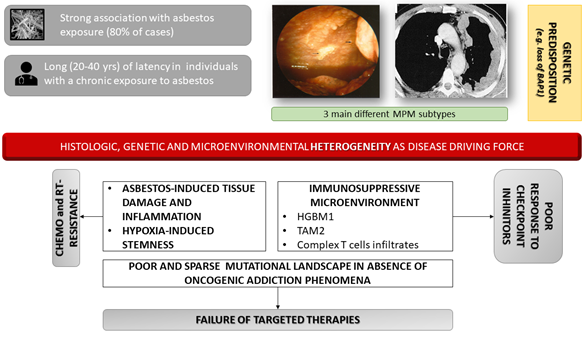
Mesothelioma is a malignancy of serosal membranes including the peritoneum, pleura, pericardium and the tunica vaginalis of the testes. Malignant mesothelioma (MM) is a rare disease with a global incidence in countries like Italy of about 1.15 per 100,000 inhabitants. Malignant Pleural Mesothelioma (MPM) is the most common form of mesothelioma, accounting for approximately 80% of disease. Although rare in the global population, mesothelioma is linked to industrial pollutants and mineral fiber exposure, with approximately 80% of cases linked to asbestos. Due to the persistent asbestos exposure in many countries, a worldwide progressive increase in MPM incidence is expected for the current and coming years. The tumor grows in a loco-regional pattern, spreading from the parietal to the visceral pleura and invading the surrounding structures that induce the clinical picture of pleural effusion, pain and dyspnea. Distant spreading and metastasis are rarely observed, and most patients die from the burden of the primary tumor. Currently, there are no effective treatments for MPM, and the prognosis is invariably poor. Some studies average the prognosis to be roughly one-year after diagnosis. The uniquely poor mutational landscape which characterizes MPM appears to derive from a selective pressure operated by the environment; thus, inflammation and immune response emerge as key players in driving MPM progression and represent promising therapeutic targets.
- asbestos
- genetics
- inflammation
Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
1. Introduction
Malignant Pleural Mesothelioma (MPM) is an aggressive cancer with very dismal prognosis from diagnosis whose pathogenesis is mainly associated with exposure to asbestos fibers. MPM was first linked to exposure to the industrial fiber asbestos in 1935 [1,2] [1][2] and it is now well documented that at least 80% cases are caused by exposure to asbestos fibers [1,3][1][3]. Due to its extreme versatility, asbestos became very popular in the 1970s and 80s to produce cements, tiles, yarn, toys, jewelry, pipe lining and more [3,4][3][4]. In addition, its temperature-resistant properties made it particularly appealing for the insulation and heating trades [3,5][3][5]. Unfortunately, these resistance properties have rendered the disposal of the carcinogenic asbestos-laden materials nearly impossible (fittingly, the word asbestos comes from the Greek word for “inextinguishable”), posing formidable epidemiological challenges [5]. MPM is still lacking effective therapies and median survival is around 13–15 months after diagnosis [5]. Growing evidence suggests that asbestos-induced damage is associated to the generation of an inflammatory microenvironment that may support tumor growth, possibly in association to genetic predisposition [6–8][6][7][8].
2. Epidemiology and Causative Agents
Though mesothelioma is rare and the production of materials with asbestos has been illegal for more than 20 years in many counties—although it is not banned in some others—the incidence of MPM is still rising. This is mainly due to the 20 to 40-year latency of the asbestos effects in an aging, genetically susceptible population. Since 1994 the World Health Organization (WHO) has been tracking epidemiologic data for Malignant Pleural Mesothelioma (MPM) [9]. From 1994 to 2008 the WHO mortality database found nearly 100,000 deaths from MPM in 83 countries with roughly 5 deaths per million. In addition, men are more frequently affected than women and the average age at death was 70 years old. Asbestos is perfectly safe in its primary state, where it is basically a type of solid rock, but is a significant health hazard when mined or worked in such a way as to produce the carcinogenic nanometer-scale fibrous particles that become an airborne material that is readily absorbed in the lungs. Asbestos fibers can be divided into two main groups: serpentines and amphiboles. Serpentine fibers have only one subtype, which are the chrysotile fibers, that are also called white asbestos due to their light color. These fibers are short and curly and make up around 95% of all commercially used asbestos. Amphibole fibers have many different kinds, including the crocidolite (blue asbestos) and amosite (brown asbestos), tremolite and others. Amphibole fibers are long and straight making them potentially more carcinogenic. Regardless, the International Agency for Research on Cancer (IARC) has classified both fibers equally as Class I carcinogens [10]. There is a known dose-response pattern of asbestos exposure with MM as well as lung cancer, but as stated by the IARC and the WHO, no safe lower threshold has been identified. Asbestos may also cause lung cancer and up to 20,000 asbestos-related lung cancers and 10,000 MM are estimated to occur annually across the population of Western Europe, Scandinavia, North America, Japan and Australia, while registrations are not available in areas that still use asbestos such as Eastern Europe, South America, Africa and the rest of Asia, including China [11,12][11][12]. Moreover, MPM has been associated with exposure to erionite, a zeolite mineral with some physical properties similar to asbestos which is widespread in some villages in Cappadocia (Turkey) and some areas of North America [13]. Comparably, a cluster of deaths from pleural mesothelioma has been reported for Biancavilla (Sicily), in Italy. Subsequent studies demonstrated that those MPM cases were related to the patient exposure to fluoro-edenite, a material extracted from quarries which features morphology and composition like that of minerals of the tremolite-actinolite series [14,15][14][15]. Thus, the awareness of the potential danger of new man-made and biopersistent fibers with similar carcinogenic properties, exemplified by carbon nanotubes should be strictly monitored to avoid novel epidemic [16]. Though asbestos is certainly the largest and most well-known cause of mesothelioma, roughly 20% of patients do not have any known exposure to asbestos. While it’s possible that these patients were unknowingly exposed, genetic analysis and other studies have led to the suspicion that chemicals such as nitrosamines, nitrosureas, potassium bromate, ferric saccharate, as well as genetic predisposition following chronic exposure to biopersistent minerals and radiation therapy [17] are all culprits [12–18][12][13][14][15][16][17][18]. Simian Virus 40 (SV40) infection was previously explored as aetiologic agent but it was not proven [19].
3. Pathologic Features
3.1. Conventional Histo-Pathology
Such variety of clinical and imaging presentation requires diagnosis to rely on immune-histochemical (IHC) markers: a mesothelioma should be positive for at least two mesothelial markers, and negative for at least two carcinoma-related markers [20][20][21]. The most effective combination of IHC markers seem to be: calretinin and cytokeratin 5/6 (or WT1) for the positive markers and CEA (carcinoembryonic antigen) and MOC-31, also known as Epithelial Specific Antigen/Ep-CAM (or B72.3, Ber-EP4, or BG-8) for the negative markers [20,21][20][21]. Though this is the current gold standard for MPM diagnosis, it is more easily applied to the epithelioid and biphasic subtypes (which comprise 75–95% of all diagnosis), and not the sarcomatous subtype. This is because the sarcomatous histotype is underrepresented in the literature due to its rarity therefore lacking specific markers [22–24][22][23][24]. Thus, an important issue is the heterogeneity of tumors (Figure 1). According to conventional morphology, MPM is divided into three main histological subtypes: epithelioid, sarcomatoid and mixed (biphasic), of which epithelioid is the most common. In the consensus statement for the 2017 diagnostic guidelines for mesothelioma, it was noted that a pleomorphic variant of MPM exists in more than 10% of epithelioid tumors, causing cells to behave similarly to the sarcomatoid and biphasic variants [25]. In ambiguous cases, a rare transitional mesothelioma (TM) pattern may be diagnosed by conventional pathology either as epithelioid, biphasic or sarcomatoid MPM. Morphologic characteristics that favor transitional pattern include sheet-like growth of cohesive, plump, elongated epithelioid cells with well-defined cell borders and a tendency to transition into spindle cells [26]. Detection of homozygous deletion of the CDKN2A(p16) gene compared to BAP1 loss through fluorescence in situ hybridization (FISH) on the spindle cell component could be useful to separate ambiguous cases from benign florid stromal reaction and distinguish true sarcomatoid component of biphasic MPM [27]. Very recently, RNA sequencing unsupervised clustering analysis revealed that TM grouped together and were closer to sarcomatoid than to epithelioid MPM [28]. Thus, rather than being separate histological entities, some authors theorize that the mutated cells of MPM progress according to the epithelial-to-mesenchymal transition (EMT). Under this model, epithelioid MPM is epithelial, sarcomatoid MPM is mesenchymal and biphasic MPM is in between the two. Interestingly, long non-coding RNA (lncRNA) fragments have been shown to play diverse roles in EMT and in aggressiveness of MPM and differential signatures which could distinguish between epithelioid and sarcomatoid differentiation have been reported [29].This theory has been supported by the worse prognosis associated with the sarcomatoid histotype as they are more differentiated from the original epithelium. Part of this switch involves the loss of important markers and regulators of cell function such as E-cadherin and β-catenin. Understanding the classification has diagnostic and prognostic importance, especially with the advent of genomic-based data. For example, Reyniès and colleagues used hierarchical clustering of transcriptomic data to divide MPM (108 frozen tumor samples) into two groups C1 and C2 based on the presence of epithelial and mesenchymal markers [30]. The C1 group corresponded to the