Corrosion inhibitors are substances that reduce or eliminate the corrosion of a metal in a certain environment. Corrosion inhibitors act by several mechanisms, including adsorption, film formation, passivation, and oxygen scavenging. Due to their toxicity, classic corrosion inhibitors affect the environment. Therefore, in recent years, more and more studies have focused on the development of eco-friendly inhibitors for the environment. In this study, ethanolic extract of Galium verum (GV) was tested for the inhibition of steel corrosion in 1 M HCl medium using electrochemical methods: open circuit potential (OCP), potentiodynamic polarization (PP), and electrochemical impedance spectroscopy (EIS). Reverse-phase liquid chromatography (HPLC) and gas chromatography mass spectrometry (MS-GC) previous studies state that GV extract contains polyphenols and other chemical species responsible for the inhibitory effect. Corrosion investigations have highlighted the influence of the concentration of the GV extract, in the range of 50 ÷ 400 ppm G.A.E./mL, as well as the influence of temperature in the range of 20 ÷ 50 °C. The corrosion inhibitory efficiency of the Galium verum ethanolic extract had a maximum value of 91.82% for a concentration of 400 ppm polyphenol content, demonstrating the inhibitory potential of this green product in an acidic environment for mild steel. Statistical calculus on the obtained values of EIS inhibitor efficiency showed that the effect of the extract becomes stronger at higher concentrations.
- Galium verum ethanolic extract
- polyphenols
- chromatography
- steel corrosion eco-inhibitor
- chlorohydric acid
- statistics
1. GV Extract—A New Corrosion Eco-Inhibitor
A notable aspect of this work is the focus on Galium verum, a plant species previously
underexplored from this perspective, or corrosion inhibitor. This study bridges gaps in
knowledge, opening avenues for further research on its multifaceted applications.
Comprehensive chromatographic analyses (HPLC, GC-MS) identified critical metabolites
responsible for bioactivity [27][1].
Substances such as kaempferol, umbelliferone, quercetin, gallic acid, vanillic acid, and
caffeic acid were found in abundance, contributing to the extract’s efficacy.
This study differs from previous works by using a plant that has not been previously
investigated as a corrosion inhibitor. In addition, a detailed characterization of the active
substances responsible for the inhibition, comparing their efficiency with that of other
natural and synthetic inhibitors. The results obtained are comparable to those reported in
the literature, but our contribution consists in highlighting the potential of a completely
new and sustainable source.
In TableTable 1 12 there is a comparison that highlights that plant-based inhibitors, and so
is GV extract, are a promising alternative for sustainable applications, although classical
inhibitors remain preferred in stringent industrial environments.
The GV extract has all the advantages of green inhibitors, extracted from plants, but
it also has some specific advantages: GC is a spontaneous plant, widespread in Europe,
Asia, and North Africa, with no environmental impact. Plant culture could be used both
for phytotherapeutic and industrial purposes.
As a corrosion inhibitor in acid media, the efficiency is very good, with a maximum
value of 91.82%, close to classical ones.

Results of analytical investigations on ethanolic GV extract proved it to be a
polyphenol-based green inhibitor [27][1]. In comparison with other chemical categories
of substances discovered in plant extracts, polyphenols from GV extract act as a mixed
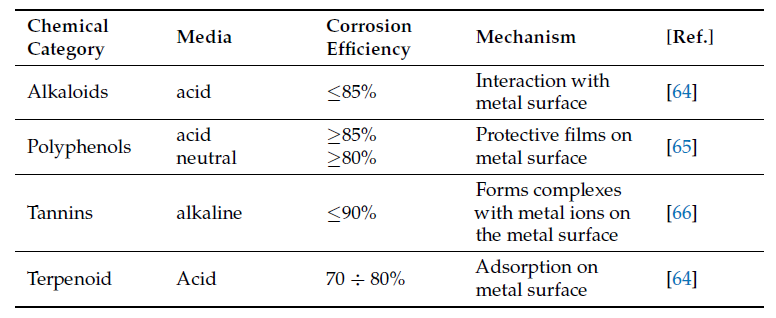
inhibitor by forming protective films on metal surfaces. The main known categories of phytochemicals
responsible for corrosion inhibition are alkaloids [64][2], phenolic compounds [65][3],
tannins [66][4], and triterpenes [64][2]. The complex chemical compositions of these plant extracts
make it very difficult to attribute the inhibitory action to a particular component or
group of components. Thus, phenolic compounds generated more interest because of their
high redox potential, which allows them to act as reducing agents, hydrogen donors, and
singlet oxygen inhibitors, and also because of their metal chelating potential [1][5]. InIn Table 2, Table 13
a comparison between different chemical categories of plant inhibitors is presented.
