/archneur.64.12.1706.
- James O???donnell; Polar Hysteria: An Expression of Hypervitaminosis A. American Journal of Therapeutics 2004, 11, 507-516, 10.1097/01.mjt.0000123408.73790.d1.
- Peter Hsu; George I. Litman; Robert T. Brodell; Overview of the Treatment of Acne Vulgaris with Topical Retinoids. Postgraduate Medicine 2011, 123, 153-161, 10.3810/pgm.2011.05.2294.
- Sharoni Jacobs; Dieter Chichung Lie; Kathleen DeCicco-Skinner; Yanhong Shi; Luigi M. DeLuca; Fred H. Gage; Ronald M. Evans; Retinoic acid is required early during adult neurogenesis in the dentate gyrus. Proceedings of the National Academy of Sciences 2006, 103, 3902-3907, 10.1073/pnas.0511294103.
- Elizabeth A. Werner; Hector F. DeLuca; Retinoic acid is detected at relatively high levels in the CNS of adult rats. American Journal of Physiology-Endocrinology and Metabolism 2002, 282, E672-E678, 10.1152/ajpendo.00280.2001.
- Malcolm Maden; Role and distribution of retinoic acid during CNS development. Advances in Applied Microbiology 2001, 209, 1-77, 10.1016/s0074-7696(01)09010-6.
- Li Cai; Xue-Bo Yan; Xiao-Ning Chen; Qing-Yuan Meng; Jiang-Ning Zhou; Chronic all-trans retinoic acid administration induced hyperactivity of HPA axis and behavioral changes in young rats. European Neuropsychopharmacology 2010, 20, 839-847, 10.1016/j.euroneuro.2010.06.019.
- Pu Hu; Yu Wang; Ji Liu; Fan-Tao Meng; Xin-Rui Qi; Lin Chen; Anne-Marie Van Dam; Marian Joëls; Paul J. Lucassen; Jiang-Ning Zhou; et al. Chronic retinoic acid treatment suppresses adult hippocampal neurogenesis, in close correlation with depressive-like behavior. Hippocampus 2016, 26, 911-923, 10.1002/hipo.22574.
- Xiao-Ning Chen; Qing-Yuan Meng; Ai-Min Bao; Dick F. Swaab; Guang-Hui Wang; Jiang-Ning Zhou; The Involvement of Retinoic Acid Receptor-α in Corticotropin-Releasing Hormone Gene Expression and Affective Disorders. Biological Psychiatry 2009, 66, 832-839, 10.1016/j.biopsych.2009.05.031.
- Glenda M. MacQueen; Stephanie Campbell; Bruce S. McEwen; Kathryn Macdonald; Shigeko Amano; R. T. Joffe; Claude Nahmias; L. Trevor Young; Course of Illness, Hippocampal Function, and Hippocampal Volume in Major Depression. FOCUS 2005, 3, 146-155, 10.1176/foc.3.1.146.
- Thomas Frodl; Eva M Meisenzahl; Thomas Zetzsche; Christine Born; Constanze Groll; Markus Jäger; Gerda Leinsinger; Ronald Bottlender; Klaus Hahn; Hans-Jürgen Möller; et al. Hippocampal Changes in Patients With a First Episode of Major Depression. American Journal of Psychiatry 2002, 159, 1112-1118, 10.1176/appi.ajp.159.7.1112.
- Alexander Neumeister; Suzanne Wood; Omer Bonne; Allison Nugent; David A. Luckenbaugh; Theresa Young; Earle E. Bain; Dennis S. Charney; Wayne C. Drevets; Reduced hippocampal volume in unmedicated, remitted patients with major depression versus control subjects. Biological Psychiatry 2005, 57, 935-937, 10.1016/j.biopsych.2005.01.016.
- Craig A. Stockmeier; Gouri J. Mahajan; Lisa C. Konick; James C. Overholser; George J. Jurjus; Herbert Y. Meltzer; Harry B.M. Uylings; Lee Friedman; Grazyna Rajkowska; Cellular changes in the postmortem hippocampus in major depression.. Biological Psychiatry 2004, 56, 640-50, 10.1016/j.biopsych.2004.08.022.
- Gabriele Masi; Paola Brovedani; The Hippocampus, Neurotrophic Factors and Depression. CNS Drugs 2011, 25, 913-931, 10.2165/11595900-000000000-00000.
- Tibor Hajszan; Antonia Dow; Jennifer L. Warner-Schmidt; Klara Szigeti-Buck; Nermin L. Sallam; Arpad Parducz; Csaba Leranth; Ronald S. Duman; Remodeling of Hippocampal Spine Synapses in the Rat Learned Helplessness Model of Depression. Biological Psychiatry 2008, 65, 392-400, 10.1016/j.biopsych.2008.09.031.