histological classification of epithelioid MPM, while the C2 group contained epithelioid, biphasic, sarcomatoid and rarer, undifferentiated types. As expected, the C1 group was associated with a better prognosis than C2. This work demonstrates the importance of taking in mind that certain MPMs with a seemingly epithelioid histotype (theoretical less aggressive behavior) had the underlying genetics of a more aggressive tumor. Epithelial-to-mesenchymal transition (EMT) results in physiological and phenotypic changes which allow epithelial cells to acquire a mesenchymal phenotype. The molecular basis of EMT involves multiple changes in expression, distribution and/or function of transducers, including extracellular matrix and plasma membrane proteins such as periostin, vimentin, integrins, matrix metalloproteinases (MMPs) and cadherins, as well. Transforming Growth Factor β (TGF-β) plays a crucial role in promoting EMT. Indeed it has been reported in vitro that asbestos might induce EMT by downregulating the expression of epithelial markers (E-cadherin, β-catenin, and occluding), and contemporarily, by upregulating mesenchymal markers, such as fibronectin, α-SMA (Alpha-smooth muscle actin), and vimentin [31]. However, the exposure of MPM cells to growth factors such as FGF2 (Fibroblast Growth Factor 2) or EGF (Epidermal Growth Factor) can induce a fibroblastoid morphology, associated to invasive properties, namely scattering, decreased cell adhesion and increased invasiveness. This behavior is mainly related to Mitogen-Activated Protein (MAP)-kinase pathway activation and quite independent of TGF-β or Phosphoinositide-3 (PI3)-kinase signaling [32]. Subsequent microarray analysis demonstrated differential expression of MMP1 (Matrix metalloproteinase-1), ESM1 (Endothelial cell-Specific Molecule 1), ETV4 (ETS Variant Transcription Factor 4), PDL1 (Programmed Death-Ligand 1) and BDKR2B (Bradykinin Receptor B2) in response to both growth factors and in epithelioid vs sarcomatoid MPM. A protein expression analysis on tissue microarray from 352 MPM samples, demonstrated that High expression of membranous EGFR (Epidermal Growth Factor Receptor), integrin β1 and nuclear p27 correlated with epithelioid differentiation whereas high expression of cytoplasmic tumoral and stromal periostin with the sarcomatoid histotype [33]. Notably low expression of periostin in the tumour cell cytoplasm were found to be independent factors for better overall survival. Similarly, high expression of PTEN (Phosphatase and tensin homolog), which is known to be implicated in EMT in cancer [34], acts as positive prognostic factor. EMT is also mediated by hypoxia inducible factor 1 (HIF-1α) through expression of EMT transcription factors such as SNAIL, SLUG, and TWIST1 [35]. In a similar fashion, by modulating cadherin activation, acts mesothelin, which expression is able to promote an EMT-associated phenotype in MPM cells [36]. Moreover, calretinin, a Ca2+-binding protein, is implicated in inducing EMT, through the increase of focal adhesion kinase (FAK) expression and/or FAK phosphorylation in MPM cells [37]. Calretinin (CR), through a feedback loop, negatively regulates septin 7, an essential cellular component implicated in the final steps of cell division, as a strong Bt-dependent gene regulatory protein binding to the promoter of CALB2 [38]. Thus, both CR and septin 7 represent active transducers in MPM genesis and promising actionable targets, as well. Notably, those cells show a higher resistance to cisplatin due to increased Wnt signaling [39].[39]
Figure 1. Genetic and inflammatory-immune circuits associated to MPM (Malignant Pleural Mesothelioma) onset and progression. Malignant pleural mesothelioma is mainly associated to asbestos exposure and can develop after long latency with no known dose threshold. Notably, it is likely that some subjects are more genetically susceptible, but absence of dose threshold is not only due to this genetic susceptibility. No driver mutations are known to affect oncogenic drivers. Moreover, the unique tumor microenvironment is involved in inducing resistance to therapies that is usually characterizes clinical setting.
3.2. Genetic Aspects
In light of the lack of mechanistic explanations and sparse treatment options, genetic analysis is providing new hope in the fight against MPM. The genetics of MPM are complex and currently under investigation. This complexity is largely due to the uncommon genetic aberrations and inter-patient variability [5]. Mutations in oncogenes known to be driver of epithelial-derived solid cancers are extremely rare as next generation sequencing (NGS) deep studies have shown [40–42] [40][41][42] although sometimes with small number of samples (22 MPMs and matched blood samples) [43] and they seem to be not prognostically relevant in the disease [44]. Most alteration found affected the p53/DNA repair and phosphatidylinositol 3-kinase pathways [45], as well as genes involved at transcription level or expression data, such as SETDB1 [46]. The mutational landscape of MPM is with a signature consistent with production of reactive oxygen species (Figure 1). Notably, asbestos is able to induce chromosome damage and genomic DNA region losses [47,48][47][48]. Many reports have confirmed that TP53 and RB tumor suppressor genes are important in maintaining genetic homeostasis in MPM [5]. Although TP53 is vital for the integrity of the genome and thus its mutation can lead to a variety of cancers, TP53 mutations do not characteristically lead to MPM. Instead, loss-of-heterozygosity (LOH) analysis demonstrated that the two areas of the chromosome most frequently altered in MPM are CDKN2A–ARF at 9p21, and NF2 at 22q12 [5,49,50][49][50]. CDKN2A encodes for a cell-cycle regulator mutated in more common cancers like melanoma, and neurofibromatosis 2 (NF2) acts as tumor suppressor that is part of the NF2/Merlin complex that makes up the NF2/Hippo pathway [51,52][51][52]. Deletions of 3p21 region, enclosing BAP1 gene are also reported in 33 MPM bioptic samples [53]. LOH of the entire 3p21 region has been reported, whereas many of the deletions described were not contiguous, but rather they alternated along normal DNA segments, as in chromothripsis [54]. Roughly 40% of mesotheliomas have an NF2 mutation, causing hypophosphorylation of the YAP ( Yes-Associated Protein) transcriptional coactivator. YAP is active in its hypophosphorylated state, where it causes transcriptional activation of cell proliferation genes like cyclin D1 (CCDN1) and growth factors like connective tissue growth factor (CTGF). TAZ, encoding for Tafazzin,is a paralog to YAP, two major effectors of Hippo pathways. MPM is one of a few cancers (it has been demonstrated on 12 out of 14 MPM samples) that harbor mutations in Hippo pathway genes [55]. Thus, it would be expected that some patients with mesothelioma would show an activating mutation in TAZ, however in vivo evidence of such mutations is lacking. Absence of activating mutation for TAZ is also true for YAP [56]. BAP1 is a nuclear protein that regulates nuclear material, cell differentiation, gluconeogenesis, transcription, apoptosis. A germ-line mutation in BAP1 is thought to cause a syndrome that includes mesothelioma, uveal and cutaneous melanoma as well as other neoplasms [57]. When several family members all get mesothelioma, despite only one member of the family working near asbestos, the high incidence can be explained by the transmission of the fibers on the skin and clothes of the one family member to the other members; however, genetic analysis suggests BAP1 mutations may induce augmented susceptibility (Figure 1). Interestingly, BAP1 mutations seem to prime for epithelial MPM more than any other type, which has important implications for screening and prognosis [58]. Signal transduction pathways and growth factors that stimulate cell survival and proliferation are commonly mutated in cancer cells. In MPM, Extracellular Regulated Kinases (ERK)-dependent phosphorylated antigen, c-MET and the mTOR pathway have all been shown to be activated/enhanced [59]. We and others demonstrated that the activation of mTOR pathway is a prognostic factor for MPM, being phospho-mTOR expression associated to poor response to chemotherapy and shorter overall survival [60]. The HGF-receptor MET has been reported to be activated in MPM due to overexpressed [61], not related to the occurrence of MET genetic lesions: i) MET gene amplification are very infrequent [62] and ii) mutations in MET gene were not found in MPM by recent NGS studies supporting that MET mutations are really rare.
Within respect to gene copy number analysis, an interesting paper by Hylebos et al. analyzed an MPM-cohort (85 cases) for which genomic microarray data available through ‘The Cancer Genome Atlas’ (TCGA) and a validation cohort of 21 cases. Losses on chromosomes 1, 3, 4, 6, 9, 13 and 22 and gains on chromosomes 1, 5, 7 and 17 were found in at least 25% and 15% of MPMs, respectively. Besides the above described M-associated genes, CDKN2A, NF2 and BAP1, other interesting (and not previously described) genes carried a copy number loss (EP300, SETD2 and PBRM1) and four cancer-associated genes showed a high frequency of amplification (TERT, FCGR2B, CD79B and PRKAR1A) [63]. In mice combinatorial deletions of Bap1, Nf2, and Cdkn2a result in aggressive mesotheliomas, defined by stem cell-like potential [64]. Previous analysis from the same group revealed gene rearrangements in other unexpected candidate genes. Among them the mitogen-activated protein kinase kinase 6 gene (MAP2K6), which encodes a kinase that phosphorylates p38 in response to stress and inducing apoptosis. Another interesting candidate was dipeptidyl-peptidase 10 gene (DPP10) which impacts on cell cycle regulation by binding specific voltage-gated potassium channels and modulating their function. Finally, amplification of dihydrofolate reductase gene (DHFR) and pterin-4-α-carbinolamine dehydratase 2 (PCBD2) an enzyme important in folate metabolism, were detected [65].