Table 12. Comparison of GV extract vs. other plant inhibitors and classical inhibitors.
Table 1. Comparison of GV extract vs. other plant inhibitors and classical inhibitors.

Table 13. Comparison of corrosion behavior of different types of chemical compounds from plant
extracts.
Table 2. Comparison of corrosion behavior of different types of chemical compounds from plant extracts.
| Chemical Category | Media | Corrosion Efficiency | Mechanism | [Ref.] |
|---|---|---|---|---|
| Alkaloids | acid | ≤85% | Interaction with metal surface | [2] |
| Polyphenols | acid neutral |
≥85% ≥80% |
Protective films on metal surface | [3] |
| Tannins | alkaline | ≤90% | Forms complexes with metal ions on the metal surface | [4] |
| Terpenoid | Acid | 70 ÷ 80% | Adsorption on metal surface | [2] |
Detailed explanation of polyphenol action as corrosion inhibitors is given below.
Derived from renewable natural resources, the GV extract could be a part of a circular
economy and reduce the utilization of classical, non-renewable, synthetic inhibitors. Because
the plant is readily available, it will involve low production costs. Being already part
of pharmacopeia, not only in Eastern Europe but also in other regions, GV provides fewer
risks to human health or the ecosystem.
The research also explored potential sustainable applications for this extract, leveraging
the eco-friendly and biodegradable nature of plant-based products.
The findings pave the way for future studies on enhancing extraction and/or investigation
techniques and broadening applications to include not only therapeutic but also
industrial fields.
These advantages make GV extract, a plant-based inhibitor, a promising solution for
industries looking to adopt greener and more sustainable practices.
Consideration on Inhibitor Corrosion Mechanism of GV Extract
2. Consideration on Inhibitor Corrosion Mechanism of GV Extract
From the electrochemical experimental results, Galium verum extract was shown to be
a mixed type of inhibitor that acts on both the anodic metal dissolution reaction and the
cathodic hydrogen evolution reaction.
At carbon steel corrosion in an acidic medium, a sequence of steps could be identified
in the corrosion mechanism [61]. Thus, the next electrode processes could be mentioned:
the anodic carbon steel dissolution reactions and the cathodic hydrogen evolution reactions:
On the steel surface a film is formed by the adsorption of GV extract, by the interaction
of iron ions and plant extract compounds.
Absorption inhibitors are substances that can selectively adsorb either the anodic
zones or the entire metal surface, forming passivation films that slow down the dissolution
of metal or stop the diffusion of the depolarizer in the cathodic areas.
Adsorption inhibitors are polar molecules that adsorb on the metal surface, inhibiting
both electrode processes but especially the cathodic process of hydrogen discharge. These
inhibitors are particularly effective in very small quantities (10−5 ÷ 10−3 mol/L) because
they act in almost monomolecular layers. The greatest effectiveness of adsorption inhibitors
is in acidic media.
Adsorption of inhibitors is a particular case, electrosorption occurring through a
substitution reaction of solvent molecules oriented towards the metal surface, and it is also
influenced by the electrical variable of the interface, i.e., the electrode potential. Adsorption
of organic molecules influences the surface tension and the differential capacitance of the
electrochemical double layer, their maximum adsorption occurring near zero charge. In
practice, adsorption inhibitors are surface-active substances or surfactants (Figure 17Figure 2).

