Brewer’s spent grains (BSG) is the main solid by-product in the brewing industry, obtained during lautering. BSG has a multitude of applications and it can be used as a valuable fedstock for production of different products. Moreover, it could also be used as a biomass for energy purposes.
- Solid Fuel
- Brewer's Spent Grain
- Hydrothermal Carbonisation
1. Introduction
The main solid by-product in the brewing industry is brewer’s spent grains (BSG) obtained during lautering [1].
Industrial-scale breweries produce high quantities of mentioned wastes and are able to deliver it constantly. According to Eurostat, 34 billion L of beer was produced in the European Union in 2019 [2]. That means that large quantities of brewer’s spent grains are produced yearly. Such by-product is rich in cellulose, hemicellulose, lignin, and proteins (Table 1). It may be feasible to use them in the neighbourhood of such factories due to the high costs of transport.
Table 1.
The approximate chemical composition of BSG in different studies (% of dry weight).
|
|
Lignin |
Cellulose |
Hemicellulose |
Ash |
Protein |
Lipids |
Phenolics |
Starch |
|
Kanauchi et al., (2001)[3] |
11.9 |
25.4 |
21.8 |
2.4 |
24.0 |
10.6 |
N.D. |
N.D. |
|
Carvalheiro et al., (2004)[4] |
21.7 |
21.9 |
29.6 |
1.2 |
24.6 |
N.D. |
N.D. |
N.D. |
|
Silva et al., (2004)[5] |
16.9 |
25.3 |
41.9 |
4.6 |
N.D. |
N.D. |
N.D. |
N.D. |
|
Mussatto and Roberto, (2006)[6] |
27.8 |
16.8 |
28.4 |
4.6 |
15.2 |
N.D. |
N.D. |
N.D. |
|
Celus et al., (2006)[7] |
N.D. |
0.3 |
22.5 |
3.3 |
26.7 |
N.D. |
N.D. |
1 |
|
Xiros et al., (2008)[8] |
11.5 |
12 |
40 |
3.3 |
14.2 |
13 |
2.0 |
2.7 |
|
Jay et al., (2008)[9] |
20–22 |
31–33 |
N.D. |
N.D. |
15–17 |
6–8 |
1.0–1.5 |
10–12 |
|
Treimo et al., (2009)[10] |
12.6 ± 0.1 |
45.9 * |
|
23.4 ± 1.4 |
N.D. |
N.D. |
7.8 ± 0.2 |
|
|
Robertson et al., (2010)[11] |
13–17 |
N.D. |
22–29 |
N.D. |
20–24 |
N.D. |
N.D. |
2–8 |
|
Khidzir et al., (2010)[12] |
56.74 ± 9.38 |
40.20 ± 17.71 |
N.D. |
2.27 ± 0.76 |
6.41 ± 0.31 |
2.50 ± 0.11 |
N.D. |
0.28 ± 0.06 |
|
Waters et al., (2012)[13] |
N.D. |
26.0 |
22.2 |
1.1 |
22.1 |
N.D. |
N.D. |
N.D. |
|
Meneses et al., (2013)[14] |
19.40 ± 0.34 |
21.73 ± 1.36 |
19.27 ± 1.18 |
4.18 ± 0.03 |
24.69 ± 1.04 |
N.D. |
N.D. |
N.D. |
|
Sobukola et al., (2012)[15] |
9.19 ± 0.011 |
60.64 ± 0.26 * |
2.48 ± 0.02 |
24.39 ± 0.46 |
6.18 ± 0.13 |
N.D. |
N.D. |
|
|
Kemppai-nen et al., (2016)[16] |
19.6 |
45 * |
4.1 |
20.3 |
N.D. |
N.D. |
N.D. |
|
|
Yu et al., (2020)[17] |
N.D. |
51.0 ± 0.7 * |
4.1 ± 0.1 |
23.4 ± 0.2 |
9.4 ± 0.1 |
N.D. |
N.D. |
|
N.D.—no data, *—all carbohydrates.