Whole transcriptome analysis has been used to identify differential gene expression and clustering predictive and prognostic signatures in cancer. Single nucleotide variants were firstly detected on four MPM frozen samples compared to one lung adenocarcinoma and one normal lung sample through pyrosequencing analysis. They occurred in a number of genes, namely x-ray repair complementing defective repair in Chinese gene (XRCC6), ARP1 actin-related protein 1 homolog A, centractin α gene (ACTR1A), ubiquinol-cytochrome c reductase core protein 1 gene (UQCRC1), proteasome 26S subunit, non-ATPase 13 gene (PSMD13), PDZK1 interaction protein 1 gene (PDZK1IP1), collagen, type V α 2 gene (COL5A2), and matrix remodeling associated 5 gene (MXRA5) which encode for proteins that were either previously linked to a possible role in tumorigenesis or were found to be overexpressed in different human tumors [66]. Profiles of alternative splicing events have been also generated, such as those involving actin γ 2 smooth muscle enteric gene (ACTG2), cyclin dependent kinase 4 (CDK4), collagen, type III, α 1 gene (COL3A1), and thioredoxin reductase 1 gene (TXNRD1) [67]. The most well-studied species of the non-coding transcriptome are microRNAs (miRNAs) are known to modulate gene expression in cancer. With respect to MPM, miR-30b was found to be overexpressed in MPM and locates to 8q24, a frequently accessed region in mesothelioma. Likewise, miR-34 and miR-429 located at 1p36, as well as miR-203 located at 14q32, were not expressed in tumor samples and represented regions frequently affected by DNA copy-number loss [68,69][68][69]. Transcriptomic analysis has been also used to assess the differential transcriptional expression of wound-healing-associated genes in MPM during the EMT process [70,71][70][71]. Overall, 30 wound-healing-related genes were significantly deregulated, among which potential targets of hsa-miR-143, hsa-miR-223, and the hsa-miR-29 miRNA family members [72]. Out of those genes, ITGAV gene expression has been found to display prognostic value, been associated to lower overall survival. A comprehensive, multi-platform, genomic study of 74 MPM samples, as part of The Cancer Genome Atlas (TCGA) showed that poor prognosis subset showed higher aurora kinase A mRNA expression in association with upregulation of PI3K and mTOR signaling pathway [73]. The integrative analysis allowed the identification of prognosis clusters. For instance, poor prognosis signature had a high score for EMT-associated gene expression, which was characterized by high mRNA expression of VIM, PECAM1 and TGFB1, and low miR-200 family expression. These tumors also displayed MSLN promoter methylation and consequent low mRNA expression of mesothelin, which is a marker of differentiated mesothelial cells, as reported in sarcomatoid MPM and the sarcomatoid components of biphasic MPM [74]. Interestingly, the mRNA expression of VISTA (V-domain Ig suppressor of T cell activation), a negative immune checkpoint regulator primarily expressed on hematopoietic cells [75], was strongly inversely correlated with EMT score, being VISTA mRNA levels were highest in the epithelioid subtypes. Moreover, an unsupervised analysis of RNA-sequencing data of 284 MPMs identified a continuum of molecular profiles associated to disease prognosis. In particular, immune and vascular pathways emerged as the major sources of molecular variation, and specific profiles were detected: a hot bad-prognosis profile, with high lymphocyte infiltration and high expression of immune checkpoints and pro-angiogenic genes; a cold bad-prognosis profile, with low lymphocyte infiltration and high expression of pro-angiogenic genes; and a VEGFR2+/VISTA+ better-prognosis profile, with high expression of immune checkpoint VISTA and pro-angiogenic gene VEGFR2 [76].
It is well known that asbestos induces MPM also involving indirect effects, mainly oxidative stress associated to reactive oxygen species production and DNA-damage. These processes ultimately increase mutation rates and promote malignant transformation [77]. ROS (Reactive Oxygen Species) exposure induces methylation of the gene promoter via a specific recognition site to which DNMT1 (DNA methyltransferase 1) and PARP1 (Poly(ADP-Ribose) Polymerase 1) are recruited, linking DNA damage and DNA methylation. Prolonged ROS exposure induces demethylation by oxidizing the 5-methylcytosine to produce 5-hydroxymethylcytosine, which is catalyzed by ten-eleven translocation methylcytosine dioxygenase (TET) family of enzymes. Hypomethylation of genomic DNA is associated with genomic instability, which in combination with genetic alterations (chromosome deletion), contribute to malignant transformation [78–80][78][79][80]. These changes entail DNA oxidation events, post-translational modifications of histones proteins, and DNA methylation. Exposure to asbestos might affect miRNAs expression through epigenetic regulation: a first example regards miR-126. Its expression increases as an adaptive response to asbestos exposure and may proceed to the loss of its expression because of DNA damage accumulation and chromosome deletion, thus leading to carcinogenesis [81]. Interestingly, miR-103 was reported to be significantly down-regulated in the blood cell fraction of 23 patients with MPM, compared to 17 subjects formerly exposed to asbestos, and 25 healthy controls. The differential expression allowed discriminating between MPM patients and asbestos-exposed controls with a sensitivity of 83% and a specificity of 71% [82]. Similarly, the expression of miR-625-3p was reported to be significantly higher in plasma/serum of 30 MPM patients and allowed to discriminate between cases and controls, defined as 14 healthy subjects and 10 subjects with asbestosis [83]. More recently, MPM-specific RNA-based biomarker panels have been detected including DNA damage regulated autophagy modulator 1 (DRAM1) and arylsulfatase A (ARSA), together with their epigenetic regulators: the microRNA (miR-2053) and the lncRNA RP1-86D1.3 [84]. Overall, these circulating signatures should have important features such as low invasiveness and high specificity, which could play a critical role in next future early detection of MPM [85]. These findings give rise to novel attention to availability of compounds that modulate epigenetic modifications, such as histone acetylation or DNA methylation in therapeutic perspective.
3.3. Asbestos-Induced Carcinogenesis Mechanisms
The current concept is that tumor grows in a loco-regional pattern, spreading from the parietal to the visceral pleura and invading the surrounding structures that induce the clinical picture of pleural fluid, pain and dyspnea. Distant spreading and metastatization is rarely observed and most patients die from the local growth of the primary pleural mass. MPM has a uniquely moderate mutational landscape which appears to derive from a selective pressure operated by the environment [86]. Mesothelial cells are found in both the parietal and visceral pleura where they can sound the inflammatory alarm in the presence of pro-inflammatory material like asbestos fibers. It’s believed that when fibers are inhaled, they travel through the airways directly to the visceral pleura or arrive there via the lymphatic system [87]. Different fiber types are found in different areas of the lung, where they can disrupt the phagocytosis of the mesothelial cells, causing cytotoxicity, damaging DNA through oxidative stress and thus lead to an inflammatory response. The fibers themselves can cause aberrant separation of chromosomes during mitosis and direct activation of tyrosine kinase receptors, in absence of driver mutations [88,89][88][89]. Support that inflammation is a key aspect of MPM pathogenesis is that the lungs contain small milky spots of lymphoid patches (Kampmeiere’s foci) on the basal parietal pleura, which is the site where mesothelioma most frequently occurs. Preference for the parietal pleura over the visceral pleura may be rooted in differences in gene expression [5]. Finally, DNA repair/checkpoint genes are important for cancer genesis; these include BRCA2, TOP2A, BIRC5 (surviving) and CHECK1 (checkpoint kinase 1) as demonstrated on tissue microarray comprising 335 MPM patients [90]. Genetics may also point to the reason why some patients are exposed to asbestos and never develop MM, while a small percentage will. Within the pleural space, fibers can cause irritation and repeated cycles of tissue damage. The endpoint of asbestos-induced damage is the generation of an inflammatory microenvironment that may support tumor growth in individuals with predisposition, for instance due to loss of BAP1. Panou et al. demonstrated, by analyzing samples from 198 patients, that a significant proportion of them carry germline mutations in cancer susceptibility genes, as BAP1, CDKN2A, TMEM127, VHL and WT1 [7]. Moreover, it appears that mutations in two genes involved in DNA repair, XRCC1 and XRCC3, along with the GSTM1 (Glutathione S-Transferase Mu 1) antioxidant/detoxifying protein, increase susceptibility in this patient population, as demonstrated on more than 220 MPM samples and matched controls [91,92][91][92]. Researching the genetic basis underlying MM is fundamental to risk stratification, diagnosis, prognosis and, especially, the development of novel treatment strategies.