Figure 172. Schematic representation of the adsorption of corrosion inhibitors competing corrosion products on the metal surface.
The adsorption mechanism is dependent on the polarizability of the molecules. The asymmetric chemical structure, formed by two parts, one polar – acting as an anchor and one non-polar, oriented towards the corrosive environment, formed by the hydrocarbon residue and which forms the actual adsorption layer, which isolates the metal and prevents the corrosion reaction. Among the most effective anchor functional groups are -CH2-OH, -COOH, -CHO, -COOR, molecules that are found in abundance in the GV extract. In a previous study [1], a chemical characterization of Galium Verum extracts using analytical techniques identified and measured the presence of polyphenols: gallic acid, catechin, vanillic acid, caffeic acid, kaempferol, quercetin, and umbelliferon and others compounds including alcohols, phenols, fatty acids, hydrocarbons, esters and other aromatic compounds.
The adsorption mechanism is dependent on the polarizability of the molecules. The asymmetric chemical structure, formed by two parts, one polar – acting as an anchor and one non-polar, oriented towards the corrosive environment, formed by the hydrocarbon residue and which forms the actual adsorption layer, which isolates the metal and prevents the corrosion reaction. Among the most effective anchor functional groups are -CH2-OH, -COOH, -CHO, -COOR, molecules that are found in abundance in the GV extract. In a previous study [27], a chemical characterization of Galium Verum extracts using analytical techniques identified and measured the presence of polyphenols: gallic acid, catechin, vanillic acid, caffeic acid, kaempferol, quercetin, and umbelliferon and others compounds including alcohols, phenols, fatty acids, hydrocarbons, esters and other aromatic compounds. Size, spatial orientation, shape and electrical charge play an important role in the inhibition mechanism. The larger the volume of a molecule (aromatic compounds for example), the greater the inhibition capacity. The nature of the metal is also important. Metals in the Fe group, having free “d” electronic levels, tend to desorb organic molecules with “π” electrons, i.e. polar molecules, containing O, N, S.
Size, spatial orientation, shape and electrical charge play an important role in the inhibition mechanism. The larger the volume of a molecule (aromatic compounds for example), the greater the inhibition capacity. The nature of the metal is also important. Metals in the Fe group, having free “d” electronic levels, tend to desorb organic molecules with “π” electrons, i.e. polar molecules, containing O, N, S.Also, the greater the number of polar groups, as in the case of polyphenols, the more intense is the corrosion inhibition.
Also, the greater the number of polar groups, as in the case of polyphenols, the more intense is the corrosion inhibition. Taking into consideration the above arguments, among the substances present in plant extract to be corrosion inhibitors, polyphenols could be considered the main actors in limiting the corrosion rate of metals.
Taking into consideration the above arguments, among the substances present in plant extract to be corrosion inhibitors, polyphenols could be considered the main actors in limiting the corrosion rate of metals3.
Conclusions
Due to the variety of existing plants and their constituent substances, in order to replace the dangerous toxic substances that have proven their effectiveness in protecting metals against corrosion, studies are being carried out to test plant extracts against corrosion. Most extracts are prepared from leaves, but they have also been reported for other parts of plants. Acidic environments are the most tested corrosive environments, being encountered in various industrial processes, and carbon steel is one of the most widely used industrial materials, therefore the protection of carbon steel is the most researched. The use of natural corrosion inhibitors, obtained from plants, brings significant environmental benefits. These biodegradable compounds reduce pollution and risks associated with synthetic chemicals, which can be toxic and difficult to break down. Furthermore, extracting inhibitors from plant sources promotes a sustainable process, avoiding the contamination of soil and water with hazardous substances. This approach helps protect ecosystems and reduce the negative impact on human health and nature. In essence, choosing nature is an important step towards a greener future.
