Supercapacitors are energy storage devices with high power density, rapid charge/discharge rate, and excellent cycle stability. Carbon-based supercapacitors are increasingly attracting attention because of their large surface area and high porosity. Carbon-based materials research has been recently centered on biomass-based materials due to the rising need to maintain a sustainable environment. Cellulose and lignin constitute the major components of lignocellulose biomass. Since they are renewable, sustainable, and readily accessible, lignin and cellulose-based supercapacitors are economically viable and environmentally friendly.
- lignin
- cellulose
- nanofiber
- electrospinning
- supercapacitor
1. Introduction
Human work, welfare and industrialization rely on power generation, the most critical source of which is fossil fuels [1][2]. However, the significant environmental problem associated with the release of CO2, SO2 and NOx into the air presents for heavy consumption of fossil fuels[32]. The Global Energy Council has predicted that by 2050, the world will need to double its current demand, www.worldenergy.org. So it is important to follow a green solution for an efficient energy infrastructure that is capable of replacing fossil fuels [2][3][4][5]. Recently versatile supercapacitors, owing to the high power density, fast charge/discharge rate, excellent cycle stability and resilient design are among the most well-examined energy storage systems. The electrochemical dual-layer capacitor (EDLC) is a special energy storage device that uses the ability to absorb the electrolyte and align at the interface of the electrolyte and conductive electrodes with large surface area and suitable porosity [65]. The EDLC's performance is based on the characteristics of the electrode (active) material selected. Due to their excellent electrical conductivity, wide surface area, high porosity, mechanical flexibility and chemical stability, Electrospun carbonaceous materials are used as electrode materials, conductive additives and supporting substrates in EDLCs [76]. Because of its vast area and high porosity, carbon-based supercapacitors are gaining ever more attention. Carbon-based materials research has recently centred on biomass materials due to rising environmental conservation needs. Polyacrylonitrile (PAN) is the most suitable precursor to high-performance carbon fibres (CFs), relative to other traditional precursors, because of its high melting point, rich carbon content and rapid speed. PAN (such as polyaniline, pitch, rayon, etc.). PAN Electrospinning with stabilisation and carbonization can be used for the production of fine and controlled fibre diameters of carbon nanofibers (CNFs) [87]. The PAN and other petroleum polymers, however, are very costly [4] and releases toxic compounds during the carbonation process [98]. Greener carbon sources must also be opted for in the manufacture of CNFs.
As the most plentiful biological macromolecules in the world, biomass celluloses (mainly from cellulose acetate, ethyl cellulose, methyl cellulose etc) are excellent precursors of CNFs due to their stability and basic properties [109]. Natural d abundance and their low prices, despite possessing unique features such as wide surfaces and the high carbon content make CNFs highly desirable in recent years [1110]. Cellulose contains carbon, hydrogen, and oxygen elements and primarily releases carbon dioxide and water by its carbonization. Cellulose has no seeming melting point and preserves its physical morphology following effective carbonization[98]. Compared to cellulose, nanofibers and films contain cellulose acetate are far more simple to electrospin because cellulose acetate solubility relies on how much the acetate group is substituted[1211]. Cellulose is however a macromolecular polysaccharide, a significant number of cellulose structured oxygen atoms have strongly been autoxidised, and thermal instability is caused by these atoms. This thermal instability contributes to the breakdown of morphology when carbonized[109]. Lignin is the second richest bio-oriented polymer. In large amounts, it is obtained as a co-product from the wood, paper and bioethanol industries[1312]. Because of its many aromatic subunits, lignin is a rich source of carbon precursor [1413][15]. Electrospun lignin-based CNFs have a large surface, excellent graphitic and pore structures and are highly stable against corrosive environments [1614]. The structural units in lignin are nevertheless non-linear and randomly linked with various C-C and Ether bonds which together lead to low flexibility of the lignin polymer[1715]. Clean lignin materials are thus relatively delicate and cannot easily be handled. Besides, it is possible to drastically reduce its tensile strength by arbitrarily scattered aliphatic chains separating aromatic units in lignin and module during thermal treatment[1816]. Another serious issue with the batch reproducibility of lignin-derived CNFs is the variety of lignin biopolymers [1816]. However, to create healthy fibers, lignin may be combined with plasticizers [1913][20][15].
CNFs are a recent research area, focused on growth in energy storage systems based on CNFs from biomass. The lignin/cellulose mix is a growing need to examine the analysis. To our knowledge, only a few papers have been written about electrospun lignin/cellulose nanofibers in energy storageInterestingly, the few studies show that lignin-based supercapacitors can be an exotic area of research interests based on carbon-biomass precursors in the field of supercapacitors. Furthermore, lignin and cellulose are green and durable and their extraction methods are simple, cost-effective and environmentally friendly. This new field must therefore be investigated very clearly. A lot of reviews have been published on electrospun lignin and nanofibers. On both lignin and cellulose nanofibers, very little systemic reporting is available. No review has been published of electro-punished nanofibers from this rich combination of biomass materials.