3.4. Inflammatory Microenvironment
Inflammation, like that created by asbestos, can prime the cellular terrain, creating a selective pressure that favors cells with an aggressive phenotype (Figure 1). Thus, inflammatory signaling molecules are also frequently enhanced in cancers, and MM is no exception. In mice, xenografts of human mesotheliomas cause inflammation before the development of the tumor [93]. Interleukins 1, 6 and 10, growth factors such as G-CSF, (HGF-Hepathocyte Growth Factor)/scatter factor, and vascular endothelial growth factor (VEGF), and chemokines like CCL2 (C-C motif ligand 2), CCL5, CXCL1 (C-X-C Motif Chemokine Ligand 1) and IFN-γ have all been increased/implicated in MPM pathogenesis. The High Mobility Group Box 1 protein (HMGB1) is a damage-associated molecular pattern (DAMP) protein and a key mediator of inflammation. It is involved in the early stages of mesothelial cell transformation upon exposure to asbestos and erionite by establishing an autocrine circuit influencing cell proliferation and survival [94]. Bianchi and colleagues demonstrated that under severe cellular stress, HMGB1 is relocated from the nucleus to the cytoplasm and then to secretory lysosomes or directly to the extracellular space. Then, the extracellular space triggers inflammation and adaptive immunological responses by switching among multiple oxidation states. Moreover, HMGB1 supports tissue repair and, by coordinating the switch of macrophages to a tissue-healing phenotype, activation and proliferation of stem cells and neoangiogenesis. Concomitantly, it enhances the immunogenicity of mutated proteins in the tumor (neoantigens) thus promoting anti-tumor responses [95]. Overall, inhibiting HMGB1 by a HMGB1 monoclonal antibodies (mAb), by the recombinant HMGB1 antagonist BoxA and by a mAb against the HMGB1 main receptor RAGE (Receptor for Advanced Glycation Endproducts) impair MPM progression in vitro and in animal models [96]. HMGB1 functions as a ‘master switch’ by which the chronic inflammation that drives mesothelioma growth is initiated and maintained. Overall HMGB1 plays a crucial role in MPM onset and progression according to the following mechanisms: i) asbestos-induced effector since its secretion by mesothelial or immune cells is highly responsive to asbestos fiber stimulation; ii) inflammatory and epithelial-to-mesenchymal transition mediator. For instance, it induces tumor necrosis factor-α secretion by macrophages thus triggering chronic peritumoral inflammation [97]. Moreover, HMGB1 can increase the expression of cadherins thus promoting cellular mesenchymal differentiation associated to malignant phenotype [98]. In this perspective the serum level of HMGB1 is considered to be a predictive biomarker for monitoring occupational workers and subjects at higher risk to develop MPM, although preliminary reports have been conducted in limited population [99,100][99][100]. Notably, it has been reported that therapeutic levels of aspirin and its metabolite salicylic acid can suppress growth, migration, invasion, wound healing, and anchorage-independent colony formation of HMGB1-secreting human mesothelioma cells [101,102][101][102]. Moreover, a number of inflammatory cells can be found in MPM-surrounding stroma. The vast majority (30%) is represented by macrophages expressing markers (like CD206) associated with tissue-healing phenotype M2. In contrast M1 macrophages (classically activated macrophages) shows pro-inflammatory, tissue destructive and anti-tumor activity. Tumor associated macrophages (TAMs) derive from circulating monocytic precursors. In MPM, monocytes are recruited by several chemokines and interleukines, such as IL-4, IL-13 and IL-10 produced by tumor infiltrating lymphocytes (TILs). The latter promote differentiation of macrophages towards an M2 phenotype [103]. A high ratio of intratumoral M2 macrophages is a negative prognostic factor in epithelioid MPM [104]. Complex T cell infiltrates are generally found. Myeloid-derived suppressor cells (MDSCs) CD33 and CD11b positive induce Tregs and produce nitric oxide and arginase, leading to loss of function of CD4+ and CD8+ T cells [105]. This immunosuppressive milieu ultimately promotes immune escape, tumor growth, invasion and angiogenesis [106]. Higher levels of TILs have been associated with better survival in MPM. Notably, numbers of CD45+ leukocytes were increased in non-epithelioid mesothelioma compared to epithelioid ones and seem to be associated with worse response to chemotherapy [107,108][107][108]. Higher fraction of FOXP3+ (Forkhead boX P3)/CD4+ Tregs have been reported in MPM, both chemotherapy-pretreated and untreated and is associated to worse prognosis [109]. In general, PD-L1-positive cells are heterogeneously present in MPM, being PD-L1 expression higher in non-epithelioid mesothelioma compared to epithelioid mesothelioma [110]. Dissecting the properties of these inflammatory cells within tumors will provide greater insights into the immunologic mechanisms of response and resistance to immunotherapy in this disease. Awad and colleagues showed, by applying flow cytometry to characterize 43 resected MPM specimens, distinct immunologic phenotypes in PD-L1–positive tumors as compared with PD-L1–negative ones, and in sarcomatoid/biphasic tumors vs epithelioid ones. Frequencies of T cells in the 38-patient cohort were highly variable, but showed a similar differentiation status and cellular composition, including a relatively high percentage of CD4+ T cells that expressed FOXP3 (~20%). In detail they found that PD-L1–positive and sarcomatoid/biphasic tumors have a significantly greater proportion of infiltrating T cells than PD-L1-negative and epithelioid tumors, respectively [111]. PD-L1-positive tumors also show significant increases in T-cell proliferation and activation, along with significant increases in Tregs and expression of T-cell-inhibitory markers, such as TIM-3 ( T cell immunoglobulin domain and mucin domain -3)[112]. The work by Klampatsa et al. extended the findings by analyzing fresh tumor and blood samples of 22 MPM cases and demonstrated high levels of the inhibitory receptor TIGIT (T cell immunoreceptor with Ig and ITIM domains) (~60%), CD39 (~20%), and CTLA4 (Cytotoxic T-Lymphocyte Antigen 4) (~25%) [113]. Overall, they found that MPM TILs were consistently hypofunctional, mainly associated with higher numbers of CD4 regulatory Tregs and with expression of TIGIT. Although the TILs showed uniformly high levels of cytokine production. The considerable immunophenotypic variability is coherent to the variable responses obtained in MPM by PD-L1 inhibitors [114], although other factors are involved: i) the abundance of infiltrating lymphocytes; ii) co-expression of multiple inhibitory receptors on T cells; iii) the influence of MDSCs and tumor-associated macrophages [115].
3.5. Serology
Non-invasive measures are coming to the surface and involve the analysis of various serum proteins using a 13-protein classifier as well as individually looking for soluble mesothelin-related peptides (SMRP), mesothelin, fibulin-3 and micro-RNAs in the plasma. The sensitivity and specificity of SMRP/Mesothelin has been debated in the literature but most studies seem to suggest that sensitivity and specificity are great enough to make them acceptable markers of tumor burden; they also correlate with disease severity as they’re most elevated in late disease states [5,116–118][116][117][118]. Using the Slow Off-rate Modified Aptamers (SOMA)-scan proteomic assay, a highly sensitive candidate 13-biomarker panel was discovered and validated (on 117 MPM samples and 142 asbestos-exposed control individuals) for the detection of MPM in the asbestos-exposed population with an accuracy of 92% and detection of 88% of Stage I and II disease [119].The 13-protein classifier uses short segments of DNA-like molecules that can bind to molecular targets and capture various inflammatory and proliferative proteins found in the serum when a patient is positive for mesothelioma. This method has promising results and potential usefulness in surveillance and diagnosis of MPM in those subjects at highest risk for the disease, but it has still not been validated for diagnosis. Fibulin-3 is an extremely sensitive and specific marker found in the plasma and in pleural effusion fluid that can identify patients with mesothelioma from those without it [120]. Osteopontin (OPN) is a glycoprotein known to be overexpressed in several human cancers. Interestingly, high circulating levels of OPN have been detected in samples from 56 MPM patients and serum OPN level may act as useful diagnostic marker for MPM patients [121]. More recently, the expression of brain-derived neurotrophic factor (BDNF), a neurotrophin, has been demonstrated in MPM. In detail high BDNF expression, at the mRNA level have been reported in tumors and at the protein level in pleural effusions (PE), thus becoming as a novel specific hallmark of MPM samples. Notably, high BDNF gene expression and PE concentration were predictive of shorter MPM patient survival in a cohort of 79 MPM tumor samples and 26 normal pleura. Moreover, BDNF activation is implicated in the PE-induced angiogenesis: this observation has potentially strong clinical implication and supports rationale of targeting angiogenesis in MPM [122]. Circulating cell-free RNA (MirRNA) fragments might serve as biomarkers in several diseases. Within respect to MPM, the MiR-625-3p has been found in the serum which has high stability and thus high diagnostic potential in a cohort of 15 MPM samples matched with 14 normal cases, weather the validation cohort was defined by 30 MPM and 10 asbestosis cases, respectively [83]. Its use as a specific and sensitive diagnostic marker is still under investigation. These markers, along with genomic data, as mentioned before, are shedding light on the possibility of new classifications and diagnostic methods.
References
- Robinson, B.M. Malignant pleural mesothelioma: an epidemiological perspective. Ann. Cardiothorac. Surg. 2012, 1, 491–496. doi:10.3978/j.issn.2225-319X.2012.11.04
- Gloyne, S. Roodhouse. Two Cases of Squamous Carcinoma of the Lung Occurring in Asbestosis. Tubercle 1935, 17, 5–10. doi:10.1016/s0041-3879(35)80795-2.
- Gilham, C.; Rake, C.; Burdett, G.; Nicholson, A.G.; Davison, L.; Franchini, A.; Carpenter, J.; Hodgson, J.; Darnton, A.; Peto, J. Pleural mesothelioma and lung cancer risks in relation to occupational history and asbestos lung burden. Occup. Environ. Med. 2016, 73, 290–299. doi:10.1136/oemed-2015-103074.
- Leigh, J.; Davidson, P.; Hendrie, L.; Berry, D. Malignant mesothelioma in Australia, 1945–2000. Am. J. Ind. Med. 2002, 41, 188–201. doi:10.1002/ajim.10047.
- Røe, O.D.; Stella, G.M. Malignant pleural mesothelioma: history, controversy and future of a manmade epidemic. Eur. Respir. Rev. 2015, 24, 115–131. doi:10.1183/09059180.00007014.
- Carbone, M.; Yang, H.; Pass, H.I.; Krausz, T.; Testa, J.R.; Gaudino, G. BAP1 and cancer. Nat. Rev. Cancer 2013, 13, 153–159. doi:10.1038/nrc3459.
- Panou, V.; Gadiraju, M.; Wolin, A.; Weipert, C.M.; Skarda, E.; Husain, A.N.; Patel, J.D.; Rose, B.; Zhang, S.R.; Weatherly, M.; et al. Frequency of Germline Mutations in Cancer Susceptibility Genes in Malignant Mesothelioma. J. Clin. Oncol. 2018; 36, 2863–2871. doi:10.1200/JCO.2018.78.5204.
- Attanoos, R.L.; Churg, A.; Galateau-Salle, F.; Gibbs, A.R.; Roggli, V.L. Malignant Mesothelioma and Its Non-Asbestos Causes. Arch. Pathol. Lab. Med. 2018, 142, 753–760. doi:10.5858/arpa.2017-0365-RA.
- Delgermaa, V.; Takahashi, K.; Park, E.K.; Le, G.V.; Hara, T.; Sorahan, T. Global mesothelioma deaths reported to the World Health Organization between 1994 and 2008. Bull. World Health Organ. 2011, 89, 716–724. doi:10.2471/BLT.11.086678.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Arsenic, metals, fibres, and dusts. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100(Pt C), 11–465.
- Mensi, C.; Riboldi, L.; De Matteis, S.; Bertazzi, P.A.; Consonni, D. Impact of an asbestos cement factory on mesothelioma incidence: global assessment of effects of occupational, familial, and environmental exposure. Environ. Int. 2015, 74, 191–199.
- Ngamwong, Y.; Tangamornsuksan, W.; Lohitnavy, O.; Chaiyakunapruk, N.; Scholfield, C.N.; Reisfeld, B.; Lohitnavy, M. Additive Synergism between Asbestos and Smoking in Lung Cancer Risk: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0135798. doi:10.1371/journal.pone.0135798.
- Carbone, M.; Baris, Y.I.; Bertino, P.; Brass, B.; Comertpay, S.; Dogan, A.U.; Gaudino, G.; Jube, S.; Kanodia, S.; Partridge, C.R.; et al. Erionite exposure in North Dakota and Turkish villages with mesothelioma. Proc. Natl. Acad. Sci. USA 2011, 108, 13618–13623. doi:10.1073/pnas.1105887108.
