Relapse after apparent remission remains a major cause of death in patients with acute myeloid leukemia (AML). On the cellular level, leukemia relapse is considered to emerge from subpopulations of therapy-resistant leukemic stem cells (LSC). Identification and targeting of LSC are thus most important goals for AML treatment. However, AML and their LSC are highly heterogeneous.
- Acute Myeloid Leukemia,Stem Cells
- heterogeneity
- relapse
1. Introduction
Acute myeloid leukemia (AML) is a devastating, rapidly-evolving disease characterized by an abnormal proliferation of poorly-differentiated cells which impairs normal hematopoiesis. AML patients suffer from cytopenia associated with recurrent infections, anemia, easy bleeding, and other manifestations [1] and show highly variable responses to therapy and survival rates. Notably, a major cause of disease progression and relapse is the persistence of therapy-resistant, clonogenic leukemic subpopulations: the leukemic stem cells (LSC) [2].
In 1994, John Dick and colleagues were the first to prove the existence of human LSC in an in vivo experimental model. Human CD34+ leukemic cells were shown to repopulate the bone marrow (BM) of severe combined immunodeficient (SCID) mice, while CD34− leukemic blasts remained non-leukemogenic [3][4][3,4]. These CD34+ cells responsible for leukemia initiation and maintenance were termed LSC. Nowadays, they are documented as cells with enhanced capacities to selectively escape chemotherapy treatments [5] as well as immune surveillance [6], thus leading to disease relapse after therapy, a major cause of death in these patients. Since AML is highly heterogeneous with respect to genetic alterations, epigenetics, and leukemia cell of origin, it is not surprising that considerable heterogeneity is also observed among surface markers of AML cells and their LSC [2], making immunological targeting of LSC a constant challenge [7][8][9][7,8,9].
Hematopoiesis is organized hierarchically with a minor subset of hematopoietic stem cells (HSC) giving rise to all blood cells during the lifespan of an individual. HSC must balance regenerative requirements (which naturally involve cell division and differentiation) with the need to protect their own genomic integrity by reducing cell division. In order to achieve this, HSC undergo highly complex fine-tuned interactions with the BM microenvironment and interact with several other cell types (e.g., osteoblasts, stromal cells, endothelial cells, adipocytes, and neural cells) via soluble factors, biophysical forces, and cell-mediated interactions [10]. Similarly, LSC also reside and are influenced by the so-called BM niche, which sustains their quiescence and protects them from genotoxic stress [11][12][11,12].
AML is also organized hierarchically and contains subpopulations of LSC that share functional and molecular properties with their cells of origin, the healthy hematopoietic stem and progenitor cells (HSPC) [4][13][14][15][4,13,14,15]. Consistent with a close relationship between these two cell types, molecules expressed on healthy HSPC, i.e., CD34, were also reported to identify LSC [16]. Functionally, the CD34 family encompasses podocalyxin and endoglycan proteins and is considered to regulate cell differentiation, adhesion, trafficking, and proliferation [17]. CD34 is expressed on the vast majority of HSC, but rare CD34− HSC giving rise to CD34+ HSPC have also been reported [16].
In 2016, the LSC17 gene expression score was defined as the molecular LSC hallmark that predicts outcome and treatment resistance in patients with AML [18]. Among the genes highlighted in the LSC17 score were e.g., CD34 and the G protein-coupled receptor GPR56, a surface protein involved in cell adhesion which was also described to mark healthy HSC [16][19][16,19]. However, great phenotypic heterogeneity is observed in AML LSC and a wide range of surface markers has been found to identify LSC in only some, but not all AML (e.g., CD93, TIM3, CD44, CD123, etc. [9][20][21][22][23][24][25][26][9,20,21,22,23,24,25,26]).
2. The Relevance of Immunomodulatory Proteins for LSC Detection
Interestingly, a variety of antigens involved in LSC identification are in fact involved in immunological processes (Figure 1, Table 1). This suggests that LSC and non-LSC may have different interactions with the immune system. This notion has been substantiated by recent work from our research group demonstrating that LSC selectively escape immune surveillance by suppressing surface expression of NKG2D ligands (NKG2DL) [6]. When compared to corresponding non-stem leukemic blasts from the same patients, LSC lack expression of such ligands for activating NKG2D receptors on natural killer (NK) cells thereby evading NK-mediated lysis. In several AML patient samples of heterogeneous genetic backgrounds, lack of NKG2DL surface expression robustly distinguished LSC from other non-stem leukemic cells [6].