2. Studies on Electrospun Lignin and Cellulose Nanofibers
2.1. Electrospinning technique
Electrospinning is a lightweight nanofabrication technique based on repulsive electrostatic forces to produce a nanofibrous viscoelastic solution. Although several modern electrospinning systems developments exist, each configuration is based on three basic components: high voltage source, viscoelastic solution dispense mode and a fibre-recovery system[2117]. Electrospinning variables may generally be grouped into three parameters: solution parameters (e.g. viscosity, surface tension, etc.), control parameters (e.g. flow rate, electric field, etc.) and environmental parameters (e.g. relative humidity, temperature etc), [2117][2218]. When an electric field is present in a liquid droplet, an electrostatic charge is deposited at the tip of the droplet[2319]. The electrostatic force generated by the charge droplets withstands the surface tension at the end of the needle at high sufficiently high pressure; thus the Taylor cone formation, which releases the jet by the spinneret if the coulombic force is above surface tension[2420]. The fibre diameter is dependent on the formation of Taylor's cone. It is associated with different experimental variables including the quality of the solvent and the viscosity of the suspension; injection speed, needle-collector distance and the voltage applied.
2.2. Studies on Electrospun Cellulose Nanofibers
The most prevalent natural macromolecule and the main component of the biomass in lignocellulose is cellulose. Its carbon content is roughly 44%, it is highly stable and porous, due to its hierarchical structure and highly functional linear rigid chains[2521][2622]. The first commercial carbon fibres were made in the late 60s (a cloth made of cellulose) [2723]. Cellulose nanofibers are made from various cellulose derivates such as cellulose acetate (CA) and ethyl cellulose (EC)[98] because they cannot dissolve in certain organic solvents. [87].
The selection of a suitable solvent is highly critical in the formation of CNF. To promote the jet elongation resulting in nanofibers formation, the correct solvent, which can evaporate quickly during the electrospinning phase, is needed. It is difficult to prepare a solution of cellulose electrospinning because in most organic solvents it is typically not soluble. The solubility of CA depends on when the acetate group is substituted, however, cells are easier to electrospin. Therefore the CA derivative is the most extensively used in the preparation of cellulose nanofibers. According to one study CA was dissolved at room temperature 12 hours before thermal treatment in acetone and dimethyl-acetamide (DMAc) for the electrolysis nanofibers CA followed by hydrolysis in NaOH / ethanol [2824]. These nanofibers were easily electrospun since DMAc and ethanol combined to generate enough surface tension, thus creating a Taylor cone. As can be seen in SEM images in figure 1, the resulting nanofibers display a morphological collapse causing a melting phenomenon. Due to the short soaking time in the DMAc/ethanol solution, morphological collapse may result in incomplete deacetylation. Increasing ZnCl2 content in the precursor solution may be ascribed to developing more fibrous morphology. Then Zncl2 shows that the powerful ions linkage of CA macromolecules improves thermal and structural stability thus facilitating dehydrogenation.
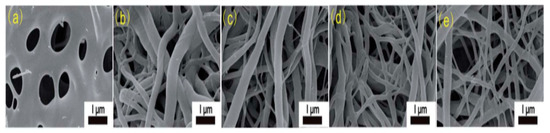
Figure 1. SEM images of electrospun cellulose acetate nanofibers (dissolved in acetone/DMAc) at different ZnCl2 content (a) CACNF-0, (b) CACNF-ZnCl2-2%, (c) CACNF-ZnCl2-5%, (d) CACNF-ZnCl2-10%, (e) CACNF-ZnCl2-20% [2824].
2.3. Studies on Electrospun Lignin Nanofibers
After cellulose, lignin is the second most abundant natural bioresource. Due to its rigid nature, it provides structural support to plants. The International lignin Institute reports that the pulp and paper industries and bio-refineries produce approximately 40 to 50 millions tonnes of lignin annually and about 300 billion metric tonnes of lignin per year globally. Lignin has a high content of carbon, approximately 60–65 per cent, suggesting the potential high yield following the production of carbon fibre [2925]. Kubo et al. have first published carbon fibres from lignin precursor material [3026]. To remove spinnable content from lignin, the authors suggested the conversion of lignin into functional polymers by an effective separation method. The prepared fibres were carbonized to transform lignin fibres to carbon fibres after thermal stabilisation. At present, nanofibers of the electrospun lignin are either prepared with lignin blending mechanically or with lignin structure modified by chemical alteration [3127][3228][3329][3430][3531]. The papers that study electrospun lines nanofibers will be reviewed in this section.