In this work, the inhibitory effect of the extract of Galium Verum (GV) was evaluated as a corrosion inhibitor for carbon steel in 1M HCl solutions. Studies were carried out in the temperature range 20 - 50°C.
The determinations of the potentiaoncl in the open circuit made at all the studied temperatures showed the displacement of the potential towardsions more positive values in the solutions with the addition of GV extract compared to the results obtained in the control solution of 1M HCl.
The results of the Tafel-potentiodynamic polarization studies showed that the presence of the GV extract changed the corrosion potential towards more positive values and the variation of the cathodic slopes was more pronounced than the anodic slopes, suggesting that the GV extract is a mixed inhibitor, predominantly cathodic. Electrochemical impedance spectroscopy studies showed an increase in polarization resistance in the presence of GV extract compared to the results obtained in its absence.Due to the variety of existing plants and their constituent substances, in order to replace the dangerous toxic substances that have proven their effectiveness in protecting metals against corrosion, studies are being carried out to test plant extracts against corrosion. Most extracts are prepared from leaves, but they have also been reported for other parts of plants. Acidic environments are the most tested corrosive environments, being encountered in various industrial processes, and carbon steel is one of the most widely used industrial materials, therefore the protection of carbon steel is the most researched. The use of natural corrosion inhibitors, obtained from plants, brings significant environmental benefits. These biodegradable compounds reduce pollution and risks associated with synthetic chemicals, which can be toxic and difficult to break down. Furthermore, extracting inhibitors from plant sources promotes a sustainable process, avoiding the contamination of soil and water with hazardous substances. This approach helps protect ecosystems and reduce the negative impact on human health and nature. In essence, choosing nature is an important step towards a greener future.
Green inhibitors have been reported to have an inhibition efficiency around 60-95% which means that Galium Verum is a very good one. The corrosion inhibitory efficiency of the Galium Verum ethanolic extract had a maximum value of 91.82%, for a concentration of 400 ppm polyphenol content, demonstrating the inhibitory potential of this green product comparable with synthetic inhibitors for steel in acid media. Obtained values of the inhibition efficiencies for mild steel in 1 M HCl were between 19.92% at the addition of 50 ppm GAE at 30 oC and 91.82% at 400 ppm GAE at the temperature of 40°C. Temperature over 40 oC decrease the inhibition efficiency of GV extract, due to less stability of polyphenols, the chemical species responsible for inhibition effect. Polyphenols and others polar molecules contained in GV-extract are adsorption corrosion inhibitors, acting after a mixed mechanism.In this work, the inhibitory effect of the extract of Galium Verum (GV) was evaluated as a corrosion inhibitor for carbon steel in 1M HCl solutions. Studies were carried out in the temperature range 20 - 50°C.
The presence of the GV extract led to the reduction of the corrosion rate, the results being comparable to the values obtained for other plant extracts, over 90%, therefore promising for the use of the GV extract as a green inhibitor of steel corrosion in acid.The determinations of the potential in the open circuit made at all the studied temperatures showed the displacement of the potential towards more positive values in the solutions with the addition of GV extract compared to the results obtained in the control solution of 1M HCl.
GV-extract proved to be a new corrosion inhibitor, with specific advantages : spontaneous flora, widespread on globe, sustainability, cost-effectiveness, high inhibitor efficiency, reduced health risks.The results of the Tafel-potentiodynamic polarization studies showed that the presence of the GV extract changed the corrosion potential towards more positive values and the variation of the cathodic slopes was more pronounced than the anodic slopes, suggesting that the GV extract is a mixed inhibitor, predominantly cathodic. Electrochemical impedance spectroscopy studies showed an increase in polarization resistance in the presence of GV extract compared to the results obtained in its absence.
