Supercapacitors are energy storage devices with high power density, rapid charge/discharge rate, and excellent cycle stability. Carbon-based supercapacitors are increasingly attracting attention because of their large surface area and high porosity. Carbon-based materials research has been recently centered on biomass-based materials due to the rising need to maintain a sustainable environment. Cellulose and lignin constitute the major components of lignocellulose biomass. Since they are renewable, sustainable, and readily accessible, lignin and cellulose-based supercapacitors are economically viable and environmentally friendly.
- lignin
- cellulose
- nanofiber
- electrospinning
- supercapacitor
1. Introduction
Human activities, security, and industrialization depend on energy production and this energy is mostly derived from fossil fuels
Human work, welfare and industrialization rely on power generation, the most critical source of which is fossil fuels
[1]. However, heavy consumption of fossil fuels poses a serious environmental challenge due to the emission of CO2, SO2, and NOx into the atmosphere
. However, the significant environmental problem associated with the release of CO2, SO2 and NOx into the air presents for heavy consumption of fossil fuels
[2]. The World Energy Council (www.worldenergy.org) has estimated that by 2050, the world would demand twice its present energy consumption. Thus, a green approach to an effective energy production system that can replace fossil fuels is necessary
. The Global Energy Council has predicted that by 2050, the world will need to double its current demand, www.worldenergy.org. So it is important to follow a green solution for an efficient energy infrastructure that is capable of replacing fossil fuels
[2][3][4]. Recently, flexible supercapacitors are among the most investigated energy storage devices due to their high-power density, rapid charge/discharge rate, excellent cycle stability, and robust nature. The electrochemical double-layer capacitor (EDLC) is a specific type of energy storage system where the capacitance is taken from electrolyte adsorption and alignment at the interface between the electrolyte and a conductive porous electrode with a wide surface area
. Recently versatile supercapacitors, owing to the high power density, fast charge/discharge rate, excellent cycle stability and resilient design are among the most well-examined energy storage systems. The electrochemical dual-layer capacitor (EDLC) is a special energy storage device that uses the ability to absorb the electrolyte and align at the interface of the electrolyte and conductive electrodes with large surface area and suitable porosity
[5]. The efficiency of the EDLC is determined by the properties of its chosen electrode (active) materials. Electrospun carbonaceous materials are used as electrode materials, conducting additives, and supporting substrates in EDLCs due to their excellent electrical conductivity, large surface area, high porosity, mechanical flexibility, and chemical stability
. The EDLC's performance is based on the characteristics of the electrode (active) material selected. Due to their excellent electrical conductivity, wide surface area, high porosity, mechanical flexibility and chemical stability, Electrospun carbonaceous materials are used as electrode materials, conductive additives and supporting substrates in EDLCs
[6]. Due to its wide surface area and high porosity, carbon-based supercapacitors draw more and more interest. Recently, carbon-based materials research focuses on biomass materials because of the growing need for sustainable environmental protection. Owing to its high melting point, rich carbon content, and quick speed in pyrolysis, polyacrylonitrile (PAN) is the most suitable precursor for manufacturing high-performance carbon fibers (CFs) as compared to other common precursors (such as polyaniline, pitch, rayon, etc.). PAN precursor electrospinning accompanied by stabilization and carbonization can be used for producing carbon nanofibers (CNFs) having finer and more regulated fiber diameters
. Because of its vast area and high porosity, carbon-based supercapacitors are gaining ever more attention. Carbon-based materials research has recently centred on biomass materials due to rising environmental conservation needs. Polyacrylonitrile (PAN) is the most suitable precursor to high-performance carbon fibres (CFs), relative to other traditional precursors, because of its high melting point, rich carbon content and rapid speed. PAN (such as polyaniline, pitch, rayon, etc.). PAN Electrospinning with stabilisation and carbonization can be used for the production of fine and controlled fibre diameters of carbon nanofibers (CNFs)
[7]. However, PAN and other petroleum-based polymers are quite expensive
. The PAN and other petroleum polymers, however, are very costly [4] and releases toxic compounds during the carbonation process
[4]. Additionally, PAN, polybenzimidazole, or pitch, as carbon sources release toxic substances during carbonization [8]. It is therefore necessary to opt for greener carbon sources for the production of CNFs.
. Greener carbon sources must also be opted for in the manufacture of CNFs.