Chemical Modification of Lignin Structure
The non-linear and arbitrarily intertwined various C-C and ether bonds, which make the lignin stiff and difficult to electrospin can be altered by introducing foreign elements to the lignin precursor solution. Tunnapat and Surawut [3127] were able to apply glycerol to the precursor solution with a simple single heated spinning device to electrospin water-soluble nanofiber. The addition of glycerol reduced precursor solution surface tension, which increases the lignin spin. The fibre diameter and BET surface area increase with a higher glycerol ratio while electrical conductivity decreases. During thermal stabilisation, decreased fibres may be caused by the evaporation of glycerol, while reduced electrical conductivity could contribute to the essential role of glycerol in modifying lignin structure, thus increasing spinability Similarly, a higher glycerol surface area can be ascribed to constant evaporation of glycerol during thermal stabilisation, thereby reducing the resulting average fibre diameter. A further report produces lignin/H3PO4 nanofibers in ethanol (core spinning solution) and pure ethanol (shell spinning solution) by coaxial electrospinning technique of Alcell lignin[1312]. The presence of H3PO4 in the precursor solution enabled the electrospinning of lignin nanofibre. H3PO4 can effectively dehydrate the lignin phenolic-OH group, thus increasing lignin spinnability to form phospholipid bonding between lignin molecules [3632]. The use of Isophorone diisocyanate (IPDI) to the spinning solution was also used for the preparation of lignin nanofibers[3632]. By forming stable covalent relations between them, IPDI may bind the adjacent lignin molecules. SEM images (not shown) in lignin nanofibers made either by phosphating or covalent bonding have no beads or demonstrate morphological collapse after carbonization. Evident to the degree of phenolic hydroxyl groups in lignin, H3PO4 and IPDI, continuously dehydrated, stable lignin fibres were formed
Physical blending of lignin with other binders
The weak electrical properties of the lignin-based electrode, despite the natural abundance of lignin and the lignin-based high surface area, contribute to poor electrochemical efficiency. Lignin can be combined with other binding polymers to enhance spinnability and morphological characteristics of lignin nanofibre-based electrode. Likewise, combining lignin with other binders improves the electrochemical properties of the nanofibers being produced. Due to the high versatility and fibre processing capability of PAN, it has been combined with lignin to produce strong plasticity nitrogen-rich nanofibers [3733]. In another study, butyric anhydride (BA) has previously been applied to the lignin/PAN precursor solution[3834]. Although PAN enhances electrospun nanofibre versatility, BA forms important bonds between hydroxyl groups in BA lignin anhydride groups. Other polymers blended with lignin to prepare electrospun lignin nanofibers include PVA [3935], PMMA [4036], PEO [4137] and PVP [1614]. Inorganic compounds can equally be added into lignin precursor solution to modify lignin structure, enabling spinnability and improving quality and performance of resultant nanofiber. For instance, SiO2 improved interface miscibility of double capillary and tailored porosity [4238],
2.4. Studies on Electrospun Lignin/Cellulose Nanofibers
The fascinating characteristics of lignin and cellulose nanofibers such as low cost, high carbon performance, wide-field, mechanical stability, and environmental sustainability make them promising candidates for carbon-based EDLC electrode materials. Lignin and cellulose nanofibers can be combined into lignin/cellulose nanofibers to optimize the advantages of lignin and cellulose biomass materials as energy storage devices [3430]. Physical blending of lignin and cellulose generally involves modifying the -OH groups phenolic group of lignin and cellulosic material (acetyl group in the case of cellulose acetate) to allow crosslinking reaction between lignin and cellulose macromolecules [3127]
Lignin/Cellulose Acetate (CA) Blend
Nanofibers Lignin/CA have been obtained in order to establish electron-deficient oxygen atoms by the continuous dehydration of phenolic lignin hydroxyl group and cellulosic hydroxyl group. These oxygenatoms can combine successfully to form stable connections between lignin and cellulose acetate, with highly electron-rich atoms of binding agents [3329]. Early attempts to prepare lignin/cellulose fibres were not successful due to the various beaded fibre formations and resulting fibre morphology meltdowns. The addition of lignin to cellulose acetate butyrate disturbed the order of nanocrystalline nature of the cellulosic phase in 1997 by Dave and Glasser [4339]. Similarly, smooth, defect-free morphology of lignin/CA nanofibers have been reported [4440]. Nevertheless, after carbonization, the fibrous morphologies of these nanofibers were completely lost due to the lignin-cellulose phase separation The SEM pictures of the prepared nanofibers are seen in Figure 2, after and before carbonization. The loss of fibrous morphology following carbonization was attributed to poor contact between the phenolic lignin group and the acetyl cellulose group, which culminated in the separation process.
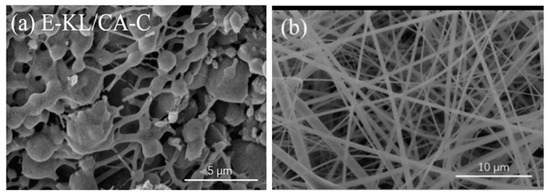
Figure 2. SEM images of electrospun lignin cellulose acetate nanofibers (a) after carbonization and (b) before carbonization. Adapted with permission from ref [4440].
Lignin/Nanocellulose (NC) Blend
Nanocellulose (also called nanoscale cellulose) is more defect-free, has greater surface area, more active functional groups, and enhanced chemical modification facility than conventional cellulose [4541]. Based on their sources, preparation methods, and fiber morphologies, NC can be divided into three categories: (1) cellulose nanocrystals, (2) cellulose nanofibrils, and (3) bacterial cellulose [4642]. Nanofibers have not yet been documented to pure electrospun lignin/NC. However, recent studies have been made about the influence of NC on electrospun lignin (mixed with other polymers such as PVA, PEO and PAN). M. Ago researched the effects of cellulose nanocrystals on electrospun lignin/PVA nanofibers interface properties [4743][4844]. They also documented that cellulose nanocrystals decrease the phase-separated domains. This may be due to lignin-PVA matrix molecular movement. Therefore we can conclude that cellulose nanocrystal may have decreased polymer dispersion below the solvent evaporation rate, decreasing the nanofibers' phase separation.