- Paoletti, L.; Batisti, D.; Bruno, C.; Di Paola, M.; Gianfagna, A.; Mastrantonio, M.; Nesti, M.; Comba, P. Unusually high incidence of malignant pleural mesothelioma in a town of eastern Sicily: an epidemiological and environmental study. Arch. Environ. Health 2000, 55, 392–398. doi:10.1080/00039890009604036.
- Comba, P.; Gianfagna, A.; Paoletti, L. Pleural mesothelioma cases in Biancavilla are related to a new fluoro-edenite fibrous amphibole. Arch. Environ. Health 2003, 58, 229–232. doi:10.3200/AEOH.58.4.229-232.
- Stella, G.M. Carbon nanotubes and pleural damage: perspectives of nanosafety in the light of asbestos experience. Biointerphases 2011, 6, P1–17. doi:10.1116/1.3582324.
- Farioli, A.; Ottone, M.; Morganti, A.G.; Compagnone, G.; Romani, F.; Cammelli, S.; Mattioli, S.; Violante, F.S. Radiation-induced mesothelioma among long-term solid cancer survivors: a longitudinal analysis of SEER database. Cancer Med. 2016, 5, 950–959. doi:10.1002/cam4.656.
- Jaurand, M.C.; Fleury-Feith, J. Pathogenesis of malignant pleural mesothelioma. Respirology 2005, 10, 2–8. doi:10.1111/j.1440-1843.2005.00694.x.
- Weiner, S.J.; Neragi-Miandoab, S. Pathogenesis of malignant pleural mesothelioma and the role of environmental and genetic factors. J. Cancer Res. Clin. Oncol. 2009, 135, 15–27. doi:10.1007/s00432-008-0444-9.
- Ordóñez, N.G. The immunohistochemical diagnosis of mesothelioma: a comparative study of epithelioid mesothelioma and lung adenocarcinoma. Am. J. Surg. Pathol. 2003, 27, 1031–1051. doi:10.1097/00000478-200308000-00001.
- Marchevsky, A.M. Application of Immunohistochemistry to the Diagnosis of Malignant Mesothelioma. Arch. Pathol. Lab. Med. 2008, 132, 397–401. doi:10.1043/1543-2165(2008)132.
- Panou, V.; Vyberg, M.; Weinreich, U.M.; Meristoudis, C.; Falkmer, U.G.; Røe, O.D. The established and future biomarkers of malignant pleural mesothelioma. Cancer Treat. Rev. 2015, 41, 486–495. doi:10.1016/j.ctrv.2015.05.001.
- Ascoli, V.; Minelli, G.; Cozzi, I.; Romeo, E.; Carnovale Scalzo, C.; Ancona, L.; Forastiere, F. Pathology reporting of malignant pleural mesothelioma first diagnosis: A population-based approach. Pathol. Res. Pract. 2016, 212, 886–892. doi:10.1016/j.prp.2016.07.010.
- Marchevsky, A.M.; LeStang, N.; Hiroshima, K.; Pelosi, G.; Attanoos, R.; Churg, A.; Chirieac, L.; Dacic, S.; Husain, A.; Khoor, A.; et al. The differential diagnosis between pleural sarcomatoid mesothelioma and spindle cell/pleomorphic (sarcomatoid) carcinomas of the lung: evidence-based guidelines from the International Mesothelioma Panel and the MESOPATH National Reference Center. Hum. Pathol. 2017, 67, 160–168. doi:10.1016/j.humpath.2017.07.015.
- Husain, A.N.; Colby, T.V.; Ordóñez, N.G.; Allen, T.C.; Attanoos, R.L.; Beasley, M.B.; Butnor, K.J.; Chirieac, L.R.; Churg, A.M.; Dacic, S.; et al. Guidelines for Pathologic Diagnosis of Malignant Mesothelioma 2017 Update of the Consensus Statement From the International Mesothelioma Interest Group. Arch. Pathol. Lab. Med. 2018, 142, 89–108. doi:10.5858/arpa.2017-0124-RA.
- Dacic, S.; Le Stang, N.; Husain, A.; Weynand, B.; Beasley, M.B.; Butnor, K.; Chapel, D.; Gibbs, A.; Klebe, S.; Lantuejoul, S.; et al. Interobserver variation in the assessment of the sarcomatoid and transitional components in biphasic mesotheliomas. Mod. Pathol. 2020, 33, 255–262. doi:10.1038/s41379-019-0320-y.
- Galateau Salle, F.; Le Stang, N.; Nicholson, A.G.; Pissaloux, D.; Churg, A.; Klebe, S.; Roggli, V.L.; Tazelaar, H.D.; Vignaud, J.M.; Attanoos, R.; et al. New Insights on Diagnostic Reproducibility of Biphasic Mesotheliomas: A Multi-Institutional Evaluation by the International Mesothelioma Panel From the MESOPATH Reference Center. J. Thorac. Oncol. 2018, 13, 1189–1203. doi:10.1016/j.jtho.2018.04.023.
- Galateau Salle, F.; Le Stang, N.; Tirode, F.; Courtiol, P.; Nicholson, A.G.; Tsao, M.S.; Tazelaar, H.D.; Churg, A.; Dacic, S.; Roggli, V.; et al. Comprehensive Molecular and Pathologic Evaluation of Transitional Mesothelioma Assisted by Deep Learning Approach: A Multi-Institutional Study of the International Mesothelioma Panel from the MESOPATH Reference Center. J. Thorac. Oncol. 2020, doi:10.1016/j.jtho.2020.01.025.
- Singh, A.S.; Heery, R.; Gray, S.G. In Silico and In Vitro Analyses of LncRNAs as Potential Regulators in the Transition from the Epithelioid to Sarcomatoid Histotype of Malignant Pleural Mesothelioma (MPM). Int. J. Mol. Sci. 2018, 19, 1297. doi:10.3390/ijms19051297.
- de Reyniès, A.; Jaurand, M.C.; Renier, A.; Couchy, G.; Hysi, I.; Elarouci, N.; Galateau-Sallé, F.; Copin, M.C.; Hofman, P.; Cazes, A.; et al. Molecular classification of malignant pleural mesothelioma: identification of a poor prognosis subgroup linked to the epithelial-to-mesenchymal transition. Clin. Cancer Res. 2014, 20, 1323–1334. doi:10.1158/1078-0432.CCR-13-2429.
- Turini, S.; Bergandi, L.; Gazzano, E.; Prato, M.; Aldieri, E. Epithelial to Mesenchymal Transition in Human Mesothelial Cells Exposed to Asbestos Fibers: Role of TGF-β as Mediator of Malignant Mesothelioma Development or Metastasis via EMT Event. Int. J. Mol. Sci. 2019, 20,150. doi: 10.3390/ijms20010150.
- Schelch, K.; Wagner, C.; Hager, S.; Pirker, C.; Siess, K.; Lang, E.; Lin, R.; Kirschner M.B.; Mohr, T.; Brcic, L.; et al. FGF2 and EGF induce epithelial-mesenchymal transition in malignant pleural mesothelioma cells via a MAPKinase/MMP1 signal. Carcinogenesis 2018, 39, 534–545. doi:10.1093/carcin/bgy018.
- Schramm, A.; Opitz, I.; Thies, S.; Seifert, B.; Moch, H.; Weder, W.; Soltermann, A. Prognostic significance of epithelial-mesenchymal transition in malignant pleural mesothelioma. Eur. J. Cardiothorac. Surg. 2010, 37, 566–572. doi:10.1016/j.ejcts.2009.08.027.
- Tamura, M.; Gu, J.; Tran, H.; Yamada, K.M. PTEN gene and integrin signaling in cancer. J. Natl. Cancer Inst. 1999; 91, 1820–1828. doi:10.1093/jnci/91.21.1820.
- Kim, M.C.; Cui, F.J.; Kim, Y. Hydrogen peroxide promotes epithelial to mesenchymal transition and stemness in human malignant mesothelioma cells. Asian Pac. J. Cancer Prev. 2013, 14, 3625–3630. doi:10.7314/apjcp.2013.14.6.3625.
- He, X.; Wang. L.; Riedel, H.; Wang, K.; Yang, Y.; Dinu, C.Z.; Rojanasakul, Y. Mesothelin promotes epithelial-to-mesenchymal transition and tumorigenicity of human lung cancer and mesothelioma cells. Mol Cancer. 2017, 16,63. doi:10.1186/s12943-017-0633-8.
- Wörthmüller, J.; Blum, W.; Pecze, L.; Salicio, V.; Schwaller, B. Calretinin promotes invasiveness and EMT in malignant mesothelioma cells involving the activation of the FAK signaling pathway. Oncotarget 2018, 9, 36256–36272. doi:10.18632/oncotarget.26332.
- Blum, W.; Pecze, L.; Rodriguez, J.W.; Steinauer, M.; Schwaller, B. Regulation of calretinin in malignant mesothelioma is mediated by septin 7 binding to the CALB2 promoter. BMC Cancer 2018, 18, 475. doi:10.1186/s12885-018-4385-7.
- Wörthmüller, J.; Salicio, V.; Oberson, A.; Blum, W.; Schwaller, B. Modulation of Calretinin Expression in Human Mesothelioma Cells Reveals the Implication of the FAK and Wnt Signaling Pathways in Conferring Chemoresistance towards Cisplatin. Int. J. Mol. Sci. 2019, 20, 5391. doi:10.3390/ijms20215391.
- Carbone, M.; Gaudino G, Yang H. Recent insights emerging from malignant mesothelioma genome sequencing. J. Thorac. Oncol. 2015, 10, 409–411. doi:10.1097/JTO.0000000000000466.
- Bueno, R.; Stawiski, E.W.; Goldstein, L.D.; Durinck, S.; De Rienzo, A.; Modrusan, Z.; Gnad, F.; Nguyen, T.T.; Jaiswal, B.S.; Chirieac, L.R.; et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat. Genet. 2016, 48, 407–416. doi:10.1038/ng.3520.
