The conservation of crop genetic resources, including their wild relatives, is of utmost importance for the future of mankind. Most crops produce orthodox seeds and can, therefore, be stored in seed genebanks. However, this is not an option for crops and species that produce recalcitrant (non-storable) seeds such as cacao, coffee and avocado, for crops that do not produce seeds at all; therefore, they are inevitably vegetatively propagated such as bananas, or crops that are predominantly clonally propagated as their seeds are not true to type, such as potato, cassava and many fruit trees. Field, in vitro and cryopreserved collections provide an alternative in such cases.
- Crop Genetic Resources
- Conservation
- Field Genebanks
1. Introduction
In the course of crop domestication, many plants have been selected for quantity and/or quality of their seed, while some have been cultivated for their roots, tubers, fruits, stems and leaves. Plant genetic resources for food and agriculture (PGRFA) are of strategic importance to ensure sustainable crop production [1], nutritious food and food security for humans and to enhance economic prosperity of the present and future generations. They comprise the sum of genes, gene combinations or genotypes which serve as a reservoir for direct use in food production systems and for breeding new varieties [2].
Since the beginning of agriculture, selection of plants and seeds for sowing, growing, harvest and storage gave rise to locally adapted varieties, so-called “landraces”, that reveal specific variations of morphological and yield characteristics and quality traits. In the mid-19th century, the rediscovery of Gregor Mendel’s work and the introduction of breeding schemes resulted in the development of high-yielding and more stress-tolerant varieties leading to higher crop yields. This laid the foundation for the green revolution taking place in the middle of the last century bringing about increased agricultural production to feed the exponentially growing world population. However, the expansion of industrial mono-cropping with the replacement of landraces by modern breeding varieties has caused the loss of 75% of plant genetic diversity, with more than 90% of crop varieties having disappeared from farmers’ fields [3]. In this era of global environmental problems, climate change, and booming population growth, it is of paramount importance that the remaining crop genetic resources are kept available to sustain the agricultural production systems, to feed the world population a healthy diet and to tackle future demanding challenges [4].
Since the 16th century, botanical gardens have collected and preserved a variation of more than 80,000 plant species in about 3,400 gardens all over the world [5]. They primarily have an interest in conserving the widest possible plant diversity and crop wild relatives that can be an important source material for breeders. PGRFA are conserved ex situ in specialized repositories, often termed genebanks that have been established since the mid-20th century. In contrast to most botanical gardens, genebanks focus on both intra- and inter-specific crop diversity. There are more than 17,000 national, regional and international institutes and organizations dealing with the conservation and sustainable use of PGRFA [5]. Currently, 711 gene banks and 16 international/regional centers in 90 countries retain more than 5.4 million accessions from over 7,051 genera, mainly focusing their conservation efforts on crop species, including landraces and crop wild relatives, breeding materials and cultivars [6].
Most of the major food crops produce orthodox seeds that tolerate intense dehydration and low temperatures, thus seed storage under dry and cool conditions is naturally the most widely adopted method for long-term ex situ conservation at relatively low costs. About 45% of the accessions stored as seeds are cereals, i.e., wheat (Triticum aestivum), triticale (Triticum secale), rice (Oryza sativa), oat (Avena sativa), rye (Secale cereale), barley (Hordeum vulgare), maize (Zea mays) and sorghum (Sorghum bicolor), followed by food legumes (15%), forages (9%) and vegetables (7%) [5].
In contrast, a large number of food crops are not storable through seeds and thus need different conservation approaches [7]. This category of plants consists of important species that produce desiccation sensitive, recalcitrant or intermediate seeds, such as coconut (Cocos nucifera), cacao (Theobroma cacao), avocado (Persea americana) and citrus (Citrus spp.) and species that are seedless such as edible banana (Musa spp.) and garlic (Allium sativum). Species including yucca (Yucca sp.) and bamboo (Bambuseae sp.) that have long life cycles and take years or decades to reproduce also fall into this category. Other species that produce orthodox seeds but require the conservation of particular gene combinations or genotypes, such as root and tuber crops, notably potato (Solanum tuberosum), cassava (Manihot esculenta), yam (Dioscorea spp.), taro (Colocasia esculenta) and several fruit and nut trees are also included. These crops are propagated vegetatively, and each genotype needs to be maintained as a clone.