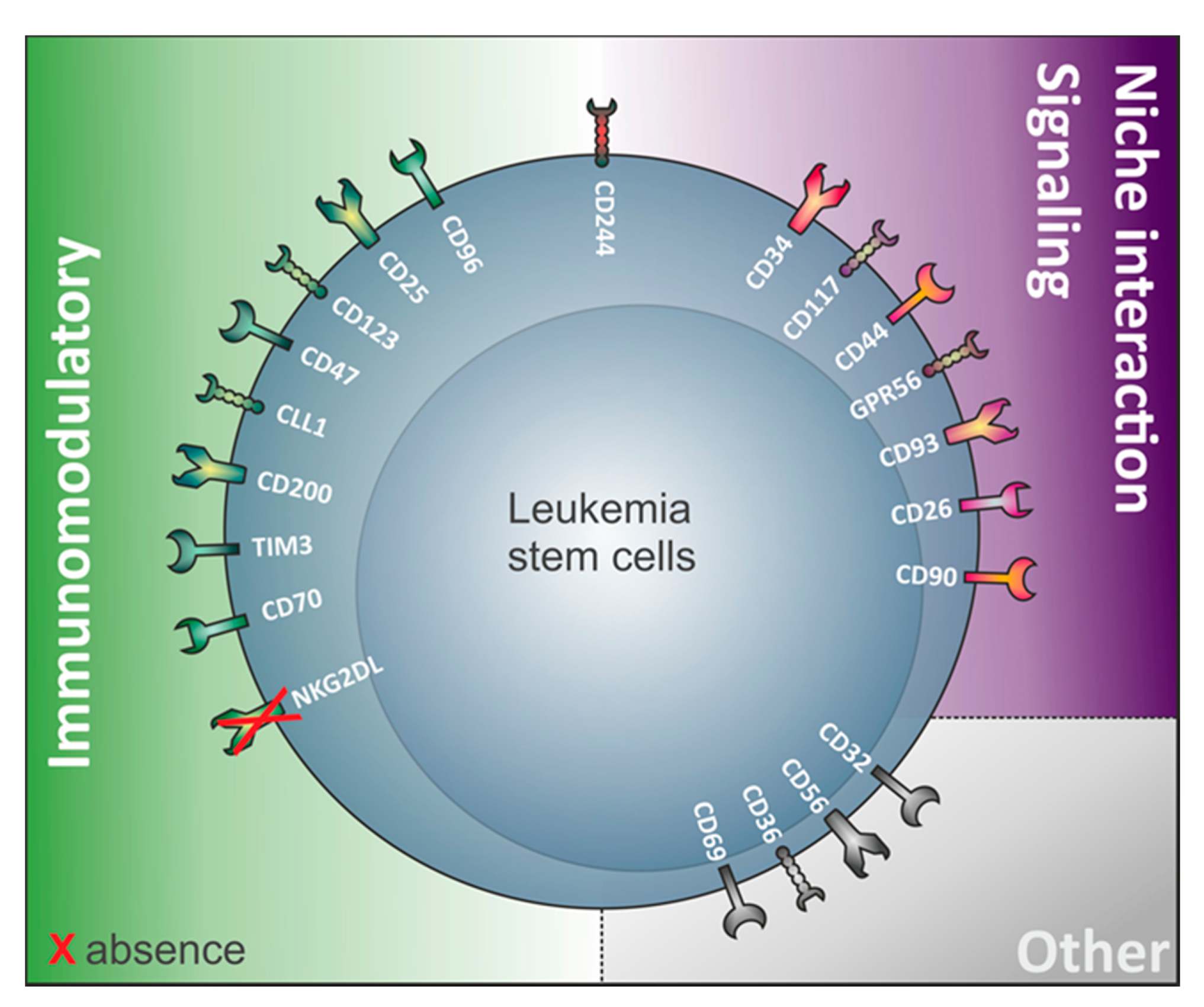
Table 1. Non-comprehensive list of human markers that can be found on LSC and their (potential) expression on the cell surface of other healthy blood cells. Highlighted in grey are the markers expressed on the cell surface of LSC from both CD34-expressing and non-expressing acute myeloid leukemia (AML). In white: markers only demonstrated to play roles in LSC from CD34 expressing AML. N.D: Not described/MPP: multipotential progenitor/MEP: megakaryocyte–erythroid progenitor).