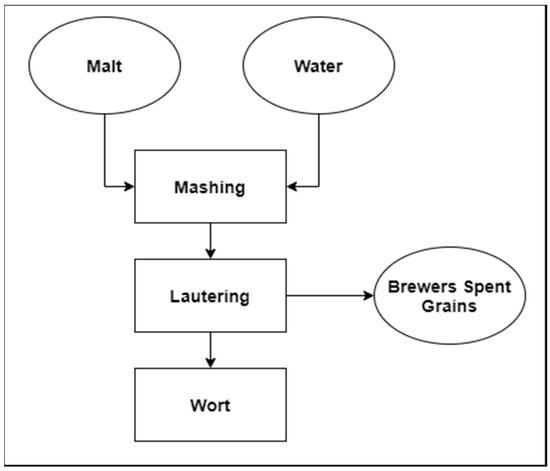
Figure 1. Diagram of beer wort production with an emphasis on the main solid by-product.
2. Thermal Valorization of BSG
2.1. BSG as a Solid Fuel
Basic fuel properties, reported by many researchers, suggest that BSG is promising as a solid fuel (Figure 2) [18][19]. Reported carbon content reported is typically ranging between 45% up to approx. 49% on the dry basis [18][19], which makes BSG not significantly different in terms of its fuel properties, in comparison to lignocellulosic biomass. Additionally, ash content varies between 2 and 6% [19][20][21], which is similar to different types of agricultural biomass [22][23][24][25][26][27][28]. However, high moisture content, exceeding 70% [18][19], is a significant obstacle in the use of BSG as a solid biofuel. Drying is possible but requires energy and bulky installations due to relatively high residence time, e.g., the order of magnitude of 100 min was reported by some studies [29] for achieving moisture reduction of 0.2 of the original value, corresponding with the moisture content of approx. 15%. Moreover, the energy required for the drying process should not be overlooked. Some studies [30] reported drying energy, for superheated steam drying of BSG, ranging between 0.65 and 1.45 MJ/kg of removed water, when latent heat recovery from steam was included in the balance [30].
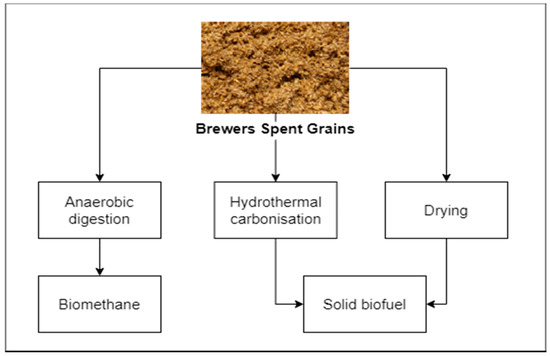
Figure 2. Energetic valorization of BSG.
2.2. Hydrothermal Carbonization as a Thermal Valorization Method for Wet Types of Biomass
HTC is a thermal valorization process, typically performed at elevated temperatures (typically 200 to 260 °C) in subcritical water, at elevated pressure [31][32]. The use of such a process can enhance mechanical dewatering, which has already been reported for various wet types of biomass [33][34][35].
The ionic constant of water is significantly increased, and water behaves as a non-polar solvent at 200–280 °C [35][36][37][38][39][40][41]. A multitude of reactions occurring at the same time, with the output of multiple different products, can be considered characteristic for HTC of complex substances such as different types of biomass [32]. The HTC process starts with hydrolysis [31]. This is followed by dehydration and decarboxylation [31][42]. Dehydration decreases the amount of hydroxyl groups (OH) [31]. The decrease in the amount of OH groups also causes a lower O/C ratio. Decarboxylation decreases the amount of carboxyl (COOH) and carbonyl (C=O) groups, also slightly decreasing the O/C ratio of the solid product [31]. This is followed by polymerization and aromatization [31][42]. A decrease in the number of hydroxyl groups is the key aspect in making hydrothermally carbonized biomass more hydrophobic, lowering its equilibrium moisture content [43] and making physical dewatering easier [31]. The ability to decrease O/C ratio is beneficial when valorization is performed, aiming at improving the results of subsequent pyrolysis [44][45][46]. Moreover, the process of hydrothermal carbonization can change the biomass in terms of the composition of the inorganic fraction [32][47]. Furthermore, some studies reported relatively easy pelletizing of hydrochars [48]. This makes hydrothermal carbonization a prospective valorization process for low-quality solid biofuels, especially when wet biomass is concerned as a potential feedstock.