3. Conclusions
Carbon nanofibre-based biomass technology is one of the most active energy storage research areas, particularly in the EDLC. Whereas studies of lignin and supercapacitors based on electrospun are considered to be adequate, very limited studies of supercapacitors based on cellulose/lignin were reported. The reported study of supercapacitors based on lignin/cellulose however suggests new research directions for supercapacitors based on biomass. Researchers have identified certain major challenges associated with the wider use of this special class of electrode materials from the papers reviewed in this study.
-
Nanofiber electrodes based on electrospun lignin and cellulose precursor have a wide area, good porosity, mechanical stability and excellent cycle stability. However, in comparison to other carbon-based materials, such as PAN, graphene etc the energy density of these electrode materials is quite low.
-
The hierarchy and complex arrangement of lignin make the electrospinning lignin to nanofibers very difficult. However, lignin can easily be mixed with additional binders to create nanofibers based on fine lignin. The electrospun lignin nanofibers are influenced by various binders in different ways.
-
The precursor solution of electrospinning lignin and cellulose formed nanofibers with segregated phases into cellulose and lignin domains. The use of an appropriate crosslinker, however, solves the phase separation problem. Thermal lignin stability and CA flexibility are presented by resulting nanofibers.
-
When extensively explored, lignin/cellulose nanofiber can serve as the ideal alternative to petroleum carbon nanofibers for next-generated supercapacitors
References
- Hassan YM, Guan BH, Chuan LK and Adam AA; Electrowetting Effect using Dielectric Nanoparticles for Enhanced Oil Recovery. Progress in Petrochemical Science 2021, 4(2), 411-414, 10.31031/PPS.2021.04.000584.York, R.; Bell, S.E. Energy transitions or additions?: Why a transition from fossil fuels requires more than the growth of renewable energy. Energy Res. Soc. Sci. 2019, 51, 40–43.
- Yarima Mudassir Hassan; Beh Hoe Guan; Hasnah Mohd Zaid; Mohammed Falalu Hamza; Muhammad Adil; Abdullahi Abbas Adam; Kurnia Hastuti; Application of Magnetic and Dielectric Nanofluids for Electromagnetic-Assistance Enhanced Oil Recovery: A Review. Crystals 2021, 11, 106, 10.3390/cryst11020106.Wang, D.-G.; Liang, Z.; Gao, S.; Qu, C.; Zou, R. Metal-organic framework-based materials for hybrid supercapacitor application. Coord. Chem. Rev. 2020, 404.
- Wang, D.-G.; Liang, Z.; Gao, S.; Qu, C.; Zou, R. Metal-organic framework-based materials for hybrid supercapacitor application. Coord. Chem. Rev. 2020, 404.Dubey, R.; Guruviah, V. Review of carbon-based electrode materials for supercapacitor energy storage. Ionics 2019, 25, 1419–1445.
- Dubey, R.; Guruviah, V. Review of carbon-based electrode materials for supercapacitor energy storage. Ionics 2019, 25, 1419–1445.Ciszewski, M.; Koszorek, A.; Radko, T.; Szatkowski, P.; Janas, D. Review of the Selected Carbon-Based Materials for Symmetric Supercapacitor Application. J. Electron. Mater. 2018, 48, 717–744.
- Ciszewski, M.; Koszorek, A.; Radko, T.; Szatkowski, P.; Janas, D. Review of the Selected Carbon-Based Materials for Symmetric Supercapacitor Application. J. Electron. Mater. 2018, 48, 717–744.Liu, C.-F.; Liu, Y.-C.; Yi, T.-Y.; Hu, C.-C. Carbon materials for high-voltage supercapacitors. Carbon 2019, 145, 529–548.
- Liu, C.-F.; Liu, Y.-C.; Yi, T.-Y.; Hu, C.-C. Carbon materials for high-voltage supercapacitors. Carbon 2019, 145, 529–548.Zhang, B.; Kang, F.; Tarascon, J.-M.; Kim, J.-K. Recent advances in electrospun carbon nanofibers and their application in electrochemical energy storage. Prog. Mater. Sci. 2016, 76, 319–380.
- Zhang, B.; Kang, F.; Tarascon, J.-M.; Kim, J.-K. Recent advances in electrospun carbon nanofibers and their application in electrochemical energy storage. Prog. Mater. Sci. 2016, 76, 319–380.Lu, X.; Wang, C.; Favier, F.; Pinna, N. Electrospun nanomaterials for supercapacitor electrodes: Designed architectures and electrochemical performance. Adv. Energy Mater. 2017, 7, 1601301.
- Lu, X.; Wang, C.; Favier, F.; Pinna, N. Electrospun nanomaterials for supercapacitor electrodes: Designed architectures and electrochemical performance. Adv. Energy Mater. 2017, 7, 1601301.Cai, J.; Niu, H.; Li, Z.; Du, Y.; Cizek, P.; Xie, Z.; Xiong, H.; Lin, T. High-Performance Supercapacitor Electrode Materials from Cellulose-Derived Carbon Nanofibers. ACS Appl. Mater. Interfaces 2015, 7, 14946–14953.