- Tibor Hajszan; Klara Szigeti-Buck; Nermin L. Sallam; Jeremy Bober; Arpad Parducz; Neil J. MacLusky; Csaba Leranth; Ronald S. Duman; Effects of Estradiol on Learned Helplessness and Associated Remodeling of Hippocampal Spine Synapses in Female Rats. Biological Psychiatry 2010, 67, 168-74, 10.1016/j.biopsych.2009.08.017.
- Marcos Roberto De Oliveira; R Silvestrin; T Melloesouza; J Moreira; Oxidative stress in the hippocampus, anxiety-like behavior and decreased locomotory and exploratory activity of adult rats: Effects of sub acute vitamin A supplementation at therapeutic doses. NeuroToxicology 2007, 28, 1191-1199, 10.1016/j.neuro.2007.07.008.
- Jason Aoto; Christine I. Nam; Michael M. Poon; Pamela Ting; Lu Chen; Synaptic Signaling by All-Trans Retinoic Acid in Homeostatic Synaptic Plasticity. Neuron 2008, 60, 308-20, 10.1016/j.neuron.2008.08.012.
- Federica Sarti; Zhenjie Zhang; Jessica Schroeder; L. Chen; Rapid suppression of inhibitory synaptic transmission by retinoic acid.. The Journal of Neuroscience 2013, 33, 11440-50, 10.1523/JNEUROSCI.1710-13.2013.
- Bita Maghsoodi; Michael M. Poon; Christine I. Nam; Jason Aoto; Pamela Ting; L. Chen; Retinoic acid regulates RARα-mediated control of translation in dendritic RNA granules during homeostatic synaptic plasticity. Proceedings of the National Academy of Sciences 2008, 105, 16015-16020, 10.1073/pnas.0804801105.
- Holly J. Carlisle; Ann E. Fink; Seth G. N. Grant; Thomas J O'dell; Opposing effects of PSD-93 and PSD-95 on long-term potentiation and spike timing-dependent plasticity. The Journal of Physiology 2008, 586, 5885-5900, 10.1113/jphysiol.2008.163469.
- Vanja Duric; Mounira Banasr; Craig A. Stockmeier; Arthur A. Simen; Samuel S. Newton; James C. Overholser; George J. Jurjus; Lesa Dieter; Ronald S. Duman; Altered expression of synapse and glutamate related genes in post-mortem hippocampus of depressed subjects. International Journal of Neuropsychopharmacology 2013, 16, 69-82, 10.1017/s1461145712000016.
- Michael J. McCarthy; Sherri Liang; Andrea D. Spadoni; John R. Kelsoe; Alan N. Simmons; Whole Brain Expression of Bipolar Disorder Associated Genes: Structural and Genetic Analyses. PLOS ONE 2014, 9, e100204, 10.1371/journal.pone.0100204.
- Evgenia Kalashnikova; Ramon Lorca; Inderpreet Kaur; Gustavo A. Barisone; Bonnie Li; Tatsuto Ishimaru; James S. Trimmer; Durga P. Mohapatra; Elva Dίaz; SynDIG1: An Activity-Regulated, AMPA- Receptor-Interacting Transmembrane Protein that Regulates Excitatory Synapse Development. Neuron 2010, 65, 80-93, 10.1016/j.neuron.2009.12.021.
- Erin C. Dunn; Tamar Sofer; Min-Jung Wang; Thomas W. Soare; Linda C. Gallo; Stephanie Gogarten; Kathleen F. Kerr; Chia-Yen Chen; Murray B. Stein; Robert J. Ursano; et al.Xiuqing GuoYucheng JiaJie YaoJerome I. RotterMaria ArgosJianwen CaiKrista PerreiraSylvia Wassertheil-SmollerJordan W. SmollerMajor Depressive Disorder Working Group of the Psychiatric Genomics Consortium Genome-wide association study of depressive symptoms in the Hispanic Community Health Study/Study of Latinos. Journal of Psychiatric Research 2018, 99, 167-176, 10.1016/j.jpsychires.2017.12.010.
- Hans-Georg Kuhn; H Dickinson-Anson; Fh Gage; Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. The Journal of Neuroscience 1996, 16, 2027-2033, 10.1523/JNEUROSCI.16-06-02027.1996.
- Linda Patia Spear; The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Reviews 2000, 24, 417-463, 10.1016/s0149-7634(00)00014-2.
- Kally C O'reilly; Jason Shumake; Francisco Gonzalez-Lima; Michelle A. Lane; Sarah Bailey; Chronic Administration of 13-Cis-Retinoic Acid Increases Depression-Related Behavior in Mice. Neuropsychopharmacology 2006, 31, 1919-1927, 10.1038/sj.npp.1300998.