Biomass cellulose (mostly from cellulose acetate, ethyl cellulose, methyl cellulose, etc.), being the most abundant biological macromolecules in nature, is a good precursor material for electrospinning CNFs due to its good flexibility and essential properties
As the most plentiful biological macromolecules in the world, biomass celluloses (mainly from cellulose acetate, ethyl cellulose, methyl cellulose etc) are excellent precursors of CNFs due to their stability and basic properties
[9]. In the last few decades, CNFs has been fascinating, due to its abundance and low cost, while at the same time having special characteristics such as large surface area and high carbon content
. Natural d abundance and their low prices, despite possessing unique features such as wide surfaces and the high carbon content make CNFs highly desirable in recent years
[10]. Cellulose consists of carbon, hydrogen, and oxygen components, and its carbonization mainly releases carbon dioxide and water. Cellulose does not have an apparent melting point and after successful carbonization, it preserves its physical morphology
. Cellulose contains carbon, hydrogen, and oxygen elements and primarily releases carbon dioxide and water by its carbonization. Cellulose has no seeming melting point and preserves its physical morphology following effective carbonization
[8]. Compared to cellulose, it is easier to electrospin cellulose acetate into nanofibers and films because the solubility of cellulose acetate depends on the degree to which the acetate group is replaced
. Compared to cellulose, nanofibers and films contain cellulose acetate are far more simple to electrospin because cellulose acetate solubility relies on how much the acetate group is substituted
[11]. However, cellulose is a macromolecular polysaccharide, a large number of oxygen atoms in cellulose structure are highly autoxidized at high temperatures, thereby causing thermal instability. This thermal instability leads to morphology collapse during carbonization
. Cellulose is however a macromolecular polysaccharide, a significant number of cellulose structured oxygen atoms have strongly been autoxidised, and thermal instability is caused by these atoms. This thermal instability contributes to the breakdown of morphology when carbonized
[9]. The second most abundant bio-based polymer is lignin. It is obtained in large quantities from the pulp, paper, and bio-ethanol industries as a coproduct
. Lignin is the second richest bio-oriented polymer. In large amounts, it is obtained as a co-product from the wood, paper and bioethanol industries
[12]. Lignin is a rich carbon source due to its many aromatic subunits
. Because of its many aromatic subunits, lignin is a rich source of carbon precursor
[13]. Electrospun lignin-based CNFs have large surface area, excellent graphitic and porous structures, and superior chemical stability against corrosive elements
. Electrospun lignin-based CNFs have a large surface, excellent graphitic and pore structures and are highly stable against corrosive environments
[14]. However, the structural units in lignin are non-linear and are randomly interconnected by numerous C-C and ether bonds, which together help to make the lignin polymer poorly flexible
. The structural units in lignin are nevertheless non-linear and randomly linked with various C-C and Ether bonds which together lead to low flexibility of the lignin polymer
[15]. Thus, clean lignin materials are comparatively delicate and cannot be easily manipulated. Furthermore, the randomly distributed aliphatic chains separating the aromatic units in lignin reduces its tensile strength and moduli dramatically during thermal treatment
. Clean lignin materials are thus relatively delicate and cannot easily be handled. Besides, it is possible to drastically reduce its tensile strength by arbitrarily scattered aliphatic chains separating aromatic units in lignin and module during thermal treatment
[16]. Diversity of lignin biopolymers is another serious problem affecting batch reproducibility of lignin derived CNFs
. Another serious issue with the batch reproducibility of lignin-derived CNFs is the variety of lignin biopolymers
[16]. Nevertheless, lignin may be mixed with plasticizers in order to obtain good fibers
. However, to create healthy fibers, lignin may be combined with plasticizers
[13] .
.
CNFs are a recent research area, focused on growth in energy storage systems based on CNFs from biomass. The lignin/cellulose mix is a growing need to examine the analysis. To our knowledge, only a few papers have been written about electrospun lignin/cellulose nanofibers in energy storageInterestingly, the few studies show that lignin-based supercapacitors can be an exotic area of research interests based on carbon-biomass precursors in the field of supercapacitors. Furthermore, lignin and cellulose are green and durable and their extraction methods are simple, cost-effective and environmentally friendly. This new field must therefore be investigated very clearly. A lot of reviews have been published on electrospun lignin and nanofibers. On both lignin and cellulose nanofibers, very little systemic reporting is available. No review has been published of electro-punished nanofibers from this rich combination of biomass materials.
The study into CNFs made from a lignin/cellulose blend is a new research field that needs to be explored due to the rising need for energy storage devices based on biomass CNFs. As far as we know, only a few papers on electrospun lignin/cellulose nanofibers for energy storage have been published. Interestingly, the few studies show that supercapacitors based on lignin/cellulose may be an exotic area of research interest in the field of supercapacitors based on carbon biomass precursors. Besides, both lignin and cellulose are green and sustainable, and their extraction methods are very simple, economical, and eco-friendly. The need to investigate this new field is, therefore, very evident. Many reviews on electrospun lignin and cellulose nanofibers have been published. However, very few systemic reports are available on both lignin and cellulose nanofibers. In particular, no review of electrospun nanofibers from a blend of these abundant biomass materials has been published.
2. Studies on Electrospun Lignin and Cellulose Nanofibers
2. Studies on Electrospun Lignin and Cellulose Nanofibers
2.1. Electrospinning technique
2.1. Studies on Electrospun Cellulose Nanofibers
Cellulose is the most abundant natural macromolecule and the main ingredient of lignocellulose biomass. It has a high carbon content of around 44%, high stability, and outstanding porosity due to its hierarchical composition and highly functional rigid linear chains
Electrospinning is a lightweight nanofabrication technique based on repulsive electrostatic forces to produce a nanofibrous viscoelastic solution. Although several modern electrospinning systems developments exist, each configuration is based on three basic components: high voltage source, viscoelastic solution dispense mode and a fibre-recovery system[17]. Electrospinning variables may generally be grouped into three parameters: solution parameters (e.g. viscosity, surface tension, etc.), control parameters (e.g. flow rate, electric field, etc.) and environmental parameters (e.g. relative humidity, temperature etc),
[17][18]. Cellulose is well known as a precursor material for the processing of CNFs. In the late 60 s rayon (a fabric made from cellulose) based fibers became the first commercial carbon fibers
. When an electric field is present in a liquid droplet, an electrostatic charge is deposited at the tip of the droplet
[19]. Because cellulose does not dissolve in many organic solvents, cellulose nanofibers are prepared from different derivatives of cellulose such as cellulose acetate (CA) and ethyl cellulose (EC)
. The electrostatic force generated by the charge droplets withstands the surface tension at the end of the needle at high sufficiently high pressure; thus the Taylor cone formation, which releases the jet by the spinneret if the coulombic force is above surface tension[20]. The fibre diameter is dependent on the formation of Taylor's cone. It is associated with different experimental variables including the quality of the solvent and the viscosity of the suspension; injection speed, needle-collector distance and the voltage applied.