- Cai, J.; Niu, H.; Li, Z.; Du, Y.; Cizek, P.; Xie, Z.; Xiong, H.; Lin, T. High-Performance Supercapacitor Electrode Materials from Cellulose-Derived Carbon Nanofibers. ACS Appl. Mater. Interfaces 2015, 7, 14946–14953.Cao, Q.; Zhu, M.; Chen, J.; Song, Y.; Li, Y.; Zhou, J. Novel Lignin-Cellulose-Based Carbon Nanofibers as High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2020, 12, 1210–1221.
- Cao, Q.; Zhu, M.; Chen, J.; Song, Y.; Li, Y.; Zhou, J. Novel Lignin-Cellulose-Based Carbon Nanofibers as High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2020, 12, 1210–1221.Cai, J.; Zhou, R.; Li, T.; He, J.; Wang, G.; Wang, H.; Xiong, H. Bamboo cellulose-derived cellulose acetate for electrospun nanofibers: Synthesis, characterization and kinetics. Cellulose 2017, 25, 391–398.
- Cai, J.; Zhou, R.; Li, T.; He, J.; Wang, G.; Wang, H.; Xiong, H. Bamboo cellulose-derived cellulose acetate for electrospun nanofibers: Synthesis, characterization and kinetics. Cellulose 2017, 25, 391–398.Wsoo, M.A.; Shahir, S.; Bohari, S.P.M.; Nayan, N.H.M.; Abd Razak, S.I. A review on the properties of electrospun cellulose acetate and its application in drug delivery systems: A new perspective. Carbohydr. Res. 2020, 491, 107978.
- Wsoo, M.A.; Shahir, S.; Bohari, S.P.M.; Nayan, N.H.M.; Abd Razak, S.I. A review on the properties of electrospun cellulose acetate and its application in drug delivery systems: A new perspective. Carbohydr. Res. 2020, 491, 107978.García-Mateos, F.J.; Ruiz-Rosas, R.; María Rosas, J.; Morallón, E.; Cazorla-Amorós, D.; Rodríguez-Mirasol, J.; Cordero, T. Activation of electrospun lignin-based carbon fibers and their performance as self-standing supercapacitor electrodes. Sep. Purif. Technol. 2020, 241, 116724.
- García-Mateos, F.J.; Ruiz-Rosas, R.; María Rosas, J.; Morallón, E.; Cazorla-Amorós, D.; Rodríguez-Mirasol, J.; Cordero, T. Activation of electrospun lignin-based carbon fibers and their performance as self-standing supercapacitor electrodes. Sep. Purif. Technol. 2020, 241, 116724.Roman, J.; Neri, W.; Derré, A.; Poulin, P. Electrospun lignin-based twisted carbon nanofibers for potential microelectrodes applications. Carbon 2019, 145, 556–564.
- John Ojur Dennis; Yas Al-Hadeethi; E. M. Mkawi; Abdullahi Adam; Bashir Abubakar Abdulkadir; Fahad Usman; Yarima Mudassir Hassan; I. Wadi; Mustapha Sani; State of the Art and New Directions on Electrospun Lignin/Cellulose Nanofibers for Supercapacitor Application: A Systematic Literature Review. Polymers 2020, 12, 2884, 10.3390/polym12122884.Ma, C.; Li, Z.; Li, J.; Fan, Q.; Wu, L.; Shi, J.; Song, Y. Lignin-based hierarchical porous carbon nanofiber films with superior performance in supercapacitors. Appl. Surf. Sci. 2018, 456, 568–576.
- Roman, J.; Neri, W.; Derré, A.; Poulin, P. Electrospun lignin-based twisted carbon nanofibers for potential microelectrodes applications. Carbon 2019, 145, 556–564.Cao, Q.; Zhang, Y.; Chen, J.; Zhu, M.; Yang, C.; Guo, H.; Song, Y.; Li, Y.; Zhou, J. Electrospun biomass based carbon nanofibers as high-performance supercapacitors. Ind. Crop. Prod. 2020, 148.
- Ma, C.; Li, Z.; Li, J.; Fan, Q.; Wu, L.; Shi, J.; Song, Y. Lignin-based hierarchical porous carbon nanofiber films with superior performance in supercapacitors. Appl. Surf. Sci. 2018, 456, 568–576.Schlee, P.; Herou, S.; Jervis, R.; Shearing, P.R.; Brett, D.J.L.; Baker, D.; Hosseinaei, O.; Tomani, P.; Murshed, M.M.; Li, Y.; et al. Free-standing supercapacitors from Kraft lignin nanofibers with remarkable volumetric energy density. Chem. Sci. 2019, 10, 2980–2988.
- Cao, Q.; Zhang, Y.; Chen, J.; Zhu, M.; Yang, C.; Guo, H.; Song, Y.; Li, Y.; Zhou, J. Electrospun biomass based carbon nanofibers as high-performance supercapacitors. Ind. Crop. Prod. 2020, 148.Patil, S.C.S.A. Electrospun Carbon Nanofibers. In Carbon Nanomaterials Sourcebook; CRC Press, Taylor and Francis Group: Boka Raton, FL, USA, 2016; pp. 208–307.