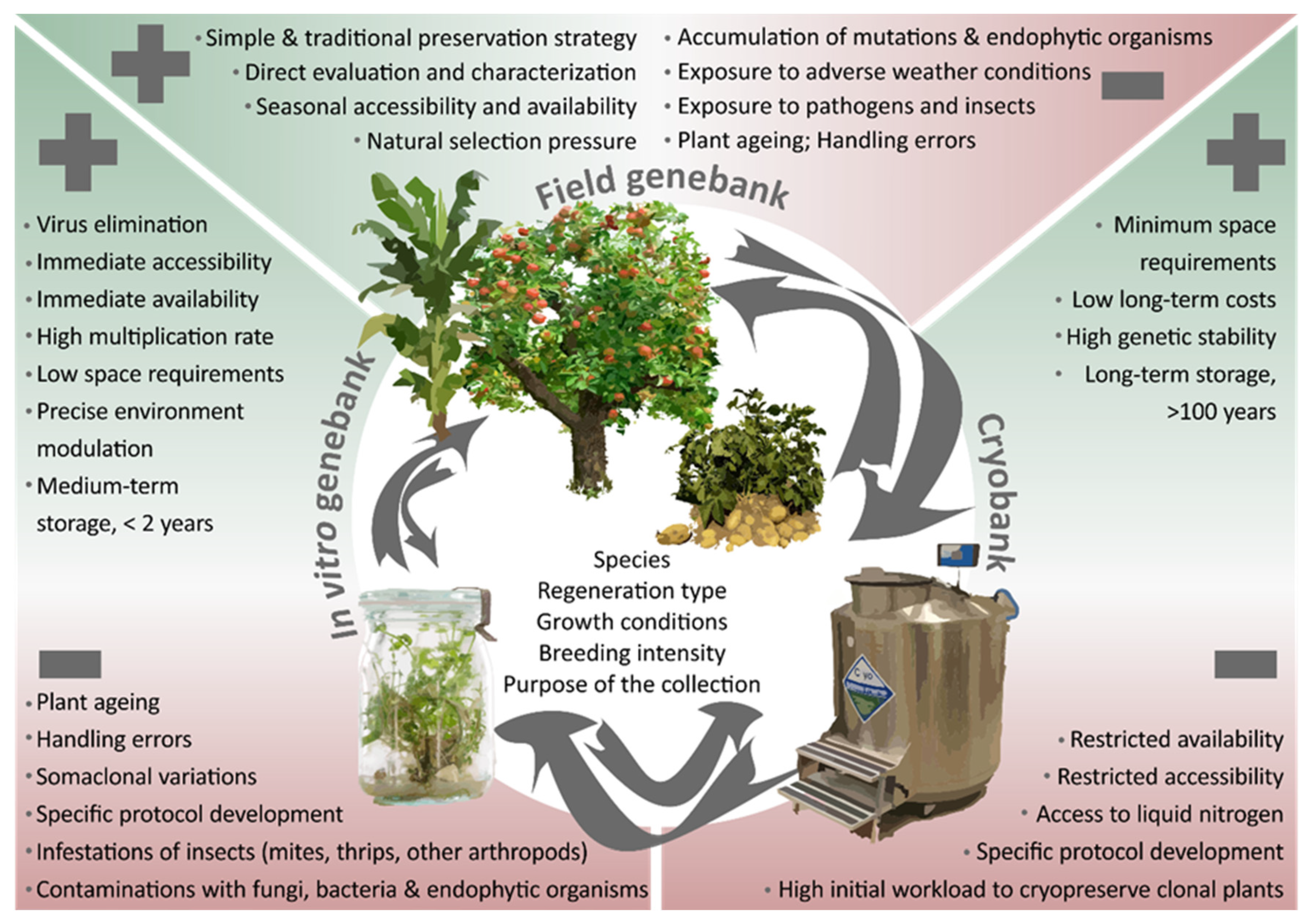
Two main ex ex situsitu conservation approaches can be distinguished for these crops: the conservation of plants in field genebanks and the maintenance of propagules in tissue culture, either (i) as active growing cultures in short- and medium-term storage (i.e., in vitro storage), or (ii) in frozen state at ultra-low temperature in liquid nitrogen for long-term storage (cryopreservation). These approaches, the challenges entailed, and prospects offered to secure crop diversity ex situ will be discussed in detail in this chapter and are depicted in Figure 1.