| Antigen | Percentage of AML Patients Expressing the Marker | Expression on Non-LSC | Expression on HSC | Expression on Other Healthy Blood Cells | Function in Healthy Conditions | References | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CLL-1 | 92 | Yes | No | Monocytes, granulocytes, CMP, GMP | Modulates the activation state of cells during inflammation processes | Bakker et al. 2004 [27] Jiang et al. 2018 [28] Daga et al. 2019 [29] Marshall et al. 2006 [30] | Bakker et al. 2004 [57] Jiang et al. 2018 [58] Daga et al. 2019 [55] Marshall et al. 2006 [29] |
||||
| CD9 | 40 | Yes | No | Monocytes, macrophages, granulocytes, DC, endothelial cells, B, T, and NK cells | Cell migration, adhesion, activation, | Brosseau et al. 2018 [31] Touzet et al. 2019 [32] Paprocka et al. 2017 [33] | Brosseau et al. 2018 [59] Touzet et al. 2019 [47] Paprocka et al. 2017 [46] |
||||
| CD25 | 10–25 | Yes | No | T cells and regulatory T cells | Important role for T cells survival | Saito et al. 2010 [34] Kageyama et al. 2018 [35] Triplett et al. 2012 [36] | Saito et al. 2010 [60] Kageyama et al. 2018 [61] Triplett et al. 2012 [31] |
||||
| CD26 | N.D | Yes | No | T, B, NK, and myeloid cells | T cell activation and proliferation, cell adhesion, metabolism | Herrmann et al. 2020 [25] Klemann et al. 2016 [37] | Herrmann et al. 2020 [25] Klemann et al. 2016 [62] |
||||
| CD32 | 35 | Yes | No | Monocytes, B and T cells | Immune cell activation | Saito et al. 2010 [34] Anania et al. 2019 [38] | Saito et al. 2010 [60] Anania et al. 2019 [30] |
||||
| CD33 | 88 | Yes | Yes | Myeloid cells, lymphocytes, NK cells, MPP, GMP, MEP | Modulates inflammatory and immune responses by reducing tyrosine kinase dependent pathways | Ehninger et al. 2014 [39] Liu et al. 2007 [40] Laszlo et al. 2014 [41] Haubner et al. 2017 [24] | Ehninger et al. 2014 [63] Liu et al. 2007 [64] Laszlo et al. 2014 [65] Haubner et al. 2017 [24] |
||||
| CD34 | 70 | Yes | Yes | Mast cells, eosinophils, neurons, fibrocytes | Regulates cell differentiation, adhesion, trafficking and proliferation | Quek et al. 2016 [42] Engelhardt et al. 2002 [16] Nielsen et al. 2008 [17] | Quek et al. 2016 [36] Engelhardt et al. 2002 [16] Nielsen et al. 2008 [17] |
||||
| CD36 | N.D | Yes | No | Platelets, monocytes, adipocytes | Fatty acid uptake, angiogenesis, PRR recognition | Silverstein et al. 2009 [43] Sachs et al. 2020 [44] Herrmann et al. 2020 [25] | Silverstein et al. 2009 [66] Sachs et al. 2020 [67] Herrmann et al. 2020 [25] |
||||
| CD38 | 5–55 (FAB subtypes) |
Yes | No | T and B cells, monocytes, NK, granulocytes, platelets, red blood cells | Regulates calcium levels and NAD+ homeostasis | Hogan et al. 2019 [45] Sarry et al. 2011 [46] Goardon et al. 2011 [47] Keyhani et al. 2000 [48] | Hogan et al. 2019 [38] Sarry et al. 2011 [35] Goardon et al. 2011 [40] Keyhani et al. 2000 [68] |
||||
| CD44 | N.D | Yes | Yes | T cells, mesenchymal cells, ectodermal cells, neuron-like cells | Cell adhesion molecule, cellular signaling | Ponta et al. 2003 [49] Jin et al. 2006 [50] Bendall et al. 2000 [51] Herrmann et al. 2020 [25] | Ponta et al. 2003 [69] Jin et al. 2006 [70] Bendall et al. 2000 [71] Herrmann et al. 2020 [25] |