2.3. The Effect of Hydrothermal Carbonization of BSG
Slight improvement in mechanical dewatering, thanks to HTC of BSG, was observed [34]. Moreover, the GC-MS analysis of the liquid HTC effluent indicated that it contains organic compounds that could be used to produce biogas in the anaerobic digestion [34]. Similarly, phenols, benzenediols, and fatty acids can be found in the liquid by-products of HTC of BSG, concluding that the release of such compounds is an effect of the presence of bound lipids in the feedstock [49]. HTC of spent grain from a big scale brewery, resulted in an improvement in fuel properties. Higher heating value (HHV) increased, accompanied by a decrease in the ash content, especially for high water: biomass ratios [50]. The study deemed low temperatures of the HTC process especially suitable, thanks to the high content of hemicellulose in the feedstock [50]. For HTC of BSG the mass yield can be determined by an indirect method [51]. Moreover, it has been confirmed that HTC can increase the heating value of BSG and decrease the O/C ratio [51], indicating its suitability as a valorization method suitable for subsequent pyrolysis.
A Py-GC-MS analysis of BSG and corresponding hydrochars were performed. It was noticed that relatively low pyrolysis temperature for spent grains resulted in a release of a significant amount of N-compounds, which was attributed to weakly bonded proteins present in the feedstock [52]. On the other hand, fewer N-compounds was released during pyrolysis of hydrochars, owing to the Maillard reactions producing more stable N-heterocyclic structures [52]. Hydrothermal carbonization, performed at temperatures between 180 and 260 °C, resulted in the removal efficiency of inorganics, ranging from almost 60% to more than 95% for K, approx. 45% to approx. 55% for P, and approx. 35% up to approx. 75% for Na [53]. Moreover, HTC performed at 180 and 220 °C, and pyrolysis at 600 °C resulted in increased BET surface for pyrochars from a two-step process, when comparing to single-step pyrolysis at the same temperature [53].
3. Extraction of High-Value Compounds from BSG
Due to the multitude of compounds contained, the brewer’s spent grain undergoes extraction processes to obtain substances with the desired properties. BSG undergoes many different extraction processes, such as alkaline hydrolysis [54], enzymatic hydrolysis [55],microwave-assisted extraction [56], solvent extraction[14], supercritical carbon dioxide extraction [57], ultrasound-assisted extraction [58], etc. The products that can be obtained by extraction are:
3.1. Arabinoxylans, Polyphenol, Antioxidants and Glucose
Arabinoxylan is a polysaccharide consisting of two pentose sugars: xylose and arabinose [59]. Among other hemicelluloses, cellulose, and lignin, it is part of the dietary fibre found in BSG. It can bind to polyphenols such as ferulic acid and p-cumaic acid. Arabinoxylans can be recovered by ultrasound-assisted extraction [58], microwave-assisted extraction[56] or HCl and ethanol extraction (after previous protein extraction) [60].
Studies show that supercritical extraction of CO2 with ethanol 60% v/v at 35 MPa, 40 °C at an extraction time of 240 min allows a good recovery of phenolic or flavonoid fractions [57][61]. The extract obtained is characterized by good antioxidant properties. Phenolic fractions can also be obtained by solvent extraction (acetone–water mixture) [14]. Good recovery of ferulic and p-coumaric acids is provided by the BSG alkaline extraction [54] and solvent extraction (acetone: water mixture) [14][62]. For ultrasound-assisted extraction of polyphenol compounds from BSG, experimental data were in good agreement with both power law and the Weibull model [63]. Ultrasound-assisted extraction achieved similar productivity, after 30 min of treatment, in comparison to enzyme hydrolysis [63]. Comparison of conventional maceration, microwave and ultrasound-assisted extraction, using BSG from light and dark beer as well as their mixtures concluded that microwave and ultrasound extraction did not improve the total polyphenol yield [64]. As the result of the use of Bacillus subtilis WX-17 to improve the nutritional value of BSG in a solid-state fermentation the total amount of unsaturated fatty acid and the total antioxidant quantity can increase by as much as 1.7 and 5.8 times, respectively[65]. Extraction of phenolic antioxidants from BSG, using acetone–water and ethanol–water mixtures as extraction solvents, achieves maximum yield at 60% (v/v) organic solvent concentration, for both solvents [66].