2. Ways of Conservation
2.1. Field Genebanks
Clonally propagated crops and their wild relatives belong to some 34 plant families, including herbs, shrubs, trees and vines [8]. Agronomically important genotypes that were selected over centuries for their specific properties can only be conserved in a vegetative mode. Consequently, the simplest and most traditional way to establish a collection is to gather specific genotypes from farmers’ fields, gardens or in the wild and then grow them in the field genebanks where they continue to grow when maintained appropriately. Even under the highest standards of management, germplasm maintained in the field can deteriorate due to a wide variety of climate conditions, ageing of the plants, diseases and pests, hence the need for timely regeneration. For example, depending on the rootstock and orchard conditions, apple (Malus sp.) trees may need to be repropagated periodically after 25–50 years [9].
Some of the largest collections were established at the beginning of the 20th century and are based on the efforts of passionate geneticists and plant explorers such as Nikolai I. Vavilov, Frank N. Meyer [10] and Hans Stubbe among others [11]. In the 1970s, the International Board for Plant Genetic Resources (IBPGR) promoted and sponsored numerous collection missions. To preserve clonal material, 23 field genebanks of nine major crops were established worldwide [12]. As such, approximately 400,000 accessions are currently held in field genebanks of international, regional and national authorities. A recent study commissioned by the Alliance of Bioversity International and the International Center for Tropical Agriculture (CIAT), the International Potato Center (CIP) and the Global Crop Diversity Trust [13] showed that of the 20 institutions surveyed, the vast majority of the clonal plant material is kept in the field. Worldwide, major genebanks maintain potato (98,285 accessions), apple (59,922), cassava (36,529), citrus (36,410), sweet potato (Ipomoea batatas, 35,478), coffee (Coffea spp., 30,483) and cacao (23,107). The largest field genebanks are located in the USA (potato, sweet potato and apple), Japan (apple, citrus and sweet potato), Russia (potato and apple) and Brazil (citrus and coffee). In addition, there are numerous smaller collections, such as for grape vine (Vitis vinifera L.), garlic (Figure 2), Jerusalem artichoke (Helianthus tuberosus L.), and Andean root crops, which are of high value as luxury foods and condiments or are of regional or religious significance.

2.2. In Vitro Collections
In vitro culture (or tissue culture) of plants is a biotechnological technique in which plant parts are isolated from in vivo plants, disinfected to free the explants from bacteria and fungi and transferred onto well-defined and sterile tissue culture media that provides the plant tissue with the necessary nutrients for growth and multiplication. In a relatively small space, the environment can be controlled precisely and plant growth can be easily observed and manipulated. In vitro approaches are commonly used for large-scale micro-propagation, reproduction purposes including embryo rescue, ploidy manipulations, protoplast fusions and somatic embryogenesis and are appropriate tools for short- and mid-term storage of plant genetic resources. Although the feasibility of using in vitro culture methods for plant genetic resources conservation was already known in the 1970s, it was only in the 1980s that the International Board for Plant Genetic Resources (IBPGR) established a working group of specialists to investigate the critical aspects of in vitro plant conservation [14][15]. Since then, in vitro collections have been setup for many vegetatively propagated crops. Additionally, Genebank Standards for PGRFA maintained in vitro were developed [1], forming the benchmark for establishing standard operating procedures and quality management systems to ensure effective, safe and efficient conservation of these genetic resources. Nowadays, in vitro collections for PGRFA comprise potato (9,700 accessions), cassava (8,700), sweet potato (6,400), yam (3,200), banana (2,000) and taro (1,200) [15][16][17]. The largest collections are maintained by international organizations such as Bioversity International, the International Center for Tropical Agriculture (CIAT), CIP, the International Institute of Tropical Agriculture (IITA) and in national institutes such as Brazilian Agricultural Research Corporation (EMBRAPA) in Brazil, CRI in Czech Republic, the IPK in Germany and the USDA-NPGS in the USA [13][17].