Electrochemical, analytical and statistical techniques from this study establish the mechanism of corrosion and the chemical species involved, but is not exhaustive, further investigations will be continued, more powerful methods should be used to provide additional information, to analyze in detail the interaction of the inhibitor with the metal surface.Green inhibitors have been reported to have an inhibition efficiency around 60-95% which means that Galium Verum is a very good one. The corrosion inhibitory efficiency of the Galium Verum ethanolic extract had a maximum value of 91.82%, for a concentration of 400 ppm polyphenol content, demonstrating the inhibitory potential of this green product comparable with synthetic inhibitors for steel in acid media. Obtained values of the inhibition efficiencies for mild steel in 1 M HCl were between 19.92% at the addition of 50 ppm GAE at 30°C and 91.82% at 400 ppm GAE at the temperature of 40°C. Temperature over 40°C decrease the inhibition efficiency of GV extract, due to less stability of polyphenols, the chemical species responsible for inhibition effect. Polyphenols and others polar molecules contained in GV-extract are adsorption corrosion inhibitors, acting after a mixed mechanism.
References
The presence of the GV extract led to the reduction of the corrosion rate, the results being comparable to the values obtained for other plant extracts, over 90%, therefore promising for the use of the GV extract as a green inhibitor of steel corrosion in acid.
- Rajni, R. Industrial applications of green chemistry: Status, Challenges and Prospects. SN Appl. Sci. 2020, 2, 263. [Google Scholar] [CrossRef]
- Lgaz, H.; Lee, H.-S. Computational Exploration of Phenolic Compounds in Corrosion Inhibition: A Case Study of Hydroxytyrosol and Tyrosol. Materials 2023, 16, 6159. [Google Scholar] [CrossRef] [PubMed]
- Al-Moubaraki, A.H.; Chaouiki, A.; Alahmari, J.M.; Al-Hammadi, W.A.; Noor, E.A.; Al-Ghamdi, A.A.; Ko, Y.G. Development of Natural Plant Extracts as Sustainable Inhibitors for Efficient Protection of Mild Steel: Experimental and First-Principles Multi-Level Computational Methods. Materials 2022, 15, 8688. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, J.K. Corrosion protection of mild steel in corrosive media, a shift from synthetic to natural corrosion inhibitors: A review. Bull. Natl. Res. Cent. 2024, 48, 26. [Google Scholar] [CrossRef]
- Gabsi, M.; Ferkous, H.; Delimi, A.; Boublia, A.; Boulechfar, C.; Kahlouche, A.; Darwish, A.S.; Lemaoui, T.; Benguerba, Y. The curious case of polyphenols as green corrosion inhibitors: A review on their extraction, design, and applications. Environ. Sci. Pollut. Res. 2023, 30, 59081–59105. [Google Scholar] [CrossRef]
- Zaher, A.; Aslam, R.; Lee, H.S.; Khafouri, A.; Boufellous, M.; Alrashdi, A.A.; Lgaz, H.; Ouhssine, M. A combined computational & electrochemical exploration of the Ammi visnaga L. extract as a green corrosion inhibitor for carbon steel in HCl solution. Arab. J. Chem. 2022, 15, 103573. [Google Scholar] [CrossRef]