||||
| CD45RA | N.D | Yes | Yes | T and B cells | CD45 isoform, cell signaling | Kersten et al. 2016 [52] Goardon et al. 2011 [47] Sarry et al. 2011 [46] Holmes 2006 [53] | Kersten et al. 2016 [39] Goardon et al. 2011 [40] Sarry et al. 2011 [35] Holmes 2006 [41] |
||||
| CD47 | N.D | Yes | Yes | Various healthy cells | “don’t eat me” signal on cells in order to prevent inappropriate phagocytosis | Majeti et al. 2009 [54] Jaiswal et al. 2009 [55] Sick et al. 2012 | Majeti et al. 2009 [34 | [56 | ] Jaiswal et al. 2009 [33 | ] | ] Sick et al. 2012 [72] |
| CD56 | Up to 20 | Yes | No | DC, T and NK cells | Linked to NK cytotoxicity | Van Acker et al. 2017 [57] Sasca et al. 2019 [58] Herrmann et al. 2020 | Van Acker et al. 2017 [73 | [25 | ] Sasca et al. 2019 [74 | ] | ] Herrmann et al. 2020 [25] |
| CD69 | N.D | N.D | No | T cells | T cell differentiation, tissue retention, and metabolic reprogramming | Cibrián et al. 2017 [59] Sachs et al. 2020 [44] Herrmann et al. 2020 | Cibrián et al. 2017 [75 | [25 | ] Sachs et al. 2020 [67 | ] | ] Herrmann et al. 2020 [25] |
| CD70 | N.D | Yes | No | DC | T and B cell activation | Riether et al. 2015 [60] Riether et al. 2017 [61] Borst et al. 2005 | Riether et al. 2015 [76 | [62 | ] Riether et al. 2017 [77 | ] | ] Borst et al. 2005 [78] |
| CD90 | 40 (in elderly patients) | Yes | Yes | Fibroblasts, neurons, endothelial cells | Maintenance of HSC, cell adhesion, matrix adhesion | Buccisano et al. 2004 [63] Blair et al. 1997 [64] Brendel et al. 1999 [65] Kisselbach et al. 2009 [66] Craig et al. 1993 [67] | Buccisano et al. 2004 [79] Blair et al. 1997 [52] Brendel et al. 1999 [50] Kisselbach et al. 2009 [80] Craig et al. 1993 [53] |
||||
| CD93 | N.D | N.D | No (only on CD34-HSC) | Myeloid and endothelial cells | Mechanism in innate host defense | Bohlson et al. 2008 [68] Iwasaki et al. 2015 [69] Sumide et al. 2018 [70] | Bohlson et al. 2008 [81] Iwasaki et al. 2015 [82] Sumide et al. 2018 [83] |
||||
| CD96 | 27 | Yes | Only 5% | T and NK cells | Inhibits NK and T cells | Fatlawi et al. 2016 [71] Georgiev et al. 2018 [72] Hosen et al. 2007 [73] | Fatlawi et al. 2016 [84] Georgiev et al. 2018 [27] Hosen et al. 2007 [85] |
||||
| CD117 | 87 | Yes | Yes | GMP | Promotes HSC growth by binding the stem cell factor | Sperling et al. 1997 [74] Geissler et al. 1991 [75] Quek et al. 2016 [76] | Sperling et al. 1997 [86 | [42] Wells et al. 1996 | ] Geissler et al. 1991 [87] Quek et al. 2016 [36] Wells et al. 1996 [88] |
||
| CD123 | 97 | Yes | No | Basophils, plasmacytoid DC | Proliferation, survival, activation, and differentiation by binding respective ligand | Yu et al. 2016 [76] Guthridge et al. 1998 [77] Bras et al. 2019 [78] Haubner et al. 2019 [24] Al-Mawali et al. 2017 [79] | Yu et al. 2016 [88] Guthridge et al. 1998 [32] Bras et al. 2019 [45] Haubner et al. 2019 [24] Al-Mawali et al. 2017 [44] |
||||
| CD200 | N.D | Yes | Yes | Myeloid, T and B cells | Immunoregulatory molecule | Ngwa et al. 2019 [80] Ho et al. 2020 | Ngwa et al. 2019 [89 | [81] | ] Ho et al. 2020 [90] |