2.2. Studies on Electrospun Cellulose Nanofibers
The most prevalent natural macromolecule and the main component of the biomass in lignocellulose is cellulose. Its carbon content is roughly 44%, it is highly stable and porous, due to its hierarchical structure and highly functional linear rigid chains[21][22]. The first commercial carbon fibres were made in the late 60s (a cloth made of cellulose) [23]. Cellulose nanofibers are made from various cellulose derivates such as cellulose acetate (CA) and ethyl cellulose (EC)
[8]. In this study, 13 articles on electrospun cellulose nanofibers meet our inclusion criteria. Therefore, this section presents a review of these 13 articles on electrospun cellulose nanofibers.
2.2 Precursor Solution for Electrospun Cellulose Nanofibers
In CNF formation, the selection of an appropriate solvent is extremely significant. The right solvent that can evaporate quickly during the electrospinning process is required to facilitate jet elongation leading to nanofiber formation. Cellulose electrospinning solution is difficult to prepare because it is normally not soluble in most organic solvents. However, cellulose acetate (CA) is much easier to electrospin because the solubility of CA depends on the degree to which acetate group is replaced. Therefore, CA is the most widely used cellulose derivative to prepare cellulose nanofibers. According to one report, CA was dissolved in acetone and dimethyl acetamide (DMAc) to electrospin CA nanofibers followed by hydrolysis in NaOH/ethanol solution at room temperature for 12 h prior to thermal treatment
because they cannot dissolve in certain organic solvents. [7].
The selection of a suitable solvent is highly critical in the formation of CNF. To promote the jet elongation resulting in nanofibers formation, the correct solvent, which can evaporate quickly during the electrospinning phase, is needed. It is difficult to prepare a solution of cellulose electrospinning because in most organic solvents it is typically not soluble. The solubility of CA depends on when the acetate group is substituted, however, cells are easier to electrospin. Therefore the CA derivative is the most extensively used in the preparation of cellulose nanofibers. According to one study CA was dissolved at room temperature 12 hours before thermal treatment in acetone and dimethyl-acetamide (DMAc) for the electrolysis nanofibers CA followed by hydrolysis in NaOH / ethanol
[2024]. These nanofibers were easily electrospun because the mixture of DMAc and ethanol provided ample surface tension to interplay with the viscoelastic force, thereby forming the Taylor cone. As can be seen in SEM images in Figure 1, the resultant nanofibers show a melting phenomenon due to morphological collapse. The morphological collapse could be due to short soaking time in DMAc/ethanol solution leading to incomplete deacetylation. Formation of higher fibrous morphology could be attributed to increasing ZnCl2 content in the precursor solution. Ths reveals that ZnCl2 increases thermal and structural stability by efficient ion interlinking of CA macromolecules, thus promoting dehydrogenation.
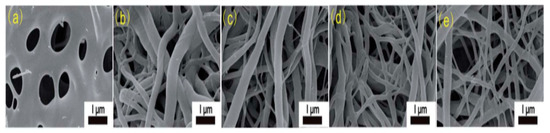
. These nanofibers were easily electrospun since DMAc and ethanol combined to generate enough surface tension, thus creating a Taylor cone. As can be seen in SEM images in figure 1, the resulting nanofibers display a morphological collapse causing a melting phenomenon. Due to the short soaking time in the DMAc/ethanol solution, morphological collapse may result in incomplete deacetylation. Increasing ZnCl2 content in the precursor solution may be ascribed to developing more fibrous morphology. Then Zncl2 shows that the powerful ions linkage of CA macromolecules improves thermal and structural stability thus facilitating dehydrogenation.

Figure 1.
SEM images of electrospun cellulose acetate nanofibers (dissolved in acetone/DMAc) at different ZnCl
2
content (
a
) CACNF-0, (
b
) CACNF-ZnCl
2
-2%, (
c
) CACNF-ZnCl
2
-5%, (
d
) CACNF-ZnCl
2
-10%, (
e
) CACNF-ZnCl
2-20%
-20% [24].
2.3. Studies on Electrospun Lignin Nanofibers
After cellulose, lignin is the second most abundant natural bioresource. Due to its rigid nature, it provides structural support to plants. The International lignin Institute reports that the pulp and paper industries and bio-refineries produce approximately 40 to 50 millions tonnes of lignin annually and about 300 billion metric tonnes of lignin per year globally. Lignin has a high content of carbon, approximately 60–65 per cent, suggesting the potential high yield following the production of carbon fibre
Studies on Electrospun Lignin Nanofibers
Lignin is the second most abundant natural bioresource material, after cellulose. It provides structural supports to plants because of its rigid nature. According to the International Lignin Institute, there are approximately 300 billion metric tons of lignin on earth with an annual estimated production of 40 to 50 million tons as by-products of the pulp and paper industries and biorefineries. Lignin has a high carbon content, almost 60–65%, which indicates a possible high yield following carbon fiber processing
. Kubo et al. have first published carbon fibres from lignin precursor material
. To remove spinnable content from lignin, the authors suggested the conversion of lignin into functional polymers by an effective separation method. The prepared fibres were carbonized to transform lignin fibres to carbon fibres after thermal stabilisation. At present, nanofibers of the electrospun lignin are either prepared with lignin blending mechanically or with lignin structure modified by chemical alteration
[2227]. The authors proposed the conversion of lignin into functional polymers by an efficient separation procedure to extract the spinnable material from lignin. After thermal stabilization, the prepared fibers were carbonized to convert lignin fibers to carbon fibers. Currently, electrospun lignin nanofibers are prepared either by physically blending lignin with other polymers or by chemically modifying lignin structure[2328][24]
29][2530][2631]. The papers that study electrospun lines nanofibers will be reviewed in this section.