- Khadom, A.A.; Abd, A.N.; Ahmed, N.A.; Kadhim, M.M.; Fadhil, A.A. Combined influence of iodide ions and Xanthium Strumarium leaves extract as eco-friendly corrosion inhibitor for low-carbon steel in hydrochloric acid. Curr. Res. Green Sustain. Chem. 2022, 5, 100278. [Google Scholar] [CrossRef]
- da Silva, M.V.L.; de Britto Policarpi, E.; Spinelli, A. Syzygium cumini leaf extract as an eco-friendly corrosion inhibitor for carbon steel in acidic medium. J. Taiwan Inst. Chem. Eng. 2021, 129, 342–349. [Google Scholar] [CrossRef]
- Prasad, D.; Dagdag, O.; Safi, Z.; Wazzan, N.; Guo, L. Cinnamoum tamala leaves extract highly efficient corrosion bio-inhibitor for low carbon steel: Applying computational and experimental studies. J. Mol. Liq. 2022, 347, 118218. [Google Scholar] [CrossRef]
- Moustafa, A.H.E.; Abdel-Rahman, H.H.; Awad, M.K.; Naby, A.A.N.A.; Seleim, S.M. Molecular dynamic simulation studies and surface characterization of carbon steel corrosion with changing green inhibitors concentrations and temperatures. Alex. Eng. J. 2022, 61, 2492–2519. [Google Scholar] [CrossRef]
- Huang, L.; Yang, K.P.; Zhao, Q.; Li, H.J.; Wang, J.Y.; Wu, Y.C. Corrosion resistance and antibacterial activity of procyanidin B2 as a novel environment-friendly inhibitor for Q235 steel in 1 M HCl solution. Bioelectrochemistry 2022, 143, 107969. [Google Scholar] [CrossRef] [PubMed]
- Zehra, B.F.; Said, A.; Eddine, H.M.; Hamid, E.; Najat, H.; Rachid, N.; Toumert, L.I. Crataegus oxyacantha leaves extract for carbon steel protection against corrosion in 1M HCl: Characterization, electrochemical, theoretical research, and surface analysis. J. Mol. Struct. 2022, 1259, 132737. [Google Scholar] [CrossRef]
- Abbout, S.; Chebabe, D.; Zouarhi, M.; Rehioui, M.; Lakbaibi, Z.; Hajjaji, N. Ceratonia Siliqua L seeds extract as eco-friendly corrosion inhibitor for carbon steel in 1 M HCl: Characterization, electrochemical, surface analysis, and theoretical studies. J. Mol. Struct. 2021, 1240, 130611. [Google Scholar] [CrossRef]
- Fernine, Y.; Salim, R.; Arrousse, N.; Haldhar, R.; El Hajjaji, F.; Kim, S.C.; Touhami, M.E.; Taleb, M. Anti-corrosion performance of Ocimum basilicum seed extract as environmental friendly inhibitors for mild steel in HCl solution: Evaluations of electrochemical, EDX, DFT and Monte Carlo. J. Mol. Liq. 2022, 355, 118867. [Google Scholar] [CrossRef]
- Zakaria, F.A.; Hamidon, T.S.; Hussin, M.H. Applicability of winged bean extracts as organic corrosion inhibitors for reinforced steel in 0.5 M HCl electrolyte. J. Indian Chem. Soc. 2022, 99, 100329. [Google Scholar] [CrossRef]
- Damej, M.; Skal, S.; Aslam, J.; Zouarhi, M.; Erramli, H.; Alrashdi, A.A.; Lee, H.S.; Lgaz, H. An environmentally friendly formulation based on Cannabis sativa L. seed oil for corrosion inhibition of E24 steel in HCl medium: Experimental and theoretical study. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128745. [Google Scholar] [CrossRef]
- Ghalib, L.; Al Jaaf, H.J.M.; Abdulghani, H.A. Temperature effect on the efficiency of Eucalyptus Camaldulensis leaves in the acid corrosion of carbon steel. Mater. Today Proc. 2021, 42, 2475–2481. [Google Scholar] [CrossRef]