||
| CD244 | N.D | Yes | Yes | GMP, HSPC, granulocytes, monocytes, DC, NK and T cells | Regulates NK, T, and DC activation state | Zhang et al. 2017 [82] Haubner et al. 2019 [24] Quek et al. 2016 [42] Agresta et al. 2018 [83] | Zhang et al. 2017 [91] Haubner et al. 2019 [24] Quek et al. 2016 [36] Agresta et al. 2018 [92] |
||||
| GPR56 | N.D | No | Yes | Central nervous system, T cells | Frontal cortex development, NK inhibition, cell migration, HSC generation | Pabst et al. 2016 [84] Daga et al. 2019 [29] Kartalaei et al. 2015 [85] Huang et al. 2018 [86] | Pabst et al. 2016 [93] Daga et al. 2019 [55] Kartalaei et al. 2015 [94] Huang et al. 2018 [95] |
||||
| NKG2DL (its absence defines LSC) |
Highly variable | Yes | No | Not expressed on healthy cells | Upregulation of NG2DL on malignant or virus-infected cells resulting in their clearance by NK cells | Paczulla et al. 2019 [6] Zingoni et al. 2018 [87] | Paczulla et al. 2019 [6] Zingoni et al. 2018 [96] |
||||
| TIM-3 | 98 | Yes | No | T cells, monocytes, macrophages, DC, and mast cells | Homeostasis-maintaining molecule of the immune system | Jan et al. 2011 [88] Haubner et al. 2019 [24] Kikushige et al. 2010 [89] Han et al. 2013 [90] | Jan et al. 2011 [97] Haubner et al. 2019 [24] Kikushige et al. 2010 [98] Han et al. 2013 [28] |
Other examples of immunomodulatory proteins involved in LSC identification (Figure 1) include the immunoglobulin superfamily member CD96, a molecule expressed on healthy T and natural killer cells with known inhibitory roles on NK cells [72][27], TIM-3 (T cell immunoglobulin mucin-3), a homeostasis-maintaining molecule of the immune system expressed on the surface of CD4+ T type 1 helper cells (Th1) and CD8+ T type 1 cytotoxic cells, monocytes/macrophages, dendritic cells (DC), and mast cells [90][28], the lectin protein CLL-1 regulating cell activation during inflammation and CD32, an immune-activating immunoglobulin Fc receptor family member showing broad expression on hematopoietic cells [30][38][29,30]. Furthermore, the interleukin-2 receptor alpha-chain CD25 commonly expressed on activated and regulatory T cells, but also found on resting memory T cells [36][31], and CD123, the interleukin-3 receptor (IL-3R) alpha chain, which is part of the IL-3R system that includes interleukin-5 receptor (IL-5R) and granulocyte-macrophage colony stimulating factor receptor (GM-CSFR), are also found on LSC. While interleukin 2 is important for survival, activation, and proliferation of T cells, the IL-3R system influences proliferation, survival, and differentiation of hematopoietic cells and is involved in immunity and inflammatory response by specifically binding respective ligands (IL-3, IL-5, and GM-CSF) [77][32].
Last but not least, the immunoglobulin-like and integrin-associated protein CD47 was identified as a novel AML LSC marker [55][33]. CD47 serves as a ligand of signal regulatory protein-1 (SIRP-1) and thereby functions as a “don’t eat me” signal, protecting LSC from macrophage phagocytosis [54][34].