3.2. Proteins
Due to the high protein content (about 20% in dry matter), BSG is a good potential source of vegetable protein for the food industry. In the case of protein extraction, the selectivity of the extraction process is crucial. Alkaline treatment of BSG, resulted in the extraction yield of 21.4% and purity of 60.2% for extracted proteins [67]. In case of a combination of alkaline pretreatment with diluted acid, a very high degree of extraction was obtained (even 95%). However, the selectivity of protein extraction process has some drawbacks, because part of lignin and hydrocarbons contained in BSG can be dissolved together with proteins [68]. Good selectivity, with lower horizontal extraction (about 65%) can be obtained with hydrothermal pretreatment, which does not require the use of chemicals [68]. Good results of the extraction of proteins from BSG (up to 80%) were achieved with the use of carboxylate salt—urea DES [69]. The disadvantage of this technology is the residual DES in the protein product, but in a case when a substitute for urea will be gained, this method could be attractive for making human nutrition products. Another promising method is the use of ultrasounds for enzymatic hydrolysis of proteins from BSG [17]. By using ultrasound pretreatment, the efficiency of protein separation is increased (from 61.6 to 69.8%), the time of enzymatic reaction is shortened (by 56%), and the cost of enzyme use can be reduced (even 73%).
References
- Esslinger, H.M. Handbook of Brewing; WILEY-VCH Verlag GmbH & Co.: Weinheim, Germany, 2015; Volume 1, ISBN 9788578110796
- Happy International Beer Day! . Eurostat. Retrieved 2020-12-19
- Osamu Kanauchi; Keiichi Mitsuyama; Yoshio Araki; Development of a Functional Germinated Barley Foodstuff from Brewer's Spent Grain for the Treatment of Ulcerative Colitis. Journal of the American Society of Brewing Chemists 2001, 59, 59-62, 10.1094/asbcj-59-0059.
- F Carvalheiro; Production of oligosaccharides by autohydrolysis of brewery's spent grain. Bioresource Technology 2004, 91, 93-100, 10.1016/s0960-8524(03)00148-2.
- Joaquim Pedro Silva; Sónia Sousa; José Carlos Rodrigues; Helena Antunes; John J. Porter; I.C. Gonçalves; Suzana Ferreira-Dias; Adsorption of acid orange 7 dye in aqueous solutions by spent brewery grains. Separation and Purification Technology 2004, 40, 309-315, 10.1016/j.seppur.2004.03.010.
- Solange I. Mussatto; Inês C Roberto; Chemical characterization and liberation of pentose sugars from brewer's spent grain. Journal of Chemical Technology & Biotechnology 2006, 81, 268-274, 10.1002/jctb.1374.
- Inge Celus; Kristof Brijs; Jan A. Delcour; The effects of malting and mashing on barley protein extractability. Journal of Cereal Science 2006, 44, 203-211, 10.1016/j.jcs.2006.06.003.
- Charilaos Xiros; Evangelos Topakas; Petros Katapodis; Paul Christakopoulos; Hydrolysis and fermentation of brewer’s spent grain by Neurospora crassa. Bioresource Technology 2008, 99, 5427-5435, 10.1016/j.biortech.2007.11.010.
- Andrew J. Jay; Mary L. Parker; Richard Faulks; Fiona Husband; Peter Wilde; Andrew C. Smith; Craig B. Faulds; K. W. Waldron; A systematic micro-dissection of brewers’ spent grain. Journal of Cereal Science 2008, 47, 357-364, 10.1016/j.jcs.2007.05.006.
- J. Treimo; Bjørge Westereng; Svein Jarle Horn; Pirkko Forssell; James A. Robertson; Craig B. Faulds; Keith W. Waldron; Johanna Buchert; Vincent G. H. Eijsink; Enzymatic Solubilization of Brewers’ Spent Grain by Combined Action of Carbohydrases and Peptidases. Journal of Agricultural and Food Chemistry 2009, 57, 3316-3324, 10.1021/jf803310f.
- James A. Robertson; Kerry J.A. I'anson; J. Treimo; Craig B. Faulds; Tim F. Brocklehurst; Vincent G.H. Eijsink; Keith W. Waldron; Profiling brewers' spent grain for composition and microbial ecology at the site of production. LWT - Food Science and Technology 2010, 43, 890-896, 10.1016/j.lwt.2010.01.019.
- Khidzir, K.M.; Noorlidah, A.; Agamuthu, P. Brewery Spent Grain: Chemical Characteristics and utilization as an Enzyme Substrate. Malays. J. Sci. 2019, 29, 41–51
- Deborah M. Waters; Fritz Jacob; Jean Titze; Elke Arendt; Emanuele Zannini; Fibre, protein and mineral fortification of wheat bread through milled and fermented brewer’s spent grain enrichment. Zeitschrift für Lebensmittel-Untersuchung und Forschung 2012, 235, 767-778, 10.1007/s00217-012-1805-9.