- Shahini, M.H.; Keramatinia, M.; Ramezanzadeh, M.; Ramezanzadeh, B.; Bahlakeh, G. Combined atomic-scale/DFT-theoretical simulations & electrochemical assessments of the chamomile flower extract as a green corrosion inhibitor for mild steel in HCl solution. J. Mol. Liq. 2021, 342, 117570. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, L.; Zhu, M.; Wang, K.; Zhang, R.; He, Z.; Lin, Y.; Leng, S.; Anadebe, V.C.; Zheng, X. Akebia trifoliate koiaz peels extract as environmentally benign corrosion inhibitor for mild steel in HCl solutions: Integrated experimental and theoretical investigations. J. Ind. Eng. Chem. 2021, 101, 227–236. [Google Scholar] [CrossRef]
- Shahmoradi, A.R.; Ranjbarghanei, M.; Javidparvar, A.A.; Guo, L.; Berdimurodov, E.; Ramezanzadeh, B. Theoretical and surface/electrochemical investigations of walnut fruit green husk extract as effective inhibitor for mild-steel corrosion in 1M HCl electrolyte. J. Mol. Liq. 2021, 338, 116550. [Google Scholar] [CrossRef]
- Hossain, N.; Chowdhury, M.A.; Iqbal, A.P.; Islam, M.S.; Omar, N.Y.S.; Saifullah, A.Z.A. Paederia Foetida leaves extract as a green corrosion inhibitor for mild steel in hydrochloric acid solution. Curr. Res. Green Sustain. Chem. 2021, 4, 100191. [Google Scholar] [CrossRef]
- Aigbogun, J.A.; Adebayo, M.A. Green inhibitor from Thaumatococcus daniellii Benn for corrosion mitigation of mild steel in 1M HCl. Curr. Res. Green Sustain. Chem. 2021, 4, 100201. [Google Scholar] [CrossRef]
- Manh, T.D.; Huynh, T.L.; Thi, B.V.; Lee, S.; Yi, J.; Nguyen Dang, N. Corrosion inhibition of mild steel in hydrochloric acid environments containing sonneratia caseolaris leaf extract. ACS Omega 2022, 7, 8874–8886. [Google Scholar] [CrossRef] [PubMed]
- Lambert, P.; Said-Ahmed, M.; Jama, C.; Lebrini, M. Molecules from Sargassum algae as Green Inhibitor for C38 in HCl Medium: Extraction, Characterization and Electrochemical Study. Coatings 2023, 13, 2076. [Google Scholar] [CrossRef]
- Prabakaran, M.; Hemapriya, V.; Kim, S.H.; Chung, I.-M. Evaluation of Antioxidant and Anticorrosion Properties of Epipremnum aureum. Arab. J. Sci. Eng. 2019, 44, 169–178. [Google Scholar] [CrossRef]
- Mauro, A.C.; Ribeiro, B.D.; Garrett, R.; Borges, R.M.; da Silva, T.U.; de Paula Machado, S.; de Araujo, J.R.; de Oliveira Massafra, S.; de Oliveira Junior, F.O.R.; D’Elia, E. Ziziphus joazeiro Stem Bark Extract as a Green Corrosion Inhibitor for Mild Steel in Acid Medium. Processes 2021, 9, 1323. [Google Scholar] [CrossRef]
- Badea, G.E.; Stănăşel, O.; Bassyouni, M.; Toderaș, M.; Petrehel, A.I.G.; Ionaș, C.D. An investigation on the antioxidant capabilities, chemical analysis and potential green applications of the yellow bedstraw (galium verum) ethanol extract. 2024. [Google Scholar] [CrossRef]
- Badea, G.E.; Dzitac, S.; Marin, L.; Petrehele, A.I.G.; Porumb, C.; Badea, P.G. An Investigation on the Electrochemical Behavior of Steel in the Presence of an Eco-inhibitor. In Proceedings of the 17th International Conference on Engineering of Modern Electric Systems (EMES), Oradea, Romania, 9–10 June 2023; pp. 1–4. [Google Scholar] [CrossRef]