Chemical Modification of Lignin Structure
The non-linear and arbitrarily intertwined various C-C and ether bonds, which make the lignin stiff and difficult to electrospin can be altered by introducing foreign elements to the lignin precursor solution. Tunnapat and Surawut
[27]. This section will analyze those articles that investigated electrospun lignin nanofibers.
Chemical Modification of Lignin Structure
Foreign elements can be introduced into lignin precursor solution to alter the non-linear and randomly entangled numerous C-C and ether bonds which makes lignin rigid and difficult to electrospin. Using a simple heated single spinneret system, Tunnapat and Surawut[23] were able to electrospin water-soluble lignin nanofiber by adding glycerol into the precursor solution. Addition of glycerol reduced surface tension of the precursor solution thereby improving the spinnability of lignin. At a higher glycerol ratio, the fiber diameter and BET surface area increase while electrical conductivity decreases. Decreased fiber diameter can be due to glycerol evaporation during thermal stabilization whereas decreased electrical conductivity may mean that glycerol plays a major role in altering the lignin structure and improving spinnability. Similarly, increased surface area at higher glycerol ratio can be attributed to successive evaporation of glycerol during thermal stabilization, reducing the resulting average fiber diameter. In another report, lignin/H
were able to apply glycerol to the precursor solution with a simple single heated spinning device to electrospin water-soluble nanofiber. The addition of glycerol reduced precursor solution surface tension, which increases the lignin spin. The fibre diameter and BET surface area increase with a higher glycerol ratio while electrical conductivity decreases. During thermal stabilisation, decreased fibres may be caused by the evaporation of glycerol, while reduced electrical conductivity could contribute to the essential role of glycerol in modifying lignin structure, thus increasing spinability Similarly, a higher glycerol surface area can be ascribed to constant evaporation of glycerol during thermal stabilisation, thereby reducing the resulting average fibre diameter. A further report produces lignin/H
3
PO
4 nanofibers were prepared via coaxial electrospinning technique of Alcell lignin in ethanol (core spinning solution) and pure ethanol (shell spinning solution)
nanofibers in ethanol (core spinning solution) and pure ethanol (shell spinning solution) by coaxial electrospinning technique of Alcell lignin
[12]. Electrospinning of lignin nanofiber was assisted by the presence of H
. The presence of H
3
PO
4 in the precursor solution. H
in the precursor solution enabled the electrospinning of lignin nanofibre. H
3
PO
4 could effectively dehydrate lignin phenolic-OH group to form phospholipid bonds between lignin molecules, thereby increasing the spinnability of lignin
can effectively dehydrate the lignin phenolic-OH group, thus increasing lignin spinnability to form phospholipid bonding between lignin molecules
. The use of Isophorone diisocyanate (IPDI) to the spinning solution was also used for the preparation of lignin nanofibers
[2932]. IPDI could link adjacent lignin molecules by establishing stable covalent bonds between them. SEM images (not shown) of lignin nanofibers prepared by either phosphating process or by covalent bonding neither have beads nor show evidence of morphological collapse after carbonization. Evidently, stable lignin fibers were formed because of continuous dehydration of phenolic hydroxyl groups in lignin by H
. By forming stable covalent relations between them, IPDI may bind the adjacent lignin molecules. SEM images (not shown) in lignin nanofibers made either by phosphating or covalent bonding have no beads or demonstrate morphological collapse after carbonization. Evident to the degree of phenolic hydroxyl groups in lignin, H
3
PO
4 and IPDI, respectively.
2.3. Studies on Electrospun Lignin/Cellulose Nanofibers
The fascinating characteristics of lignin and cellulose nanofibers such as low cost, high carbon performance, wide-field, mechanical stability, and environmental sustainability make them promising candidates for carbon-based EDLC electrode materials. Lignin and cellulose nanofibers can be combined into lignin/cellulose nanofibers to optimize the advantages of lignin and cellulose biomass materials as energy storage devices
and IPDI, continuously dehydrated, stable lignin fibres were formed
Physical blending of lignin with other binders
The weak electrical properties of the lignin-based electrode, despite the natural abundance of lignin and the lignin-based high surface area, contribute to poor electrochemical efficiency. Lignin can be combined with other binding polymers to enhance spinnability and morphological characteristics of lignin nanofibre-based electrode. Likewise, combining lignin with other binders improves the electrochemical properties of the nanofibers being produced. Due to the high versatility and fibre processing capability of PAN, it has been combined with lignin to produce strong plasticity nitrogen-rich nanofibers [33]. In another study, butyric anhydride (BA) has previously been applied to the lignin/PAN precursor solution[34]. Although PAN enhances electrospun nanofibre versatility, BA forms important bonds between hydroxyl groups in BA lignin anhydride groups. Other polymers blended with lignin to prepare electrospun lignin nanofibers include PVA [35], PMMA [36], PEO
and PVP
[2314]. To the best of our knowledge, only eight studies on carbon nanofibers derived from the physical blending of electrospun lignin and cellulose precursors were reported.