- Nuno G. T. Meneses; Silvia Martins; José A. Teixeira; Solange I. Mussatto; Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Separation and Purification Technology 2013, 108, 152-158, 10.1016/j.seppur.2013.02.015.
- O.P. Sobukola; J.M. Babajide; O. Ogunsade; EFFECT OF BREWERS SPENT GRAIN ADDITION AND EXTRUSION PARAMETERS ON SOME PROPERTIES OF EXTRUDED YAM STARCH-BASED PASTA. Journal of Food Processing and Preservation 2012, 37, 734-743, 10.1111/j.1745-4549.2012.00711.x.
- K. Kemppainen; K. Rommi; U. Holopainen; K. Kruus; Steam explosion of Brewer’s spent grain improves enzymatic digestibility of carbohydrates and affects solubility and stability of proteins. Applied Biochemistry and Biotechnology 2016, 180, 94-108, 10.1007/s12010-016-2085-9.
- Dajun Yu; Yewei Sun; Wenjun Wang; Sean F. O’Keefe; Andrew P. Neilson; Hao Feng; Zhiwu Wang; Haibo Huang; Recovery of protein hydrolysates from brewer’s spent grain using enzyme and ultrasonication. International Journal of Food Science & Technology 2019, 55, 357-368, 10.1111/ijfs.14314.
- Sperandio, G.; Amoriello, T.; Carbone, K.; Fedrizzi, M.; Monteleone, A.; Tarangioli, S.; Pagano, M. Increasing the value of spent grain from craft microbreweries for energy purposes. Chem. Eng. Trans. 2017, 58, 487–492.
- Enweremadu, C.; Waheed, M.A.; Adekunle, A.A.; Adeala, A. The Energy Potential of Brewer’s Spent Grain for Breweries in Nigeria. Eng. Appl. Sci. 2008, 3, 175–177.
- Ancuța Chetrariu; Adriana Dabija; Brewer’s Spent Grains: Possibilities of Valorization, a Review. Applied Sciences 2020, 10, 5619, 10.3390/app10165619.
- Solange I. Mussatto; Brewer's spent grain: a valuable feedstock for industrial applications. Journal of the Science of Food and Agriculture 2014, 94, 1264-1275, 10.1002/jsfa.6486.
- N. Nikolopoulos; M. Agraniotis; I. Violidakis; E. Karampinis; Panagiotis Grammelis; Ch. Papapavlou; S. Tzivenis; E. Kakaras; Parametric investigation of a renewable alternative for utilities adopting the co-firing lignite/biomass concept. Fuel 2013, 113, 873-897, 10.1016/j.fuel.2013.03.034.
- Romanowska-Duda, Z.; Piotrowski, K.; Wolska, B.; Debowski, M.; Zielinski, M.; Dziugan, P.; Szufa, S. Stimulating Effect of Ash from Sorghum on the Growth of Lemnaceae—A New Source of Energy Biomass; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 341–349. ISBN 9783030138882
- Agnieszka Bala-Litwiniak; Monika Zajemska; Computational and experimental study of pine and sunflower husk pellet combustion and co-combustion with oats in domestic boiler. Renewable Energy 2020, 162, 151-159, 10.1016/j.renene.2020.07.139.
- Szufa, S.; Adrian, Ł.; Piersa, P.; Romanowska-Duda, Z.; Grzesik, M.; Cebula, A.; Kowalczyk, S. Experimental Studies on Energy Crops Torrefaction Process Using Batch Reactor to Estimate Torrefaction Temperature and Residence Time. In Renewable Energy Sources: Engineering, Technology, Innovation; Springer: Berlin/Heidelberg, Germany, 2018; pp. 365–373. ISBN 978-3-319-72370-9
- Szymon Szufa; Grzegorz Wielgosiński; Piotr Piersa; Justyna Czerwińska; Maciej Dzikuć; Łukasz Adrian; Wiktoria Lewandowska; M. Marczak; Torrefaction of Straw from Oats and Maize for Use as a Fuel and Additive to Organic Fertilizers—TGA Analysis, Kinetics as Products for Agricultural Purposes. Energies 2020, 13, 2064, 10.3390/en13082064.
- Marcin Jewiarz; Marek Wróbel; Krzysztof Mudryk; Szymon Szufa; Impact of the Drying Temperature and Grinding Technique on Biomass Grindability. Energies 2020, 13, 3392, 10.3390/en13133392.