2.3. Cryopreserved Collections
Cryopreservation (or storage of biological material at ultra-low temperatures) is the obvious solution to the above-mentioned limitations, since at these conditions metabolic, physical and chemical alterations are unlikely to occur, even after hundreds of years of storage. Usually, cryogenic storage takes place in liquid nitrogen (−196°) or its vapor phase (between −140 and −180 °C). The main hurdle associated with cryopreservation is the formation of lethal ice crystals. Complete drying plant tissues, thus preventing the formation of ice crystals is not an option since the presence of water is inevitably linked with life. The only way to avoid ice crystal formation of a watery solution is by making use of the physical phase called “vitrification”, i.e., the solidification of a liquid forming an amorphous “or glassy” structure. All cryopreservation procedures developed for biological materials are based on optimizing the chance for vitrification. To attain this, two conditions must be met: (i) application of ultra-rapid cooling rates, limiting the time period that an ice crystal can form before all molecules are immobilized by the ultra-low temperature, and (ii) concentrating the cell solution resulting in relatively more molecules in interference with the organization of water molecules turning into ice crystals. Since the first report by Akira Sakai in 1965 [18] on the survival of plant tissues exposed to liquid nitrogen, a wide variety of plant cryopreservation protocols have been established, among
them dormant bud cryopreservation, classical (slow) freezing, encapsulation-dehydration, and a range of vitrifications solution-based protocols (for an overview, see [19]). Currently, dormant bud cryopreservation and droplet vitrification are commonly applied (Table 1).
Table 1. Cryopreservation methods used in world’s largest crop genebanks that use cryopreservation for storage of their vegetatively propagated germplasm.
| Institute | Country | Crop | Cryopreservation Method | Number of Accessions | Ref |
|---|---|---|---|---|---|
| AFOCEL | France | Elm | • Dormant bud freezing | 440 | [20] |
| Bioversity International, Leuven | Belgium | Banana | • Droplet vitrification | 1100 | Panis, personal communication, 2020 |
| Crop Research Institute, Prague | Czech Republic | garlic | • Droplet vitrification | 157 | [21] |
| International Center for Tropical Agriculture (CIAT), Cali | Colombia | cassava | • Droplet vitrification • Encapsulation/dehydration |
480 | [20] |
| International Institute of Tropical Agriculture (IITA), Ibadan | Nigeria | Yam | • Droplet vitrification | 27 | [22] |
| International Potato Center (CIP), Lima | Peru | Potato | • Straw vitrification • Droplet vitrification |
3264 (Situation 14 October 2020) | [23] |
| Julius Kühn-Institut (JKI), Institut für Züchtungsforschung an Obst, Dresden | Germany | Strawberry | • Vitrification | 194 | [24] |
| Leibniz Institute of Plant Genetics and Crop Plant Research (IPK), Gatersleben | Germany | Potato, | • Droplet freezing • Droplet vitrification |
1818 | Nagel, personal communication, 2020 |
| Leibniz Institute of Plant Genetics and Crop Plant Research (IPK), Gatersleben | Germany | Mint | • Droplet vitrification | 157 | Nagel, personal communication, 2020 |
| Leibniz Institute of Plant Genetics and Crop Plant Research (IPK), Gatersleben | Germany | Garlic and shallot | • Droplet vitrification | 213 | Nagel, personal communication, 2020 |
| National Agrobiodiversity Center (NAAS), RDA, Suwon | South Korea | Garlic | • Droplet vitrification | 1158 | [25] |
| National Institute of Agrobiological Sciences (NIAS), Tsukuba | Japan | Mulberry | • Dormant bud freezing | 1236 | [20] |
| Tissue Culture and Cryopreservation Unit, NBPGR, Delhi | India | Mulberry | • Dormant bud freezing | 329 | [20] |
| USDA-ARS, Ford Collins and Corvallis | USA | Citrus | • Droplet vitrification | 451 | [26] |
| USDA-ARS, Ford Collins and Corvallis | USA | Apple | • Dormant bud freezing | 2155 | [27] |
3. Conclusions
The objective of a genebank is to conserve crop genetic resources. The advantage of a field genebank is that characterization and evaluation can be performed on mature plants at limited additional cost. Yet, the method has significant restrictions regarding its security, costs, and sustainability [28]. In this respect, in in vitrovitro conservation offers advantages over field conservation and is recognized as an invaluable complementary approach to secure the diversity of the collection. With the potential that tissue culture protocols can be developed for practically any plant species, the technique has been successfully applied over the past decades for short- to medium-term conservation of a wide range of crop species [29]. While offering the possibility of disease elimination and rapid clonal propagation of healthy plants, it is a suitable method for exchanging germplasm accommodating national and international phytosanitary requirements and regulations. However, the possible occurrence of genetic instability as a result of enhanced selection pressure under in in vitrovitro conditions compared to in vivo may be an obstacle to its use for long-term preservation of plant germplasm [30]. The chance that somatic mutations occur in tissues maintained at the ultra-low storage temperature of liquid nitrogen (−196 °C) is very slim since all metabolic processes are suspended [31]. In this regard, cryopreservation is the best option for unlimited and secure conservation of regenerative tissues in the long term. Nevertheless, the latter also requires adequate infrastructure, reliable electricity provision and well-trained staff.