Lignin/Cellulose Acetate (CA) Blend
Lignin/CA nanofibers were obtained from continuous dehydration of phenolic hydroxyl group of lignin and cellulosic hydroxyl group to create electron-deficient oxygen atoms. These oxygen atoms can successfully combine with electron-rich atoms from binding agents to form stable bonds between lignin and cellulose acetate
. Inorganic compounds can equally be added into lignin precursor solution to modify lignin structure, enabling spinnability and improving quality and performance of resultant nanofiber. For instance, SiO2 improved interface miscibility of double capillary and tailored porosity
[2538]. Early attempts to prepare lignin/cellulose fibers met with little success due to the numerous formations of beaded fibers and subsequent collapse of fiber morphology. In 1997, Dave and Glasser reported that adding lignin to cellulose acetate butyrate disrupted the nanocrystalline order of the cellulosic phase
,
2.4. Studies on Electrospun Lignin/Cellulose Nanofibers
The fascinating characteristics of lignin and cellulose nanofibers such as low cost, high carbon performance, wide-field, mechanical stability, and environmental sustainability make them promising candidates for carbon-based EDLC electrode materials. Lignin and cellulose nanofibers can be combined into lignin/cellulose nanofibers to optimize the advantages of lignin and cellulose biomass materials as energy storage devices
[30]. Similarly, electrospun lignin/CA nanofibers with smooth and defect-free morphology were reported
. Physical blending of lignin and cellulose generally involves modifying the -OH groups phenolic group of lignin and cellulosic material (acetyl group in the case of cellulose acetate) to allow crosslinking reaction between lignin and cellulose macromolecules [27]
Lignin/Cellulose Acetate (CA) Blend
Nanofibers Lignin/CA have been obtained in order to establish electron-deficient oxygen atoms by the continuous dehydration of phenolic lignin hydroxyl group and cellulosic hydroxyl group. These oxygenatoms can combine successfully to form stable connections between lignin and cellulose acetate, with highly electron-rich atoms of binding agents [29]. Early attempts to prepare lignin/cellulose fibres were not successful due to the various beaded fibre formations and resulting fibre morphology meltdowns. The addition of lignin to cellulose acetate butyrate disturbed the order of nanocrystalline nature of the cellulosic phase in 1997 by Dave and Glasser [39]. Similarly, smooth, defect-free morphology of lignin/CA nanofibers have been reported
[3140]. However, fibrous morphologies of these nanofibers were completely lost after carbonization due to phase separation between lignin and cellulose macromolecules. The SEM images of the prepared nanofibers after and before carbonization are shown in Figure 2. Loss of fibrous morphology after carbonization was obviously due to weak interaction between lignin phenolic group and cellulose acetyl group resulting in phase separation.
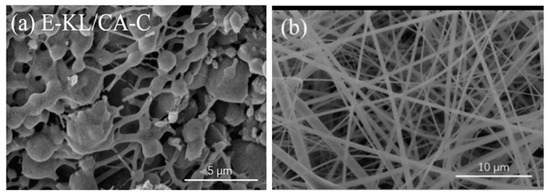
. Nevertheless, after carbonization, the fibrous morphologies of these nanofibers were completely lost due to the lignin-cellulose phase separation The SEM pictures of the prepared nanofibers are seen in Figure 2, after and before carbonization. The loss of fibrous morphology following carbonization was attributed to poor contact between the phenolic lignin group and the acetyl cellulose group, which culminated in the separation process.

Figure 2.
SEM images of electrospun lignin cellulose acetate nanofibers (
a
) after carbonization and (
b) before carbonization. Adapted with permission from ref
) before carbonization. Adapted with permission from ref [40].
Lignin/Nanocellulose (NC) Blend
Nanocellulose (also called nanoscale cellulose) is more defect-free, has greater surface area, more active functional groups, and enhanced chemical modification facility than conventional cellulose [41]. Based on their sources, preparation methods, and fiber morphologies, NC can be divided into three categories: (1) cellulose nanocrystals, (2) cellulose nanofibrils, and (3) bacterial cellulose [42]. Nanofibers have not yet been documented to pure electrospun lignin/NC. However, recent studies have been made about the influence of NC on electrospun lignin (mixed with other polymers such as PVA, PEO and PAN). M. Ago researched the effects of cellulose nanocrystals on electrospun lignin/PVA nanofibers interface properties [43][3144]
.
3. Conclusions
Biomass carbon nanofiber technology is one of the most active research areas in energy storage devices, especially EDLCs. The main objective of this systematic review is to highlight the state of the art research on electrospun lignin and cellulose nanofibers for application in supercapacitors and to provide directions of future research on the area. A rigorous scientific approach was employed to screen the eligibility of included articles and taxonomy of literature was provided from the most relevant articles included in the literature review. Apart from the articles in the taxonomy of literature, nine other articles were also included in our critical review and development of the research framework. These articles did not strictly meet our inclusion criteria, but they contain vital information that helped in developing the research framework. From our included literature, four main categories were discussed: reviews, studies on electrospun lignin nanofibers, cellulose nanofibers, and lignin/cellulose nanofibers. While studies on electrospun lignin and cellulose-based supercapacitors are considered adequate, very limited studies were reported on lignin/cellulose based supercapacitors. However, the reported studies on lignin/cellulose-based supercapacitors indicate a new research direction for biomass-based supercapacitors. From the articles reviewed in this study, researchers stated some major challenges related to large scale application of these special class of electrode materials. In the same vein, recommendations were given to mitigate these challenges. Specifically, comments and suggestions include:
-
Electrospun lignin and cellulose nanofiber-based electrodes have a wide surface area, good porosity, mechanical stability, and excellent cycle stability. However, the energy density of these electrode materials is quite low compared to other carbon-based electrode materials like PAN, graphene, etc. Similarly, carbon nanofibers produced from cellulosic precursors undergo morphological collapse during carbonization due to the low thermal stability of cellulose derivatives. Deacetylation of CA prior to thermal treatment improves the thermal stability of CA-based carbon nanofibers.
-
Electrospinning lignin into nanofibers is unrealistic due to the hierarchical and entangled structure of lignin. However, lignin can be easily blended with other binders to produce fine lignin-based nanofibers. Different binders influence electrospun lignin nanofiber differently.