- Kiyotani, K.; Park, J.-H.; Inoue, H.; Husain, A.; Olugbile, S.; Zewde, M.; Nakamura, Y.; Vigneswaran, W.T. Integrated analysis of somatic mutations and immune microenvironment in malignant pleural mesothelioma. OncoImmunology 2017, 6, e1278330. doi: 10.1080/2162402X.2016.1278330.
- Guo, G.; Chmielecki, J.; Goparaju, C.; Heguy, A.; Dolgalev, I.; Carbone, M.; Seepo, S.; Meyerson, M.; Pass, H.I. Whole-exome sequencing reveals frequent genetic alterations in BAP1, NF2, CDKN2A, and CUL1 in malignant pleural mesothelioma. Cancer Res. 2014, 75, 264–269. doi:10.1158/0008-5472.CAN-14-1008.
- Mezzapelle, R.; Miglio, U.; Rena, O.; Paganotti, A.; Allegrini, S.; Antona, J.; Molinari, F.; Frattini, M.; Monga, G.; Alabiso, O.; et al. Mutation analysis of the EGFR gene and downstream signalling pathway in histologic samples of malignant pleural mesothelioma. Br. J. Cancer. 2013, 108, 1743−1749. doi:10.1038/bjc.2013.130.
- Kim, J.E.; Kim, D.; Hong, Y.S.; Kim, K.-P.; Yoon, Y.K.; Lee, D.H.; Kim, S.-W.; Chun, S.-M.; Jang, S.J.; Kim, T.W. Mutational Profiling of Malignant Mesothelioma Revealed Potential Therapeutic Targets in EGFR and NRAS. Transl. Oncol. 2018, 11, 268–274, doi:10.1016/j.tranon.2018.01.005.
- Kang, H.C.; Kim, H.K.; Lee, S.; Mendez, P.; Kim, J.W.; Woodard, G.; Yoon, J.H.; Jen, K.Y.; Fang, L.T.; Jones, K.; et al. Whole exome and targeted deep sequencing identify genome-wide allelic loss and frequent SETDB1 mutations in malignant pleural mesotheliomas. Oncotarget 2016, 7, 8321–8331. doi:10.18632/oncotarget.7032.
- Solbes, E.; Harper, R.W. Biological responses to asbestos inhalation and pathogenesis of asbestos-related benign and malignant disease. J. Investig. Med. 2018, 66, 721–727. doi:10.1136/jim-2017-000628.
- Xu, A.; Smilenov, L.B.; He, P.; Masumura, K.; Nohmi, T.; Yu, Z.; Hei, T.K. New insight into intrachromosomal deletions induced by chrysotile in the gpt delta transgenic mutation assay. Environ. Health Perspect. 2007, 115, 87–92. doi:10.1289/ehp.9425.
- Thurneysen, C.; Opitz, I.; Kurtz, S.; Weder, W.; Stahel, R.A.; Felley-Bosco, E. Functional inactivation of NF2/merlin in human mesothelioma. Lung Cancer 2009, 64, 140–147. doi:10.1016/j.lungcan.2008.08.014.
- Cheng, J.Q.; Lee, W.C.; A Klein, M.; Cheng, G.Z.; Jhanwar, S.C.; Testa, J.R. Frequent mutations of NF2 and allelic loss from chromosome band 22q12 in malignant mesothelioma: evidence for a two-hit mechanism of NF2 inactivation. Genes, Chromosom. Cancer 1999, 24, 238–242.
- Felley-Bosco, E. Special Issue on Mechanisms of Mesothelioma Heterogeneity: Highlights and Open Questions. Int. J. Mol. Sci. 2018, 19, 3560. doi:10.3390/ijms19113560.
- Sekido, Y. Targeting the Hippo Pathway Is a New Potential Therapeutic Modality for Malignant Mesothelioma. Cancers (Basel). 2018, 10, 90. doi:10.3390/cancers10040090.
- Yoshikawa, Y.; Emi, M.; Hashimoto-Tamaoki, T.; Ohmuraya, M.; Sato, A.; Tsujimura, T.; Hasegawa, S.; Nakano, T.; Nasu, M.; Pastorino, S.; et al. High-density array-CGH with targeted NGS unmask multiple noncontiguous minute deletions on chromosome 3p21 in mesothelioma. Proc. Natl. Acad. Sci. USA. 2016, 113, 13432–13437. doi:10.1073/pnas.1612074113.
- Tubio, J.M.C.; Estivill, X. Cancer: When catastrophe strikes a cell. Nature 2011, 470, 476–477. doi:10.1038/470476a.
- Sekido, Y.; I Pass, H.; Bader, S.; Mew, D.J.; Christman, M.F.; Gazdar, A.F.; Minna, J.D. Neurofibromatosis type 2 (NF2) gene is somatically mutated in mesothelioma but not in lung cancer. Cancer Res. 1995, 55, 1227–1231.
- Pulito, C.; Korita, E.; Sacconi, A.; Valerio, M.; Casadei, L.; Sardo, F.L.; Mori, F.; Ferraiuolo, M.; Grasso, G.; Maidecchi, A.; et al. Dropwort-induced metabolic reprogramming restrains YAP/TAZ/TEAD oncogenic axis in mesothelioma. J Exp Clin Cancer Res. J. Exp. Clin. Cancer Res. 2019, 38, 349. doi:10.1186/s13046-019-1352-3.
- Walpole, S.; Pritchard, A.; Cebulla, C.; Pilarski, R.; Stautberg, M.; Davidorf, F.H.; De La Fouchardière, A.; Cabaret, O.; Golmard, L.; Stoppa-Lyonnet, D.; et al. Comprehensive Study of the Clinical Phenotype of Germline BAP1 Variant-Carrying Families Worldwide. J. Natl. Cancer Inst. 2018, 110, 1328–1341. doi:10.1093/jnci/djy171.
- Cheung, M.; Testa, J.R. BAP1, a tumor suppressor gene driving malignant mesothelioma. Transl. Lung Cancer Res. 2017, 6, 270–278. doi:10.21037/tlcr.2017.05.03.
- Pignochino, Y.; Dell’Aglio, C.; Inghilleri, S.; Zorzetto, M.; Basiricò, M.; Capozzi, F.; Canta, M.; Piloni, D.; Cemmi, F.; Sangiolo, D.; et al. The combination of sorafenib and everolimus shows antitumor activity in preclinical models of malignant pleural mesothelioma. BMC Cancer 2015, 15, 374. doi:10.1186/s12885-015-1363-1.
- Bitanihirwe, B.K.; Meerang, M.; Friess, M.; Soltermann, A.; Frischknecht, L.; Thies, S.; Felley-Bosco, E.; Tsao, M.-S.; Allo, G.; De Perrot, M.; et al. PI3K/mTOR signaling in mesothelioma patients treated with induction chemotherapy followed by extrapleural pneumonectomy. J. Thorac. Oncol. 2014, 9, 239–247. doi:10.1097/JTO.0000000000000055.
- Kanteti, R.; Riehm, J.J.; Dhanasingh, I.; Lennon, F.E.; Mirzapoiazova, T.; Mambetsariev, B.; Kindler, H.L.; Salgia, R. PI3 Kinase Pathway and MET Inhibition is Efficacious in Malignant Pleural Mesothelioma. Sci. Rep. 2016, 6, 32992. doi:10.1038/srep32992.
- Bois, M.C.; Mansfield, A.; Sukov, W.R.; Jenkins, S.M.; Moser, J.C.; Sattler, C.A.; Smith, C.; Molina, J.R.; Peikert, T.; Roden, A.C. c-Met expression and MET amplification in malignant pleural mesothelioma. Ann. Diagn. Pathol. 2016, 23, 1–7. doi:10.1016/j.anndiagpath.2016.04.007.
- Hylebos, M.; Van Camp, G.; Vandeweyer, G.; Fransen, E.; Beyens, M.; Cornelissen, R.; Suls, A.; Pauwels, P.; Van Meerbeeck, J.P.; De Beeck, K.O. Large-scale copy number analysis reveals variations in genes not previously associated with malignant pleural mesothelioma. Oncotarget 2017, 8, 113673–113686. doi:10.18632/oncotarget.22817.
- Kukuyan, A.-M.; Sementino, E.; Kadariya, Y.; Menges, C.W.; Cheung, M.; Tan, Y.; Cai, K.Q.; Slifker, M.; Peri, S.; Klein-Szanto, A.J.; et al. Inactivation of Bap1 Cooperates with Losses of Nf2 and Cdkn2a to Drive the Development of Pleural Malignant Mesothelioma in Conditional Mouse Models. Cancer Res. 2019, 79, 4113–4123. doi:10.1158/0008-5472.CAN-18-4093.
- Hylebos, M.; Van Camp, G.; Van Meerbeeck, J.P.; De Beeck, K.O. The Genetic Landscape of Malignant Pleural Mesothelioma: Results from Massively Parallel Sequencing. J. Thorac. Oncol. 2016, 11, 1615–1626. doi:10.1016/j.jtho.2016.05.020.
- Dong, L.; De Rienzo, A.; Maulik, G.; Glickman, J.N.; Chirieac , L.R.; Hartman, M.L.; Taillon, B.E.; Du, L.; Bouffard, P.; Kingsmore, S.F.; et al. Transcriptome sequencing of malignant pleural mesothelioma tumors. Proc. Natl. Acad. Sci. USA 2008, 105, 3521–3526. doi:10.1073/pnas.0712399105.
- Dong, L.; Jensen, R.V.; De Rienzo, A.; Gordon, G.J.; Xu, Y.; Sugarbaker, D.J.; Bueno, R. Differentially expressed alternatively spliced genes in malignant pleural mesothelioma identified using massively parallel transcriptome sequencing. BMC Med. Genet. 2009, 10, 149. doi:10.1186/1471-2350-10-149.
- Taniguchi, T.; Karnan, S.; Fukui, T.; Yokoyama, T.; Tagawa, H.; Yokoi, K.; Ueda, Y.; Mitsudomi, T.; Horio, Y.; Hida, T.; et al. Genomic profiling of malignant pleural mesothelioma with array-based comparative genomic hybridization shows frequent non-random chromosomal alteration regions including JUN amplification on 1p32. Cancer Sci. 2007, 98, 438–446. doi:10.1111/j.1349-7006.2006.00386.x.