The global COVID-19 pandemic has reminded us that particularly clonal ex ex situsitu collections in the field and in vitro that require continuous maintenance are vulnerable and insufficiently secured. “Essential” activities for maintenance of the germplasm in gene banks were temporarily interrupted for concerns out of human safety and health reasons, hence putting valuable genetic resources at risk of loss. While the initial costs of developing a crop- or species-specific cryopreservation protocol [32] and labor input for processing the samples in liquid nitrogen are high, once established, the maintenance of a cryopreserved collection requires very little input of resources including human intervention, making it probably the most cost-effective and secure option for long-term conservation.
References
- Genebank Standards for Plant Genetic Resources for Food and Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014.
- Gepts, P.; Hancock, J. The future of plant breeding. Crop Sci. 2006, 46, 1630–1634.
- Building on Gender, Agrobiodiversity and Local Knowledge; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004.
- Thrupp, L.A. Linking agricultural biodiversity and food security: The valuable role of agrobiodiversity for sustainable agriculture. Int. Aff. 2000, 76, 265–281.
- The State of the World’s Biodiversity for Food and Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019.
- WIEWS—World Information and Early Warning System on Plant Genetic Resources for Food and Agriculture; Food and Agriculture Orgnization of the United Nations: Rome, Italy, 2020.
- Rajasekharan, P.E.; Sahijram, L. In vitro conservation of plant germplasm. In Plant Biology and Biotechnology; Bahadur, B., Venkat Rajam, M., Sahijram, L., Krishnamurthy, K., Eds.; Springer: New Delhi, India, 2015.
- McKey, D.; Elias, M.; Pujol, B.; Duputie, A. The evolutionary ecology of clonally propagated domesticated plants. New Phytol. 2010, 186, 318–332.
- Bramel, P.J.; Volk, G.M. A Global Strategy for the Conservation and Use of Apple Genetic Resources; Global Crop Diversity Trust: Bonn, Germany, 2019.
- Damania, A.B. History, achievements, and current status of genetic resources conservation. Agron. J. 2008, 100, S27–S39.
- Müntz, K.; Wobus, U. Das Institut Gatersleben und Seine Geschichte Genetik und Kulturpflanzenforschung in Drei Politischen Systemen; Springer spectrum: Berlin/Heidelberg, Germany, 2013; p. 459.
- Hawkes, J.G.; Maxted, N.; Ford-Lloyd, B.V. The Ex Situ Conservation of Plant Genetic Resources; Springer: Dordrecht, The Netherlands, 2000.
- Acker, J.P.; Adkins, S.; Alves, A.; Horna, D.; Toll, J. Feasibility Study for a Safety Backup Cryopreservation Facility; Bioversity International: Maccarese-Stazione, Italy, 2017.
- Withers, L.A. Tissue Culture Storage for Genetic Conservation; IBPGR: Rome, Italy, 1980.
- Benson, E.E.; Harding, K.; Debouck, D.; Dumet, D.; Escobar, R.; Mafla, G.; Panis, B.; Panta, A.; Tay, D.; Vandenhouwe, I.; et al. Refinement and Standardization of Storage Procedures for Clonal Crops = Global Public Goods Phase 2: Part 1. Project Landscape and General Status of Clonal Crop In Vitro Conservation Technologies; System-wide Genetic Resources Programme: Rome, Italy, 2011.