-
Electrospinning lignin and cellulose precursor solution resulted in nanofiber with phases separated into lignin and cellulose domains. However, incorporating a suitable crosslinker resolves the problem of phase separation. Resultant nanofibers exhibit thermal stability of lignin and flexibility of CA.
-
Lignin/cellulose nanofiber, if explored extensively, can serve as ideal electrode materials for next-generation supercapacitors as an alternative to petroleum-based carbon nanofibers
. They also documented that cellulose nanocrystals decrease the phase-separated domains. This may be due to lignin-PVA matrix molecular movement. Therefore we can conclude that cellulose nanocrystal may have decreased polymer dispersion below the solvent evaporation rate, decreasing the nanofibers' phase separation.
3. Conclusions
Carbon nanofibre-based biomass technology is one of the most active energy storage research areas, particularly in the EDLC. Whereas studies of lignin and supercapacitors based on electrospun are considered to be adequate, very limited studies of supercapacitors based on cellulose/lignin were reported. The reported study of supercapacitors based on lignin/cellulose however suggests new research directions for supercapacitors based on biomass. Researchers have identified certain major challenges associated with the wider use of this special class of electrode materials from the papers reviewed in this study.
-
Nanofiber electrodes based on electrospun lignin and cellulose precursor have a wide area, good porosity, mechanical stability and excellent cycle stability. However, in comparison to other carbon-based materials, such as PAN, graphene etc the energy density of these electrode materials is quite low.
-
The hierarchy and complex arrangement of lignin make the electrospinning lignin to nanofibers very difficult. However, lignin can easily be mixed with additional binders to create nanofibers based on fine lignin. The electrospun lignin nanofibers are influenced by various binders in different ways.
-
The precursor solution of electrospinning lignin and cellulose formed nanofibers with segregated phases into cellulose and lignin domains. The use of an appropriate crosslinker, however, solves the phase separation problem. Thermal lignin stability and CA flexibility are presented by resulting nanofibers.
-
When extensively explored, lignin/cellulose nanofiber can serve as the ideal alternative to petroleum carbon nanofibers for next-generated supercapacitors
References
- York, R.; Bell, S.E. Energy transitions or additions?: Why a transition from fossil fuels requires more than the growth of renewable energy. Energy Res. Soc. Sci. 2019, 51, 40–43.
- Wang, D.-G.; Liang, Z.; Gao, S.; Qu, C.; Zou, R. Metal-organic framework-based materials for hybrid supercapacitor application. Coord. Chem. Rev. 2020, 404.
- Dubey, R.; Guruviah, V. Review of carbon-based electrode materials for supercapacitor energy storage. Ionics 2019, 25, 1419–1445.
- Ciszewski, M.; Koszorek, A.; Radko, T.; Szatkowski, P.; Janas, D. Review of the Selected Carbon-Based Materials for Symmetric Supercapacitor Application. J. Electron. Mater. 2018, 48, 717–744.
- Liu, C.-F.; Liu, Y.-C.; Yi, T.-Y.; Hu, C.-C. Carbon materials for high-voltage supercapacitors. Carbon 2019, 145, 529–548.
- Zhang, B.; Kang, F.; Tarascon, J.-M.; Kim, J.-K. Recent advances in electrospun carbon nanofibers and their application in electrochemical energy storage. Prog. Mater. Sci. 2016, 76, 319–380.
- Lu, X.; Wang, C.; Favier, F.; Pinna, N. Electrospun nanomaterials for supercapacitor electrodes: Designed architectures and electrochemical performance. Adv. Energy Mater. 2017, 7, 1601301.
- Cai, J.; Niu, H.; Li, Z.; Du, Y.; Cizek, P.; Xie, Z.; Xiong, H.; Lin, T. High-Performance Supercapacitor Electrode Materials from Cellulose-Derived Carbon Nanofibers. ACS Appl. Mater. Interfaces 2015, 7, 14946–14953.
- Cao, Q.; Zhu, M.; Chen, J.; Song, Y.; Li, Y.; Zhou, J. Novel Lignin-Cellulose-Based Carbon Nanofibers as High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2020, 12, 1210–1221.
- Cai, J.; Zhou, R.; Li, T.; He, J.; Wang, G.; Wang, H.; Xiong, H. Bamboo cellulose-derived cellulose acetate for electrospun nanofibers: Synthesis, characterization and kinetics. Cellulose 2017, 25, 391–398.
- Wsoo, M.A.; Shahir, S.; Bohari, S.P.M.; Nayan, N.H.M.; Abd Razak, S.I. A review on the properties of electrospun cellulose acetate and its application in drug delivery systems: A new perspective. Carbohydr. Res. 2020, 491, 107978.
- García-Mateos, F.J.; Ruiz-Rosas, R.; María Rosas, J.; Morallón, E.; Cazorla-Amorós, D.; Rodríguez-Mirasol, J.; Cordero, T. Activation of electrospun lignin-based carbon fibers and their performance as self-standing supercapacitor electrodes. Sep. Purif. Technol. 2020, 241, 116724.
- Roman, J.; Neri, W.; Derré, A.; Poulin, P. Electrospun lignin-based twisted carbon nanofibers for potential microelectrodes applications. Carbon 2019, 145, 556–564.
- Ma, C.; Li, Z.; Li, J.; Fan, Q.; Wu, L.; Shi, J.; Song, Y. Lignin-based hierarchical porous carbon nanofiber films with superior performance in supercapacitors. Appl. Surf. Sci. 2018, 456, 568–576.
- Cao, Q.; Zhang, Y.; Chen, J.; Zhu, M.; Yang, C.; Guo, H.; Song, Y.; Li, Y.; Zhou, J. Electrospun biomass based carbon nanofibers as high-performance supercapacitors. Ind. Crop. Prod. 2020, 148.