- Sage, A.P.; Martinez, V.; Minatel, B.; Pewarchuk, M.; Marshall, E.A.; Macaulay, G.M.; Hubaux, R.; Pearson, D.D.; Goodarzi, A.A.; Dellaire, G.; et al. Genomics and Epigenetics of Malignant Mesothelioma. High Throughput 2018, 7, 20. doi:10.3390/ht7030020.
- Blum, Y.; Jaurand, M.-C.; De Reyniès, A.; Jean, D. Unraveling the cellular heterogeneity of malignant pleural mesothelioma through a deconvolution approach. Mol. Cell. Oncol. 2019, 6, 1610322. doi:10.1080/23723556.2019.1610322.
- Mutsaers, S.; Birnie, K.; Lansley, S.; Herrick, S.E.; Lim, C.B.; Prêle, C.M. Mesothelial cells in tissue repair and fibrosis. Front. Pharmacol. 2015, 6, 113. doi:10.3389/fphar.2015.00113.
- Rouka, E.; Beltsios, E.; Goundaroulis, D.; Vavougios, G.D.; Solenov, E.; Hatzoglou, C.; Gourgoulianis, K.I.; Zarogiannis, S.G. In Silico Transcriptomic Analysis of Wound-Healing-Associated Genes in Malignant Pleural Mesothelioma. Medicina (Kaunas) 2019, 55, 267. doi:10.3390/medicina55060267.
- Hmeljak, J.; Sanchez-Vega, F.; Hoadley, K.A.; Shih, J.; Stewart, C.; Heiman, D.; Tarpey, P.; Danilova, L.V.; Drill, E.; Gibb, E.A.; et al. Integrative Molecular Characterization of Malignant Pleural Mesothelioma. Cancer Discov. 2018, 8, 1548–1565. doi:10.1158/2159-8290.CD-18-0804.
- Tan, K.; Kajino, K.; Momose, S.; Masaoka, A.; Sasahara, K.; Shiomi, K.; Izumi, H.; Abe, M.; Ohtsuji, N.; Wang, T.; et al. Mesothelin (MSLN) promoter is hypomethylated in malignant mesothelioma, but its expression is not associated with methylation status of the promoter. Hum. Pathol. 2010, 41, 1330–1338. doi:10.1016/j.humpath.2010.03.002.
- Nowak, E.C.; Lines, J.L.; Varn, F.S.; Deng, J.; Sarde, A.; Mabaera, R.; Kuta, A.; Le Mercier, I.; Cheng, C.; Noelle, R.J. Immunoregulatory functions of VISTA. Immunol. Rev. 2017, 276, 66–79. doi:10.1111/imr.12525.
- Alcala, N.; Mangiante, L.; Le Stang, N.; Gustafson, C.E.; Boyault, S.; Damiola, F.; Alcala, K.; Brevet, M.; Thivolet-Bejui, F.; Blanc-Fournier, C.; et al. Redefining malignant pleural mesothelioma types as a continuum uncovers immune-vascular interactions. EBioMedicine 2019, 48, 191–202. doi:10.1016/j.ebiom.2019.09.003.
- Benedetti, S.; Nuvoli, B.; Catalani, S.; Galati, R. Reactive oxygen species a double-edged sword for mesothelioma. Oncotarget 2015, 6, 16848–16865. doi:10.18632/oncotarget.4253.
- Valinluck, V.; Sowers, L.C. Endogenous cytosine damage products alter the site selectivity of human DNA maintenance methyltransferase DNMT1. Cancer Res. 2007, 67, 946–950. doi:10.1158/0008-5472.CAN-06-3123.
- De Vos, M.; El Ramy, R.; Quénet, D.; Wolf, P.; Spada, F.; Magroun, N.; Babbio, F.; Schreiber, V.; Leonhardt, H.; Bonapace, I.M.; et al. Poly(ADP-ribose) polymerase 1 (PARP1) associates with E3 ubiquitin-protein ligase UHRF1 and modulates UHRF1 biological functions. J. Boil. Chem. 2014, 289, 16223–16238. doi:10.1074/jbc.M113.527424.
- Zampieri, M.; Passananti, C.; Calabrese, R.; Perilli, M.; Corbi, N.; De Cave, F.; Guastafierro, T.; Bacalini, M.G.; Reale, A.; Amicosante, G.; et al. Parp1 Localizes within the Dnmt1 Promoter and Protects Its Unmethylated State by Its Enzymatic Activity. PLoS ONE 2009, 4, e4717. doi:10.1371/journal.pone.0004717.
- Tomasetti, M.; Gaetani, S.; Monaco, F.; Neuzil, J.; Santarelli, L. Epigenetic Regulation of miRNA Expression in Malignant Mesothelioma: miRNAs as Biomarkers of Early Diagnosis and Therapy. Front. Oncol. 2019, 9, 1293. doi:10.3389/fonc.2019.01293.
- Weber, D.G.; Johnen, G.; Bryk, O.; Jockel, K.-H.; Brüning, T. Identification of miRNA-103 in the cellular fraction of human peripheral blood as a potential biomarker for malignant mesothelioma--a pilot study. PLoS ONE 2012, 7, e30221. doi:10.1371/journal.pone.0030221.
- Kirschner, M.B.; Cheng, Y.Y.; Badrian, B.; Kao, S.C.; Creaney, J.; Edelman, J.J.B.; Armstrong, N.J.; Vallely, M.P.; Musk, A.W.; Robinson, B.W.; et al. Increased Circulating miR-625-3p: A Potential Biomarker for Patients With Malignant Pleural Mesothelioma. J. Thorac. Oncol. 2012, 7, 1184–1191. doi:10.1097/JTO.0b013e3182572e83.
- Matboli, M.; Shafei, A.E.; Ali, M.A.; Gaber, A.I.; Galal, A.; Tarek, O.; Marei, M.; Khairy, E.; El-Khazragy, N.; Anber, N.; et al. Clinical significance of serum DRAM1 mRNA, ARSA mRNA, hsa‐miR‐2053 and lncRNA‐RP1‐86D1.3 axis expression in malignant pleural mesothelioma. J. Cell. Biochem. 2018, 120, 3203–3211. doi:10.1002/jcb.27586.
- Ferrari, L.; Carugno, M.; Mensi, C.; Pesatori, A.C. Circulating Epigenetic Biomarkers in Malignant Pleural Mesothelioma: State of the Art and critical Evaluation. Front. Oncol. 2020, 10, 445. doi:10.3389/fonc.2020.00445.
- Blum, Y.; Meiller, C.; Quetel, L.; Elarouci, N.; Ayadi, M.; Tashtanbaeva, D.; Armenoult, L.; Montagne, F.; Tranchant, R.; Renier, A.; et al. Dissecting heterogeneity in malignant pleural mesothelioma through histo-molecular gradients for clinical applications. Nat. Commun. 2019, 10, 1333. doi:10.1038/s41467-019-09307-6.
- Miserocchi, G.; Sancini, G.; Mantegazza, F.; Chiappino, G. Translocation pathways for inhaled asbestos fibers. Environ. Health 2008, 7, 4. doi:10.1186/1476-069X-7-4.
- Krismann, M.; Muller, K.M.; Jaworska, M.; Johnen, G. Severe chromosomal aberrations in pleural mesotheliomas with unusual mesodermal features. Comparative genomic hybridization evidence for a mesothelioma subgroup. J. Mol. Diagn. 2000, 2, 209–216. doi:10.1016/S1525-1578(10)60639-3.
- Scattone, A.; Pennella, A.; Gentile, M.; Musti, M.; Nazzaro, P.; Buonadonna, A.L.; Marzullo, A.; Cavone, D.; Pollice, L.; Serio, G. Comparative genomic hybridisation in malignant deciduoid mesothelioma. J. Clin. Pathol. 2006, 59, 764–769. doi:10.1136/jcp.2005.026435.
- Arulananda, S.; Thapa, B.; Walkiewicz, M.; Zapparoli, G.V.; Williams, D.S.; Dobrovic, A.; John, T. Mismatch Repair Protein Defects and Microsatellite Instability in Malignant Pleural Mesothelioma. J. Thorac. Oncol. 2018, 13, 1588–1594. doi:10.1016/j.jtho.2018.07.015.
- Betti, M.; Ferrante, D.; Padoan, M.; Guarrera, S.; Giordano, M.; Aspesi, A.; Mirabelli, D.; Casadio, C.; Ardissone, F.; Ruffini, E.; et al. XRCC1 and ERCC1 variants modify malignant mesothelioma risk: a case-control study. Mutat. Res. Mol. Mech. Mutagen. 2011, 708, 11–20. doi:10.1016/j.mrfmmm.2011.01.001.
- Dianzani, I.; Gibello, L.; Biava, A.; Giordano, M.; Bertolotti, M.; Betti, M.; Ferrante, D.; Guarrera, S.; Betta, G.; Mirabelli, D.; et al. Polymorphisms in DNA repair genes as risk factors for asbestos-related malignant mesothelioma in a general population study. Mutat. Res. Mol. Mech. Mutagen. 2006, 599, 124–134. doi:10.1016/j.mrfmmm.2006.02.005.
- Hillegass, J.M.; Shukla, A.; Lathrop, S.A.; MacPherson, M.B.; Beuschel, S.L.; Butnor, K.J.; Testa, J.R.; Pass, H.I.; Carbone, M.; Steele, C.; et al. Inflammation precedes the development of human malignant mesotheliomas in a SCID mouse xenograft model. Ann. N. Y. Acad. Sci. 2010, 1203, 7–14.
- ube, S.; Rivera, Z.S.; Bianchi, M.E.; Powers, A.; Wang, E.; Pagano, I.; Pass, H.I.; Gaudino, G.; Carbone, M.; Yang, H. Cancer cell secretion of the DAMP protein HMGB1 supports progression in malignant mesothelioma. Cancer Res. 2012, 72, 3290–3301. doi:10.1158/0008-5472.CAN-11-3481.