- Schlee, P.; Herou, S.; Jervis, R.; Shearing, P.R.; Brett, D.J.L.; Baker, D.; Hosseinaei, O.; Tomani, P.; Murshed, M.M.; Li, Y.; et al. Free-standing supercapacitors from Kraft lignin nanofibers with remarkable volumetric energy density. Chem. Sci. 2019, 10, 2980–2988.Anindyajati, A.; Boughton, P.; Ruys, A.J. Modelling and Optimization of Polycaprolactone Ultrafine-Fibres Electrospinning Process Using Response Surface Methodology. Materials 2018, 11, 441.
- A.A. Adam; M. Musa; M. Sani; Enhanced optical transmittance of spray deposited zinc oxide thin films for optoelectronic applications. Bayero Journal of Pure and Applied Sciences 2020, 12, 1-5, 10.4314/bajopas.v12i1.1.Mitchell, G.R. Electrospinning Principles, Practice and Possibilities; RSC Polymer Chemistry Series; The Royal Society of Chemistry: London, UK, 2015; pp. P001–P004.
- Abdullahi Abbas Adam, Kandasamy Ramamurthi, Mahesh Mudaliar Margoni; A Study on Low Cost-Highly Transparent and Conductive Molybdenum Doped Zinc Oxide Thin Films Deposited by Spray Pyrolysis Technique. American Journal of Materials Research 2018, 5(3), 40-45.Cavaliere, S. Electrospinning for Advanced Energy and Environmental Applications; CRC Press, Taylor and Francis Group: Boka Raton, FL, USA, 2015.
- Patil, S.C.S.A. Electrospun Carbon Nanofibers. In Carbon Nanomaterials Sourcebook; CRC Press, Taylor and Francis Group: Boka Raton, FL, USA, 2016; pp. 208–307.Hajlane, A.; Kaddami, H.; Joffe, R. Chemical modification of regenerated cellulose fibres by cellulose nano-crystals: Towards hierarchical structure for structural composites reinforcement. Ind. Crops Prod. 2017, 100, 41–50.
- Anindyajati, A.; Boughton, P.; Ruys, A.J. Modelling and Optimization of Polycaprolactone Ultrafine-Fibres Electrospinning Process Using Response Surface Methodology. Materials 2018, 11, 441.Segmehl, J.S.; Studer, V.; Keplinger, T.; Burgert, I.J.M. Characterization of wood derived hierarchical cellulose scaffolds for multifunctional applications. Materials 2018, 11, 517.
- Mitchell, G.R. Electrospinning Principles, Practice and Possibilities; RSC Polymer Chemistry Series; The Royal Society of Chemistry: London, UK, 2015; pp. P001–P004.Milbrandt, A.; Booth, S. Carbon Fiber from Biomass; National Renewable Energy Laboratory(NREL): Golden, CO, USA, 2016.
- Cavaliere, S. Electrospinning for Advanced Energy and Environmental Applications; CRC Press, Taylor and Francis Group: Boka Raton, FL, USA, 2015.Fan, Q.; Ma, C.; Wu, L.; Wei, C.; Wang, H.; Song, Y.; Shi, J. Preparation of cellulose acetate derived carbon nanofibers by ZnCl2 activation as a supercapacitor electrode. RSC Adv. 2019, 9, 6419–6428.
- Hajlane, A.; Kaddami, H.; Joffe, R. Chemical modification of regenerated cellulose fibres by cellulose nano-crystals: Towards hierarchical structure for structural composites reinforcement. Ind. Crops Prod. 2017, 100, 41–50.Composites World. Carbon Fiber 2014 Conference Report. Available online: https://www.compositesworld.com/articles/carbon-fiber-2014-conference-report (accessed on 25 November 2020).
- Segmehl, J.S.; Studer, V.; Keplinger, T.; Burgert, I.J.M. Characterization of wood derived hierarchical cellulose scaffolds for multifunctional applications. Materials 2018, 11, 517.Kubo, S.; Uraki, Y.; Sano, Y.J.C. Preparation of carbon fibers from softwood lignin by atmospheric acetic acid pulping. Carbon 1998, 36, 1119–1124.
- Milbrandt, A.; Booth, S. Carbon Fiber from Biomass; National Renewable Energy Laboratory(NREL): Golden, CO, USA, 2016.Azwar, E.; Mahari, W.A.W.; Chuah, J.H.; Vo, D.V.N.; Ma, N.L.; Lam, W.H.; Lam, S.S. Transformation of biomass into carbon nanofiber for supercapacitor application—A review. Int. J. Hydrog. Energy 2018, 43, 20811–20821.
- Fan, Q.; Ma, C.; Wu, L.; Wei, C.; Wang, H.; Song, Y.; Shi, J. Preparation of cellulose acetate derived carbon nanofibers by ZnCl2 activation as a supercapacitor electrode. RSC Adv. 2019, 9, 6419–6428.Dallmeyer, I.; Ko, F.; Kadla, J.F. Electrospinning of technical lignins for the production of fibrous networks. J. Wood Chem. Technol. 2010, 30, 315–329.