- Thuat T. Trinh; Sebastian Werle; Khanh-Quang Tran; Aneta Magdziarz; Szymon Sobek; Marta Pogrzeba; Energy crops for sustainable phytoremediation – Thermal decomposition kinetics. Energy Procedia 2019, 158, 873-878, 10.1016/j.egypro.2019.01.224.
- José Ignacio Arranz; Teresa Miranda; Francisco José Sepúlveda; Irene Montero; Carmen Victoria Rojas; Analysis of Drying of Brewers’ Spent Grain. Proceedings 2018, 2, 1467, 10.3390/proceedings2231467.
- L.K. Stroem; D.K. Desai; A.F.A. Hoadley; Superheated steam drying of Brewer’s spent grain in a rotary drum. Advanced Powder Technology 2009, 20, 240-244, 10.1016/j.apt.2009.03.009.
- Axel Funke; Felix Ziegler; Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels, Bioproducts and Biorefining 2010, 4, 160-177, 10.1002/bbb.198.
- Moscicki, K.J.; Niedzwiecki, L.; Owczarek, P.; Wnukowski, M. Commoditization of wet and high ash biomass: Wet torrefaction—A review. J. Power Technol. 2017, 97, 354–369
- Ningbo Gao; Zongyang Li; Cui Quan; Norbert Miskolczi; Attila Egedy; A new method combining hydrothermal carbonization and mechanical compression in-situ for sewage sludge dewatering: Bench-scale verification. Journal of Analytical and Applied Pyrolysis 2019, 139, 187-195, 10.1016/j.jaap.2019.02.003.
- Mateusz Jackowski; Damian Semba; Anna Trusek; M. Wnukowski; Lukasz Niedzwiecki; Marcin Baranowski; Krystian Krochmalny; Halina Pawlak-Kruczek; Hydrothermal Carbonization of Brewery’s Spent Grains for the Production of Solid Biofuels. Beverages 2019, 5, 12, 10.3390/beverages5010012.
- Shule Wang; H. Persson; Weihong Yang; Pär Göran Jönsson; Pyrolysis study of hydrothermal carbonization-treated digested sewage sludge using a Py-GC/MS and a bench-scale pyrolyzer. Fuel 2020, 262, 116335, 10.1016/j.fuel.2019.116335.
- Małgorzata Wilk; Aneta Magdziarz; Kandasamy Jayaraman; Monika Szymańska-Chargot; Iskender Gökalp; Hydrothermal carbonization characteristics of sewage sludge and lignocellulosic biomass. A comparative study. Biomass and Bioenergy 2019, 120, 166-175, 10.1016/j.biombioe.2018.11.016.
- Aneta Magdziarz; Małgorzata Wilk; Mariusz Wądrzyk; Pyrolysis of hydrochar derived from biomass – Experimental investigation. Fuel 2020, 267, 117246, 10.1016/j.fuel.2020.117246.
- M. Toufiq Reza; Joan G. Lynam; M. Helal Uddin; Charles J. Coronella; Hydrothermal carbonization: Fate of inorganics. Biomass and Bioenergy 2013, 49, 86-94, 10.1016/j.biombioe.2012.12.004.
- C.I. Aragón-Briceño; Oliver R. Grasham; Andrew B. Ross; Valerie Dupont; M.A. Camargo-Valero; Hydrothermal carbonization of sewage digestate at wastewater treatment works: Influence of solid loading on characteristics of hydrochar, process water and plant energetics. Renewable Energy 2020, 157, 959-973, 10.1016/j.renene.2020.05.021.
- Marija Mihajlović; Jelena Petrović; Snežana Maletić; Marijana Kragulj Isakovski; Mirjana D. Stojanović; Zorica Lopičić; Snežana Trifunović; Hydrothermal carbonization of Miscanthus × giganteus : Structural and fuel properties of hydrochars and organic profile with the ecotoxicological assessment of the liquid phase. Energy Conversion and Management 2018, 159, 254-263, 10.1016/j.enconman.2018.01.003.
- S.A. Shafie; K.A. Al-Attab; Z.A. Zainal; Effect of hydrothermal and vapothermal carbonization of wet biomass waste on bound moisture removal and combustion characteristics. Applied Thermal Engineering 2018, 139, 187-195, 10.1016/j.applthermaleng.2018.02.073.
- M. Toufiq Reza; Janet Andert; Benjamin Wirth; Daniela Busch; Judith Pielert; Joan G. Lynam; Jan Mumme; Hydrothermal Carbonization of Biomass for Energy and Crop Production. Applied Bioenergy 2014, 1, 11-29, 10.2478/apbi-2014-0001.