- Bianchi, M.E.; Crippa, M.; Manfredi, A.A.; Mezzapelle, R.; Rovere Querini, P.; Venereau, E. High-mobility group box 1 protein orchestrates responses to tissue damage via inflammation, innate and adaptive immunity, and tissue repair. Immunol. Rev. 2017, 280, 74–82. doi:10.1111/imr.12601.
- Mukherjee, A.; Vasquez, K.M. Targeting chromosomal architectural HMGB proteins could be the next frontier in cancer therapy Cancer Res. 2020,doi:10.1158/0008-5472.CAN-19-3066.
- Yang, H.; Rivera, Z.; Jube, S.; Nasu, M.; Bertino, P.; Goparaju, C.; Franzoso, G.; Lotze, M.T.; Krausz, T.; Pass, H.I.; et al. Programmed necrosis induced by asbestos in human mesothelial cells causes high-mobility group box 1 protein release and resultant inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 12611–12616. doi:10.1073/pnas.100654210.
- Qi, F.; Okimoto, G.; Jube, S.; Napolitano, A.; Pass, H.I.; Laczko, R.; DeMay, R.M.; Khan, G.; I Tiirikainen, M.; Rinaudo, C.; et al. Continuous exposure to chrysotile asbestos can cause transformation of human mesothelial cells via HMGB1 and TNF-α signaling. Am. J. Pathol. 2013, 183, 1654–1666. doi:10.1016/j.ajpath.2013.07.029.
- Napolitano, A.; Antoine, D.J.; Pellegrini, L.; Baumann, F.; Pagano, I.S.; Pastorino, S.; Goparaju, C.M.; Prokrym, K.; Canino, C.; Pass, H.I.; et al. HMGB1 and Its Hyperacetylated Isoform are Sensitive and Specific Serum Biomarkers to Detect Asbestos Exposure and to Identify Mesothelioma Patients. Version 2. Clin. Cancer Res. 2016, 22, 3087–3096. doi:10.1158/1078-0432.CCR-15-1130.
- Tabata, C.; Shibata, E.; Tabata, R.; Kanemura, S.; Mikami, K.; Nogi, Y.; Masachika, E.; Nishizaki, T.; Nakano, T. Serum HMGB1 as a prognostic marker for malignant pleural mesothelioma. BMC Cancer 2013, 13, 205. doi:10.1186/1471-2407-13-205.
- Yang, H.; Pellegrini, L.; Napolitano, A.; Giorgi, C.; Jube, S.; Preti, A.; Jennings, C.J.; De Marchis, F.; Flores, E.G.; Larson, D.; et al. Aspirin delays mesothelioma growth by inhibiting HMGB1-mediated tumor progression. Cell Death Dis. 2015, 6, e1786. doi:10.1038/cddis.2015.153.
- Wang, Y.; Jiang, Z.; Yan, J.; Ying, S. HMGB1 as a Potential Biomarker and Therapeutic Target for Malignant Mesothelioma. Dis. Markers 2019, 2019, 4183157. doi:10.1155/2019/4183157.
- Minnema-Luiting, J.; Vroman, H.; Aerts, J.; Cornelissen, R. Heterogeneity in Immune Cell Content in Malignant Pleural Mesothelioma. Int. J. Mol. Sci. 2018, 19, 1041. doi:10.3390/ijms19041041.
- Cornelissen, R.; Lievense, L.A.; Maat, A.P.; Hendriks, R.W.; Hoogsteden, H.C.; Bogers, A.J.; Hegmans, J.P.; Aerts, J.G. Ratio of intratumoral macrophage phenotypes is a prognostic factor in epithelioid malignant pleural mesothelioma. PLoS ONE 2014, 9, e106742.
- Yap, T.A.; Aerts, J.G.; Popat, S.; Fennell, D.A. Novel insights into mesothelioma biology and implications for therapy. Nat. Rev. Cancer 2017, 17, 475–488.
- Burt, B.M.; Rodig, S.J.; Tilleman, T.R.; Elbardissi, A.W.; Bueno, R.; Sugarbaker, D.J. Circulating and tumor-infiltrating myeloid cells predict survival in human pleural mesothelioma. Cancer 2011; 117, 5234–5244.
- Suzuki, K.; Kadota, K.; Sima, C.S.; Sadelain, M.; Rusch, V.W.; Travis, W.D.; Adusumilli, P.S. Chronic inflammation in tumor stroma is an independent predictor of prolonged survival in epithelioid malignant pleural mesothelioma patients. Cancer Immunol. Immunother. 2011; 60, 1721–1728.
- Marcq, E.; Siozopoulou, V.; De Waele, J.; Van Audenaerde, J.; Zwaenepoel, K.; Santermans, E.; Hens, N.; Pauwels, P.; van Meerbeeck, J.P.; Smits, E.L. Prognostic and predictive aspects of the tumor immune microenvironment and immune checkpoints in malignant pleural mesothelioma. OncoImmunology 2016, 6, e1261241.
- Nishikawa, H.; Sakaguchi, S. Regulatory T cells in cancer immunotherapy. Curr. Opin. Immunol. 2014, 27, 1–7.
- Khanna, S.; Thomas, A.; Abate-Daga, D.; Zhang, J.; Morrow, B.; Steinberg, S.M.; Orlandi, A.; Ferroni, P.; Schlom, J.; Ferroni, P.; et al. Malignant Mesothelioma Effusions Are Infiltrated by CD3+ T Cells Highly Expressing PD-L1 and the PD-L1+ Tumor Cells within These Effusions Are Susceptible to ADCC by the Anti-PD-L1 Antibody Avelumab. J. Thorac. Oncol. 2016, 11, 1993–2005. doi:10.1016/j.jtho.2016.07.033.
- Awad, M.M.; Jones, R.E.; Liu, H.; Lizotte, P.H.; Ivanova, E.V.; Kulkarni, M.; Herter-Sprie, G.S.; Liao, X.; Santos, A.A.; Bittinger, M.A.; et al. Cytotoxic T Cells in PD-L1-Positive Malignant Pleural Mesotheliomas Are Counterbalanced by Distinct Immunosuppressive Factors. Cancer Immunol. Res. 2016, 4, 1038–1048. doi:10.1158/2326-6066.CIR-16-0171.
- Sakuishi, K.; Apetoh, L.; Sullivan, J.M.; Blazar, B.R.; Kuchroo, V.K.; Anderson, A.C. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 2010, 207, 2187–2194; Erratum in: J Exp Med. 2011, 208, 1331. doi:10.1084/jem.20100643.
- Klampatsa, A.; O’Brien, S.M.; Thompson, J.C.; Rao, A.S.; Stadanlick, J.E.; Martinez, M.; Liousia, M.; Cantu, E.; Cengel, K.; Moon, E.K.; et al. Phenotypic and functional analysis of malignant mesothelioma tumor-infiltrating lymphocytes. OncoImmunology 2019, 8, e1638211. doi:10.1080/2162402X.2019.1638211.
- Forde, P.M.; Scherpereel, A.; Tsao, A.S. Use of Immune Checkpoint Inhibitors in Mesothelioma. Curr. Treat. Options Oncol. 2019, 20, 18. doi:10.1007/s11864-019-0613-x.
- Marvel, D.; Gabrilovich, D.I. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J. Clin. Invest. 2015, 125, 3356–3364. doi:10.1172/JCI80005.
- Cristaudo, A.; Bonotti, A.; Guglielmi, G.; Fallahi, P.; Foddis, R. Serum mesothelin and other biomarkers: what have we learned in the last decade? J. Thorac. Dis. 2018, 10(Suppl. 2), S353–S359. doi:10.21037/jtd.2017.10.132.
- Creaney, J.; Dick, I.M.; Meniawy, T.; Leong, S.L.; Leon, J.S.; Demelker, Y.; Segal, A.; Musk, A.W.; Lee, Y.C.G.; Skates, S.J.; et al. Comparison of fibulin-3 and mesothelin as markers in malignant mesothelioma. Thorax 2014, 69, 895–902. doi:10.1136/thoraxjnl-2014-205205.
- Arnold, D.T.; De Fonseka, D.; Hamilton, F.W.; Rahman, N.M.; Maskell, N.A. Prognostication and monitoring of mesothelioma using biomarkers: a systematic review. Br. J. Cancer 2017, 116, 731–741. doi:10.1038/bjc.2017.22.
- Ostroff, R.; Mehan, M.R.; Stewart, A.; Ayers, D.; Brody, E.N.; Williams, S.A.; Levin, S.; Black, B.; Harbut, M.; Carbone, M.; et al. Early detection of malignant pleural mesothelioma in asbestos-exposed individuals with a noninvasive proteomics-based surveillance tool. PLoS ONE. 2012, 7, e46091. doi:10.1371/journal.pone.0046091.
- Pei, D.; Li, Y.; Liu, X.; Yan, S.; Guo, X.; Xu, X.; Guo, X. Diagnostic and prognostic utilities of humoral fibulin-3 in malignant pleural mesothelioma: Evidence from a meta-analysis.Oncotarget. Oncotarget 2017, 8, 13030–13038. doi:10.18632/oncotarget.14712.
- Bonotti, A.; Simonini, S.; Pantani, E.; Giusti, L.; Donadio, E.; Mazzoni, M.R.; Chella, A.; Marconi, L.; Ambrosino, N.; Lucchi, M.; et al. Serum mesothelin, osteopontin and vimentin: useful markers for clinical monitoring of malignant pleural mesothelioma. Int. J. Boil. Markers 2017, 32, e126–e131. doi:10.5301/jbm.5000229.
- Smeele, P.; d'Almeida, S.M.; Meiller, C.; Chéné, A.L.; Liddell, C.; Cellerin, L.; Montagne, F.; Deshayes, S.; Benziane, S.; Copin, M.C.; et al. Brain-derived neurotrophic factor, a new soluble biomarker for malignant pleural mesothelioma involved in angiogenesis. Mol. Cancer 2018, 17, 148. doi:10.1186/s12943-018-0891-0.