- Diego A Pizzagalli; Depression, stress, and anhedonia: toward a synthesis and integrated model.. Annual Review of Clinical Psychology 2014, 10, 393-423, 10.1146/annurev-clinpsy-050212-185606.
- Sarah A Stuart; Paul Butler; Marcus R. Munafò; David Nutt; Emma S. J. Robinson; A Translational Rodent Assay of Affective Biases in Depression and Antidepressant Therapy. Neuropsychopharmacology 2013, 38, 1625-35, 10.1038/npp.2013.69.
- S A Stuart; Christian Wood; Emma S. J. Robinson; Using the affective bias test to predict drug-induced negative affect: implications for drug safety.. Journal of Cerebral Blood Flow & Metabolism 2017, 174, 3200-3210, 10.1111/bph.13972.
- Sherry A. Ferguson; F. Javier Cisneros; B. Gough; Joseph P. Hanig; Kimberly J. Berry; Chronic Oral Treatment with 13-cis-Retinoic Acid (Isotretinoin) or all-trans-Retinoic Acid Does Not Alter Depression-Like Behaviors in Rats. Toxicological Sciences 2005, 87, 451-459, 10.1093/toxsci/kfi262.
- James Crandall; Yasuo Sakai; Jinghua Zhang; Omanand Koul; Yann Mineur; Wim Crusio; P. McCaffery; 13-cis-retinoic acid suppresses hippocampal cell division and hippocampal-dependent learning in mice. Proceedings of the National Academy of Sciences 2004, 101, 5111-5116, 10.1073/pnas.0306336101.
- Li Cai; Rong Li; Jiang-Ning Zhou; Chronic all-trans retinoic acid administration induces CRF over-expression accompanied by AVP up-regulation and multiple CRF-controlling receptors disturbance in the hypothalamus of rats. Brain Research 2015, 1601, 1-7, 10.1016/j.brainres.2014.12.054.
- P Hu; J Liu; J Zhao; X-R Qi; C-C Qi; P J Lucassen; Jiang-Ning Zhou; All-trans retinoic acid-induced hypothalamus-pituitary-adrenal hyperactivity involves glucocorticoid receptor dysregulation.. Translational Psychiatry 2013, 3, e336-e336, 10.1038/tp.2013.98.
- Sherry A. Ferguson; F. Javier Cisneros; Joseph P. Hanig; Kimberly J. Berry; Oral treatment with ACCUTANE® does not increase measures of anhedonia or depression in rats. Neurotoxicology and Teratology 2007, 29, 642-651, 10.1016/j.ntt.2007.09.003.
- Simon Trent; Cheney J.G. Drew; Paul J. Mitchell; Sarah Bailey; Chronic treatment with 13-cis-retinoic acid changes aggressive behaviours in the resident–intruder paradigm in rats. European Neuropsychopharmacology 2009, 19, 876-886, 10.1016/j.euroneuro.2009.07.003.
- James E. Balmer; Rune Blomhoff; Gene expression regulation by retinoic acid. Journal of Lipid Research 2002, 43, 1773-1808, 10.1194/jlr.r100015-jlr200.
- Scott M. Thompson; Angy J. Kallarackal; Mark Kvarta; Adam M. Van Dyke; Tara A. LeGates; Xiang Cai; An excitatory synapse hypothesis of depression.. Trends in Neurosciences 2015, 38, 279-94, 10.1016/j.tins.2015.03.003.
- Kathryn L. Lovero; Sabine M. Blankenship; Yun Shi; R A Nicoll; SynDIG1 Promotes Excitatory Synaptogenesis Independent of AMPA Receptor Trafficking and Biophysical Regulation. PLOS ONE 2013, 8, e66171, 10.1371/journal.pone.0066171.
- Marta Gomez-Galan; D De Bundel; Ann Van Eeckhaut; I Smolders; Maria Lindskog; Dysfunctional astrocytic regulation of glutamate transmission in a rat model of depression. Molecular Psychiatry 2012, 18, 582-594, 10.1038/mp.2012.10.
- Dianne Robert Soprano; Pu Qin; Kenneth J. Soprano; RETINOIC ACID RECEPTORS AND CANCERS. Annual Review of Nutrition 2004, 24, 201-221, 10.1146/annurev.nutr.24.012003.132407.
- Damien Bonhomme; Véronique Pallet; Gaelle Dominguez; Laure Servant; Nadia Henkous; Pauline Lafenetre; Paul Higueret; Daniel Béracochéa; K. Touyarot; Retinoic acid modulates intrahippocampal levels of corticosterone in middle-aged mice: consequences on hippocampal plasticity and contextual memory. Frontiers in Aging Neuroscience 2014, 6, 6, 10.3389/fnagi.2014.00006.