- Tapas C. Acharjee; Charles J. Coronella; Victor R Vasquez; Effect of thermal pretreatment on equilibrium moisture content of lignocellulosic biomass. Bioresource Technology 2011, 102, 4849-4854, 10.1016/j.biortech.2011.01.018.
- Alexander Charnchai Louwes; Ruben B. Halfwerk; Eddy A. Bramer; Gerrit Brem; Experimental Study on Fast Pyrolysis of Raw and Torrefied Woody Biomass. Energy Technology 2020, 8, 1900799, 10.1002/ente.201900799.
- Alexander Charnchai Louwes; Lucia Basile; Riza Yukananto; J.C. Bhagwandas; Eduard A. Bramer; Gerrit Brem; Torrefied biomass as feed for fast pyrolysis: An experimental study and chain analysis. Biomass and Bioenergy 2017, 105, 116-126, 10.1016/j.biombioe.2017.06.009.
- A.V. Bridgwater; Review of fast pyrolysis of biomass and product upgrading. Biomass and Bioenergy 2012, 38, 68-94, 10.1016/j.biombioe.2011.01.048.
- M. Wnukowski; Paweł Owczarek; Łukasz Niedźwiecki; WET TORREFACTION OF MISCANTHUS – CHARACTERIZATION OF HYDROCHARS IN VIEW OF HANDLING, STORAGE AND COMBUSTION PROPERTIES. Journal of Ecological Engineering 2015, 16, 161-167, 10.12911/22998993/2950.
- Maurizio Volpe; Dominik Wüst; Fabio Merzari; Michela Lucian; Gianni Andreottola; Andrea Kruse; Luca Fiori; One stage olive mill waste streams valorisation via hydrothermal carbonisation. Waste Management 2018, 80, 224-234, 10.1016/j.wasman.2018.09.021.
- J. Poerschmann; B. Weiner; H. Wedwitschka; I. Baskyr; R. Koehler; F.-D. Kopinke; Characterization of biocoals and dissolved organic matter phases obtained upon hydrothermal carbonization of brewer’s spent grain. Bioresource Technology 2014, 164, 162-169, 10.1016/j.biortech.2014.04.052.
- Pablo J. Arauzo; Maciej P. Olszewski; A. Kruse; Hydrothermal Carbonization Brewer’s Spent Grains with the Focus on Improving the Degradation of the Feedstock. Energies 2018, 11, 3226, 10.3390/en11113226.
- Mateusz Jackowski; Łukasz Niedźwiecki; Magdalena Lech; M. Wnukowski; Amit Arora; Monika Tkaczuk-Serafin; Marcin Baranowski; Krystian Krochmalny; Vivek K. Veetil; Przemysław Seruga; et al.Anna TrusekHalina Pawlak-Kruczek HTC of Wet Residues of the Brewing Process: Comprehensive Characterization of Produced Beer, Spent Grain and Valorized Residues. Energies 2020, 13, 2058, 10.3390/en13082058.
- Maciej P. Olszewski; P.J. Arauzo; Mariusz Wądrzyk; A. Kruse; Py-GC-MS of hydrochars produced from brewer’s spent grains. Journal of Analytical and Applied Pyrolysis 2019, 140, 255-263, 10.1016/j.jaap.2019.04.002.
- Maciej P. Olszewski; Sabina A. Nicolae; Pablo J. Arauzo; Maria-Magdalena Titirici; Andrea Kruse; Wet and dry? Influence of hydrothermal carbonization on the pyrolysis of spent grains. Journal of Cleaner Production 2020, 260, 121101, 10.1016/j.jclepro.2020.121101.
- Solange I. Mussatto; G. Dragone; I.C. Roberto; Ferulic and p-coumaric acids extraction by alkaline hydrolysis of brewer's spent grain. Industrial Crops and Products 2007, 25, 231-237, 10.1016/j.indcrop.2006.11.001.
- Solange I. Mussatto; Marcela Fernandes; Adriane M. F. Milagres; Inês C. Roberto; Effect of hemicellulose and lignin on enzymatic hydrolysis of cellulose from brewer's spent grain. Enzyme and Microbial Technology 2008, 43, 124-129, 10.1016/j.enzmictec.2007.11.006.