- Composites World. Carbon Fiber 2014 Conference Report. Available online: https://www.compositesworld.com/articles/carbon-fiber-2014-conference-report (accessed on 25 November 2020).Lallave, M.; Bedia, J.; Ruiz-Rosas, R.; Rodríguez-Mirasol, J.; Cordero, T.; Otero, J.C.; Marquez, M.; Barrero, A.; Loscertales, I.G. Filled and hollow carbon nanofibers by coaxial electrospinning of alcell lignin without binder polymers. Adv. Mater. 2007, 19, 4292–4296.
- Kubo, S.; Uraki, Y.; Sano, Y.J.C. Preparation of carbon fibers from softwood lignin by atmospheric acetic acid pulping. Carbon 1998, 36, 1119–1124.Wang, Y.; Qu, Q.; Gao, S.; Tang, G.; Liu, K.; He, S.; Huang, C. Biomass derived carbon as binder-free electrode materials for supercapacitors. Carbon 2019, 155, 706–726.
- Azwar, E.; Mahari, W.A.W.; Chuah, J.H.; Vo, D.V.N.; Ma, N.L.; Lam, W.H.; Lam, S.S. Transformation of biomass into carbon nanofiber for supercapacitor application—A review. Int. J. Hydrog. Energy 2018, 43, 20811–20821.Herou, S.; Schlee, P.; Jorge, A.B.; Titirici, M. Biomass-derived electrodes for flexible supercapacitors. Curr. Opin. Green Sustain. Chem. 2018, 9, 18–24.
- Dallmeyer, I.; Ko, F.; Kadla, J.F. Electrospinning of technical lignins for the production of fibrous networks. J. Wood Chem. Technol. 2010, 30, 315–329.Zhu, M.; Liu, H.; Cao, Q.; Zheng, H.; Xu, D.; Guo, H.; Wang, S.; Li, Y.; Zhou, J. Electrospun Lignin-Based Carbon Nanofibers as Supercapacitor Electrodes. ACS Sustain. Chem. Eng. 2020, 8, 12831–12841.
- Lallave, M.; Bedia, J.; Ruiz-Rosas, R.; Rodríguez-Mirasol, J.; Cordero, T.; Otero, J.C.; Marquez, M.; Barrero, A.; Loscertales, I.G. Filled and hollow carbon nanofibers by coaxial electrospinning of alcell lignin without binder polymers. Adv. Mater. 2007, 19, 4292–4296.Dai, Z.; Ren, P.G.; Jin, Y.L.; Zhang, H.; Ren, F.; Zhang, Q. Nitrogen-sulphur Co-doped graphenes modified electrospun lignin/polyacrylonitrile-based carbon nanofiber as high performance supercapacitor. J. Power Sources 2019, 437.
- Wang, Y.; Qu, Q.; Gao, S.; Tang, G.; Liu, K.; He, S.; Huang, C. Biomass derived carbon as binder-free electrode materials for supercapacitors. Carbon 2019, 155, 706–726.Dai, Z.; Ren, P.G.; He, W.; Hou, X.; Ren, F.; Zhang, Q.; Jin, Y.L. Boosting the electrochemical performance of nitrogen-oxygen co-doped carbon nanofibers based supercapacitors through esterification of lignin precursor. Renew. Energy 2020, 162, 613–623.
- Herou, S.; Schlee, P.; Jorge, A.B.; Titirici, M. Biomass-derived electrodes for flexible supercapacitors. Curr. Opin. Green Sustain. Chem. 2018, 9, 18–24.Lai, C.; Zhou, Z.; Zhang, L.; Wang, X.; Zhou, Q.; Zhao, Y.; Wang, Y.; Wu, X.F.; Zhu, Z.; Fong, H. Free-standing and mechanically flexible mats consisting of electrospun carbon nanofibers made from a natural product of alkali lignin as binder-free electrodes for high-performance supercapacitors. J. Power Sources 2014, 247, 134–141.
- Zhu, M.; Liu, H.; Cao, Q.; Zheng, H.; Xu, D.; Guo, H.; Wang, S.; Li, Y.; Zhou, J. Electrospun Lignin-Based Carbon Nanofibers as Supercapacitor Electrodes. ACS Sustain. Chem. Eng. 2020, 8, 12831–12841.Cao, M.; Cheng, W.; Ni, X.; Hu, Y.; Han, G. Lignin-based multi-channels carbon nanofibers @ SnO2 nanocomposites for high-performance supercapacitors. Electrochim. Acta 2020, 345, 136172.
- Dai, Z.; Ren, P.G.; Jin, Y.L.; Zhang, H.; Ren, F.; Zhang, Q. Nitrogen-sulphur Co-doped graphenes modified electrospun lignin/polyacrylonitrile-based carbon nanofiber as high performance supercapacitor. J. Power Sources 2019, 437.Yu, B.; Gele, A.; Wang, L. Iron oxide/lignin-based hollow carbon nanofibers nanocomposite as an application electrode materials for supercapacitors. Int. J. Biol. Macromol. 2018, 118, 478–484.