- Schlee, P.; Herou, S.; Jervis, R.; Shearing, P.R.; Brett, D.J.L.; Baker, D.; Hosseinaei, O.; Tomani, P.; Murshed, M.M.; Li, Y.; et al. Free-standing supercapacitors from Kraft lignin nanofibers with remarkable volumetric energy density. Chem. Sci. 2019, 10, 2980–2988.
- Hajlane, A.; Kaddami, H.; Joffe, R. Chemical modification of regenerated cellulose fibres by cellulose nano-crystals: Towards hierarchical structure for structural composites reinforcement. Ind. Crops Prod. 2017, 100, 41–50.Patil, S.C.S.A. Electrospun Carbon Nanofibers. In Carbon Nanomaterials Sourcebook; CRC Press, Taylor and Francis Group: Boka Raton, FL, USA, 2016; pp. 208–307.
- Segmehl, J.S.; Studer, V.; Keplinger, T.; Burgert, I.J.M. Characterization of wood derived hierarchical cellulose scaffolds for multifunctional applications. Materials 2018, 11, 517.Anindyajati, A.; Boughton, P.; Ruys, A.J. Modelling and Optimization of Polycaprolactone Ultrafine-Fibres Electrospinning Process Using Response Surface Methodology. Materials 2018, 11, 441.
- Milbrandt, A.; Booth, S. Carbon Fiber from Biomass; National Renewable Energy Laboratory(NREL): Golden, CO, USA, 2016.Mitchell, G.R. Electrospinning Principles, Practice and Possibilities; RSC Polymer Chemistry Series; The Royal Society of Chemistry: London, UK, 2015; pp. P001–P004.
- Fan, Q.; Ma, C.; Wu, L.; Wei, C.; Wang, H.; Song, Y.; Shi, J. Preparation of cellulose acetate derived carbon nanofibers by ZnCl2 activation as a supercapacitor electrode. RSC Adv. 2019, 9, 6419–6428.Cavaliere, S. Electrospinning for Advanced Energy and Environmental Applications; CRC Press, Taylor and Francis Group: Boka Raton, FL, USA, 2015.
- Composites World. Carbon Fiber 2014 Conference Report. Available online: https://www.compositesworld.com/articles/carbon-fiber-2014-conference-report (accessed on 25 November 2020).Hajlane, A.; Kaddami, H.; Joffe, R. Chemical modification of regenerated cellulose fibres by cellulose nano-crystals: Towards hierarchical structure for structural composites reinforcement. Ind. Crops Prod. 2017, 100, 41–50.
- Kubo, S.; Uraki, Y.; Sano, Y.J.C. Preparation of carbon fibers from softwood lignin by atmospheric acetic acid pulping. Carbon 1998, 36, 1119–1124.Segmehl, J.S.; Studer, V.; Keplinger, T.; Burgert, I.J.M. Characterization of wood derived hierarchical cellulose scaffolds for multifunctional applications. Materials 2018, 11, 517.
- Azwar, E.; Mahari, W.A.W.; Chuah, J.H.; Vo, D.V.N.; Ma, N.L.; Lam, W.H.; Lam, S.S. Transformation of biomass into carbon nanofiber for supercapacitor application—A review. Int. J. Hydrog. Energy 2018, 43, 20811–20821.Milbrandt, A.; Booth, S. Carbon Fiber from Biomass; National Renewable Energy Laboratory(NREL): Golden, CO, USA, 2016.
- Dallmeyer, I.; Ko, F.; Kadla, J.F. Electrospinning of technical lignins for the production of fibrous networks. J. Wood Chem. Technol. 2010, 30, 315–329.Fan, Q.; Ma, C.; Wu, L.; Wei, C.; Wang, H.; Song, Y.; Shi, J. Preparation of cellulose acetate derived carbon nanofibers by ZnCl2 activation as a supercapacitor electrode. RSC Adv. 2019, 9, 6419–6428.
- Lallave, M.; Bedia, J.; Ruiz-Rosas, R.; Rodríguez-Mirasol, J.; Cordero, T.; Otero, J.C.; Marquez, M.; Barrero, A.; Loscertales, I.G. Filled and hollow carbon nanofibers by coaxial electrospinning of alcell lignin without binder polymers. Adv. Mater. 2007, 19, 4292–4296.Composites World. Carbon Fiber 2014 Conference Report. Available online: https://www.compositesworld.com/articles/carbon-fiber-2014-conference-report (accessed on 25 November 2020).
- Wang, Y.; Qu, Q.; Gao, S.; Tang, G.; Liu, K.; He, S.; Huang, C. Biomass derived carbon as binder-free electrode materials for supercapacitors. Carbon 2019, 155, 706–726.Kubo, S.; Uraki, Y.; Sano, Y.J.C. Preparation of carbon fibers from softwood lignin by atmospheric acetic acid pulping. Carbon 1998, 36, 1119–1124.
- Herou, S.; Schlee, P.; Jorge, A.B.; Titirici, M. Biomass-derived electrodes for flexible supercapacitors. Curr. Opin. Green Sustain. Chem. 2018, 9, 18–24.Azwar, E.; Mahari, W.A.W.; Chuah, J.H.; Vo, D.V.N.; Ma, N.L.; Lam, W.H.; Lam, S.S. Transformation of biomass into carbon nanofiber for supercapacitor application—A review. Int. J. Hydrog. Energy 2018, 43, 20811–20821.