- Elisabete Coelho; M. Angélica M. Rocha; Jorge A. Saraiva; Manuel A. Coimbra; Microwave superheated water and dilute alkali extraction of brewers’ spent grain arabinoxylans and arabinoxylo-oligosaccharides. Carbohydrate Polymers 2014, 99, 415-422, 10.1016/j.carbpol.2013.09.003.
- Sara Spinelli; Amalia Conte; Lucia Lecce; Lucia Padalino; Matteo Alessandro Del Nobile; Supercritical carbon dioxide extraction of brewer's spent grain. The Journal of Supercritical Fluids 2016, 107, 69-74, 10.1016/j.supflu.2015.08.017.
- Sofia F. Reis; Elisabete Coelho; Manuel A. Coimbra; Nissreen Abu-Ghannam; Improved efficiency of brewer’s spent grain arabinoxylans by ultrasound-assisted extraction. Ultrasonics Sonochemistry 2015, 24, 155-164, 10.1016/j.ultsonch.2014.10.010.
- Łukasiak, J.; Olsen, K.; Georgiou, C.A.; Georgakopoulos, D.G. Cereal arabinoxylans: Advances in structure and physicochemical properties. Eur. Food Res. Technol. 2013, 237, 33–48
- Elsa Vieira; M. Angélica M. Rocha; Elisabete Coelho; Olívia Pinho; Jorge A. Saraiva; Isabel M.P.L.V.O. Ferreira; Manuel A. Coimbra; Valuation of brewer's spent grain using a fully recyclable integrated process for extraction of proteins and arabinoxylans. Industrial Crops and Products 2014, 52, 136-143, 10.1016/j.indcrop.2013.10.012.
- Vaida Kitrytė; Andrius Šaduikis; Petras Rimantas Venskutonis; Assessment of antioxidant capacity of brewer’s spent grain and its supercritical carbon dioxide extract as sources of valuable dietary ingredients. Journal of Food Engineering 2015, 167, 18-24, 10.1016/j.jfoodeng.2014.12.005.
- Luís F. Guido; Manuela M. Moreira; Techniques for Extraction of Brewer’s Spent Grain Polyphenols: a Review. Food and Bioprocess Technology 2017, 10, 1192-1209, 10.1007/s11947-017-1913-4.
- Patricia Alonso-Riaño; María Teresa Sanz; Beatriz Blanco; Sagrario Beltrán; Ester Trigueros; Óscar Benito-Román; Water Ultrasound-Assisted Extraction of Polyphenol Compounds from Brewer’s Spent Grain: Kinetic Study, Extract Characterization, and Concentration. Antioxidants 2020, 9, 265, 10.3390/antiox9030265.
- Rares I. Birsan; Peter J Wilde; Keith W. Waldron; Dilip K. Rai; Recovery of Polyphenols from Brewer’s Spent Grains. Antioxidants 2019, 8, 380, 10.3390/antiox8090380.
- Yong Xing Tan; Wai Kit Mok; Jaslyn Jie Lin Lee; Jaejung Kim; Wei Ning Chen; Solid State Fermentation of Brewers’ Spent Grains for Improved Nutritional Profile Using Bacillus subtilis WX-17. Fermentation 2019, 5, 52, 10.3390/fermentation5030052.
- Antonio Zuorro; Annalaura Iannone; Roberto Lavecchia; Water–Organic Solvent Extraction of Phenolic Antioxidants from Brewers’ Spent Grain. Processes 2019, 7, 126, 10.3390/pr7030126.
- Lin Du; Pablo J. Arauzo; Maria Fernanda Meza Zavala; Zebin Cao; Maciej P. Olszewski; A. Kruse; Towards the Properties of Different Biomass-Derived Proteins via Various Extraction Methods. Molecules 2020, 25, 488, 10.3390/molecules25030488.
- Fen Qin; Astrid Z. Johansen; Solange I. Mussatto; Evaluation of different pretreatment strategies for protein extraction from brewer’s spent grains. Industrial Crops and Products 2018, 125, 443-453, 10.1016/j.indcrop.2018.09.017.
- Ronny Wahlström; Katariina Rommi; Pia Willberg-Keyriläinen; Dilek Ercili-Cura; Ulla Holopainen-Mantila; Jaakko Hiltunen; Outi Mäkinen; Heli Nygren; Atte Mikkelson; Lauri Kuutti; et al. High Yield Protein Extraction from Brewer's Spent Grain with Novel Carboxylate Salt - Urea Aqueous Deep Eutectic Solvents. ChemistrySelect 2017, 2, 9355-9363, 10.1002/slct.201701492.