- Dai, Z.; Ren, P.G.; He, W.; Hou, X.; Ren, F.; Zhang, Q.; Jin, Y.L. Boosting the electrochemical performance of nitrogen-oxygen co-doped carbon nanofibers based supercapacitors through esterification of lignin precursor. Renew. Energy 2020, 162, 613–623.Wang, J.; Tang, J.; Xu, Y.; Ding, B.; Chang, Z.; Wang, Y.; Hao, X.; Dou, H.; Kim, J.H.; Zhang, X.; et al. Interface miscibility induced double-capillary carbon nanofibers for flexible electric double layer capacitors. Nano Energy 2016, 28, 232–240.
- Lai, C.; Zhou, Z.; Zhang, L.; Wang, X.; Zhou, Q.; Zhao, Y.; Wang, Y.; Wu, X.F.; Zhu, Z.; Fong, H. Free-standing and mechanically flexible mats consisting of electrospun carbon nanofibers made from a natural product of alkali lignin as binder-free electrodes for high-performance supercapacitors. J. Power Sources 2014, 247, 134–141.Davé, V.; Glasser, W.G. Cellulose-based fibres from liquid crystalline solutions: 5. Processing and morphology of CAB blends with lignin. Polymer 1997, 38, 2121–2126.
- Cao, M.; Cheng, W.; Ni, X.; Hu, Y.; Han, G. Lignin-based multi-channels carbon nanofibers @ SnO2 nanocomposites for high-performance supercapacitors. Electrochim. Acta 2020, 345, 136172.Jia, H.; Sun, N.; Dirican, M.; Li, Y.; Chen, C.; Zhu, P.; Yan, C.; Zang, J.; Guo, J.; Tao, J.; et al. Electrospun Kraft Lignin/Cellulose Acetate-Derived Nanocarbon Network as an Anode for High-Performance Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 44368–44375.
- Yu, B.; Gele, A.; Wang, L. Iron oxide/lignin-based hollow carbon nanofibers nanocomposite as an application electrode materials for supercapacitors. Int. J. Biol. Macromol. 2018, 118, 478–484.Guo, R.; Zhang, L.; Lu, Y.; Zhang, X.; Yang, D. Research progress of nanocellulose for electrochemical energy storage: A review. J. Energy Chem. 2020, 51, 342–361.
- Wang, J.; Tang, J.; Xu, Y.; Ding, B.; Chang, Z.; Wang, Y.; Hao, X.; Dou, H.; Kim, J.H.; Zhang, X.; et al. Interface miscibility induced double-capillary carbon nanofibers for flexible electric double layer capacitors. Nano Energy 2016, 28, 232–240.Tayeb, P.; Tayeb, A.H. Nanocellulose applications in sustainable electrochemical and piezoelectric systems: A review. Carbohydr. Polym. 2019, 224, 115149.
- Davé, V.; Glasser, W.G. Cellulose-based fibres from liquid crystalline solutions: 5. Processing and morphology of CAB blends with lignin. Polymer 1997, 38, 2121–2126.Ago, M.; Jakes, J.E.; Johansson, L.S.; Park, S.; Rojas, O.J. Interfacial properties of lignin-based electrospun nanofibers and films reinforced with cellulose nanocrystals. ACS Appl. Mater. Interfaces 2012, 4, 6849–6856.
- Jia, H.; Sun, N.; Dirican, M.; Li, Y.; Chen, C.; Zhu, P.; Yan, C.; Zang, J.; Guo, J.; Tao, J.; et al. Electrospun Kraft Lignin/Cellulose Acetate-Derived Nanocarbon Network as an Anode for High-Performance Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 44368–44375.Ago, M.; Okajima, K.; Jakes, J.E.; Park, S.; Rojas, O.J. Lignin-Based Electrospun Nanofibers Reinforced with Cellulose Nanocrystals. Biomacromolecules 2012, 13, 918–926.
- Guo, R.; Zhang, L.; Lu, Y.; Zhang, X.; Yang, D. Research progress of nanocellulose for electrochemical energy storage: A review. J. Energy Chem. 2020, 51, 342–361.
- Tayeb, P.; Tayeb, A.H. Nanocellulose applications in sustainable electrochemical and piezoelectric systems: A review. Carbohydr. Polym. 2019, 224, 115149.
- Ago, M.; Jakes, J.E.; Johansson, L.S.; Park, S.; Rojas, O.J. Interfacial properties of lignin-based electrospun nanofibers and films reinforced with cellulose nanocrystals. ACS Appl. Mater. Interfaces 2012, 4, 6849–6856.
- Ago, M.; Okajima, K.; Jakes, J.E.; Park, S.; Rojas, O.J. Lignin-Based Electrospun Nanofibers Reinforced with Cellulose Nanocrystals. Biomacromolecules 2012, 13, 918–926.
- A.A. Adam; M. Musa; M. Sani; Enhanced optical transmittance of spray deposited zinc oxide thin films for optoelectronic applications. Bayero Journal of Pure and Applied Sciences 2020, 12, 1-5, 10.4314/bajopas.v12i1.1.