- Costes, L.; Laoutid, F.; Aguedo, M.; Richel, A.; Brohez, S.; Delvosalle, C.; Dubois, P. Phosphorus and nitrogen derivatization as efficient route for improvement of lignin flame retardant action in PLA. Eur. Polym. J. 2016, 84, 652–667.Dallmeyer, I.; Ko, F.; Kadla, J.F. Electrospinning of technical lignins for the production of fibrous networks. J. Wood Chem. Technol. 2010, 30, 315–329.
- Zhu, M.; Liu, H.; Cao, Q.; Zheng, H.; Xu, D.; Guo, H.; Wang, S.; Li, Y.; Zhou, J. Electrospun Lignin-Based Carbon Nanofibers as Supercapacitor Electrodes. ACS Sustain. Chem. Eng. 2020, 8, 12831–12841.Lallave, M.; Bedia, J.; Ruiz-Rosas, R.; Rodríguez-Mirasol, J.; Cordero, T.; Otero, J.C.; Marquez, M.; Barrero, A.; Loscertales, I.G. Filled and hollow carbon nanofibers by coaxial electrospinning of alcell lignin without binder polymers. Adv. Mater. 2007, 19, 4292–4296.
- Davé, V.; Glasser, W.G. Cellulose-based fibres from liquid crystalline solutions: 5. Processing and morphology of CAB blends with lignin. Polymer 1997, 38, 2121–2126.Wang, Y.; Qu, Q.; Gao, S.; Tang, G.; Liu, K.; He, S.; Huang, C. Biomass derived carbon as binder-free electrode materials for supercapacitors. Carbon 2019, 155, 706–726.
- Jia, H.; Sun, N.; Dirican, M.; Li, Y.; Chen, C.; Zhu, P.; Yan, C.; Zang, J.; Guo, J.; Tao, J.; et al. Electrospun Kraft Lignin/Cellulose Acetate-Derived Nanocarbon Network as an Anode for High-Performance Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 44368–44375.Herou, S.; Schlee, P.; Jorge, A.B.; Titirici, M. Biomass-derived electrodes for flexible supercapacitors. Curr. Opin. Green Sustain. Chem. 2018, 9, 18–24.
- Zhu, M.; Liu, H.; Cao, Q.; Zheng, H.; Xu, D.; Guo, H.; Wang, S.; Li, Y.; Zhou, J. Electrospun Lignin-Based Carbon Nanofibers as Supercapacitor Electrodes. ACS Sustain. Chem. Eng. 2020, 8, 12831–12841.
- Dai, Z.; Ren, P.G.; Jin, Y.L.; Zhang, H.; Ren, F.; Zhang, Q. Nitrogen-sulphur Co-doped graphenes modified electrospun lignin/polyacrylonitrile-based carbon nanofiber as high performance supercapacitor. J. Power Sources 2019, 437.
- Dai, Z.; Ren, P.G.; He, W.; Hou, X.; Ren, F.; Zhang, Q.; Jin, Y.L. Boosting the electrochemical performance of nitrogen-oxygen co-doped carbon nanofibers based supercapacitors through esterification of lignin precursor. Renew. Energy 2020, 162, 613–623.
- Lai, C.; Zhou, Z.; Zhang, L.; Wang, X.; Zhou, Q.; Zhao, Y.; Wang, Y.; Wu, X.F.; Zhu, Z.; Fong, H. Free-standing and mechanically flexible mats consisting of electrospun carbon nanofibers made from a natural product of alkali lignin as binder-free electrodes for high-performance supercapacitors. J. Power Sources 2014, 247, 134–141.
- Cao, M.; Cheng, W.; Ni, X.; Hu, Y.; Han, G. Lignin-based multi-channels carbon nanofibers @ SnO2 nanocomposites for high-performance supercapacitors. Electrochim. Acta 2020, 345, 136172.
- Yu, B.; Gele, A.; Wang, L. Iron oxide/lignin-based hollow carbon nanofibers nanocomposite as an application electrode materials for supercapacitors. Int. J. Biol. Macromol. 2018, 118, 478–484.
- Wang, J.; Tang, J.; Xu, Y.; Ding, B.; Chang, Z.; Wang, Y.; Hao, X.; Dou, H.; Kim, J.H.; Zhang, X.; et al. Interface miscibility induced double-capillary carbon nanofibers for flexible electric double layer capacitors. Nano Energy 2016, 28, 232–240.
- Davé, V.; Glasser, W.G. Cellulose-based fibres from liquid crystalline solutions: 5. Processing and morphology of CAB blends with lignin. Polymer 1997, 38, 2121–2126.
- Jia, H.; Sun, N.; Dirican, M.; Li, Y.; Chen, C.; Zhu, P.; Yan, C.; Zang, J.; Guo, J.; Tao, J.; et al. Electrospun Kraft Lignin/Cellulose Acetate-Derived Nanocarbon Network as an Anode for High-Performance Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 44368–44375.
- Guo, R.; Zhang, L.; Lu, Y.; Zhang, X.; Yang, D. Research progress of nanocellulose for electrochemical energy storage: A review. J. Energy Chem. 2020, 51, 342–361.
- Tayeb, P.; Tayeb, A.H. Nanocellulose applications in sustainable electrochemical and piezoelectric systems: A review. Carbohydr. Polym. 2019, 224, 115149.
- Ago, M.; Jakes, J.E.; Johansson, L.S.; Park, S.; Rojas, O.J. Interfacial properties of lignin-based electrospun nanofibers and films reinforced with cellulose nanocrystals. ACS Appl. Mater. Interfaces 2012, 4, 6849–6856.
- Ago, M.; Okajima, K.; Jakes, J.E.; Park, S.; Rojas, O.J. Lignin-Based Electrospun Nanofibers Reinforced with Cellulose Nanocrystals. Biomacromolecules 2012, 13, 918–926.