- Genesys. Available online: https://www.genesys-pgr.org/ (accessed on 20 November 2020).
- Bamberg, J.B.; Martin, M.W.; Abad, J.; Jenderek, M.M.; Tanner, J.; Donnelly, D.J.; Nassar, A.M.K.; Veilleux, R.E.; Novy, R.G. In vitro technology at the US Potato Genebank. In Vitro Cell Dev. Plant 2016, 52, 213–225.
- Sakai, A. Survival of plant tissue at super-low temperature III. relation between effective prefreezing temperatures and the degree of front hardiness. Plant Physiol. 1965, 40, 882–887.
- Panis, B. Sixty years of plant cryopreservation: From freezing hardy mulberry twigs to establishing reference crop collections for future generations. In Proceedings of the III International Symposium on Plant Cryopreservation, Bangkok, Thailand, 26–28 March 2018; pp. 1–8.
- Niino, T.; Arizaga, M.V. Cryopreservation for preservation of potato genetic resources. Breed Sci. 2015, 65, 41–52.
- Wang, M.R.; Lambardi, M.; Engelmann, F.; Pathirana, R.; Panis, B.; Volk, G.M.; Wang, Q.C. Advances in cryopreservation of in vitro-derived propagules: Technologies and explant sources. Plant Cell Tissue Org. 2020.
- Dumet, D.; Diebiru, E.; Adeyemi, A.; Akinyemi, O.; Gueye, B.; Franco, J. Cryopreservation for the ‘in perpetuity’ conservation of yam and cassava genetic resources. Cryoletters 2013, 34, 107–118.
- CIP. Cryopreservation. Available online: https://cipotato.org/genebankcip/process/cryopreservation/ (accessed on 14 October 2020).
- Hofer, M.; Hanke, M.V. Cryopreservation of fruit germplasm. In Vitro Cell Dev. Plant 2017, 53, 372–381.
- Kim, H.H.; Popova, E.; Shin, D.J.; Yi, J.Y.; Kim, C.H.; Lee, J.S.; Yoon, M.K.; Engelmann, F. Cryobanking of Korean Allium Germplasm Collections: Results from a 10 Year Experience. Cryoletters 2012, 33, 45–57.
- Volk, G.M.; Jenderek, M.M.; Walters, C.; Bonnart, R.; Shepherd, A.; Skogerboe, D.; Hall, B.D.; Moreland, B.; Krueger, R.; Polek, M. Implementation of Citrus shoot tip cryopreservation in the USDA-ARS National Plant Germplasm System. In Proceedings of the III International Symposium on Plant Cryopreservation, Bangkok, Thailand, 26–28 March 2018.
- Jenderek, M.M.; Tanner, J.D.; Ambruzs, B.D.; West, M.; Postman, J.D.; Hummer, K.E. Twig pre-harvest temperature significantly influences effective cryopreservation of Vaccinium dormant buds. Cryobiology 2017, 74, 154–159.
- Reed, B.M.; Engelmann, F.; Dulloo, M.E.; Engels, J.M.M. Technical Guidelines for the Management of Field and In Vitro Germplasm Collections; International Plant Genetics Resources Institute: Rome, Italy, 2004.
- Ashmore, S.E. Status Report on the Development and Application of In Vitro Techniques for the Conservation and Use of Plant Genetic Resources; International Plant Genetic Resources Institute: Rome, Italy, 1997.
- da Silva, R.L.; Ferreira, C.F.; da Silva Ledo, C.A.; de Souza, E.H.; da Silva, P.H.; de Carvalho Costa, M.A.P.; Souza, F.V.D. Viability and genetic stability of pineapple germplasm after 10 years of in vitro conservation. Plant Cell Tissue Organ Cult. (PCTOC) 2016, 127, 123–133.
- Kaviani, B. Conservation of plant genetic resources by cryopreservation. Aust. J. Crop Sci. 2011, 5, 778–800.
- Pence, V.C. Tissue cryopreservation for plant conservation: Potential and challenges. Int. J. Plant Sci. 2014, 175, 40–45.