- Bavcon, J.; Malovrh, K.; Tomšič, M.; Ravnjak, B. In Situ Conservation of Dry Meadows. Land 2024, 13, 315. [Google Scholar] [CrossRef]
- Kim, E.-J.; Lee, S.-H.; Kim, S.-H.; Park, J.-H.; You, Y.-H. Changes in Competitors, Stress Tolerators, and Ruderals (CSR) Ecological Strategies after the Introduction of Shrubs and Trees in Disturbed Semiarid Steppe Grasslands in Hulunbuir, Inner Mongolia. Biology 2023, 12, 1479. [Google Scholar] [CrossRef]
- Hategan, A.R.; Puscas, R.; Cristea, G.; Dehelean, A.; Guyon, F.; Molnar, A.J.; Mirel, V.; Magdas, D.A. Opportunities and Constraints in Applying Artificial Neural Networks (ANNs) in Food Authentication. Honey—A Case Study. Appl. Sci. 2021, 11, 6723. [Google Scholar] [CrossRef]
- Tava, A.; Biazzi, E.; Ronga, D.; Avato, P. Identification of the Volatile Components of Galium verum L. and Cruciata leavipes Opiz from the Western Italian Alps. Molecules 2020, 25, 2333. [Google Scholar] [CrossRef]
- Mocan, A.; Diuzheva, A.; Bădărău, S.; Moldovan, C.; Andruch, V.; Carradori, S.; Campestre, C.; Tartaglia, A.; De Simone, M.; Vodnar, D.; et al. Liquid Phase and Microwave-Assisted Extractions for Multicomponent Phenolic Pattern Determination of Five Romanian Galium Species Coupled with Bioassays. Molecules 2019, 24, 1226. [Google Scholar] [CrossRef] [PubMed]
- Laanet, P.R.; Saar-Reismaa, P.; Jõul, P.; Bragina, O.; Vaher, M. Phytochemical Screening and Antioxidant Activity of Selected Estonian Galium Species. Molecules 2023, 28, 2867–2884. [Google Scholar] [CrossRef]
- Turcov, D.; Barna, A.S.; Trifan, A.; Blaga, A.C.; Tanasă, A.M.; Suteu, D. Antioxidants from Galium verum as Ingredients for the Design of New Dermatocosmetic Products. Plants 2022, 11, 2454. [Google Scholar] [CrossRef]
- Bradic, J.; Andjic, M.; Novakovic, J.; Kocovic, A.; Tomovic, M.; Petrovic, A.; Nikolic, M.; Mitrovic, S.; Jakovljevic, V.; Pecarski, D. Lady’s Bedstraw as a Powerful Antioxidant for Attenuation of Doxorubicin-Induced Cardiotoxicity. Antioxidants 2023, 12, 1277. [Google Scholar] [CrossRef]
- Semenescu, A.-D.; Moacă, E.-A.; Iftode, A.; Dehelean, C.-A.; Tchiakpe-Antal, D.-S.; Vlase, L.; Vlase, A.-M.; Muntean, D.; Chioibaş, R. Phytochemical and Nutraceutical Screening of Ethanol and Ethyl Acetate Phases of Romanian Galium verum Herba (Rubiaceae). Molecules 2023, 28, 7804. [Google Scholar] [CrossRef] [PubMed]
- Shinkovenko, I.L.; Kashpur, N.V.; Ilyina, T.V.; Kovalyova, A.M.; Goryacha, O.V.; Koshovyi, O.M.; Toryanyk, E.L.; Kryvoruchko, O.V. The immunomodulatory activity of the extracts and complexes of biologically active compounds of Galium verum L. herb. Čes. Slov. Farm. 2018, 67, 25–29. [Google Scholar] [CrossRef]
- Kanso, M.A.; Hijazi, M.A.; El-Lakany, A.; Aboul-Ela, M. Review on phytochemical constituents and pharmacological activities of genus Galium. J. Appl. Pharm. Sci. 2024, 14, 46–56. [Google Scholar] [CrossRef]
- Farcas, A.D.; Mot, A.C.; Zagrean-Tuza, C.; Toma, V.; Cimpoiu, C.; Hosu, A.; Parvu, M.; Roman, I.; Silaghi-Dumitrescu, R. Chemomapping and biochemical-modulatory and antioxidant/prooxidant effect of Galium verum extract during acute restraint and dark stres in female rats. PLoS ONE 2018, 13, e0200022. [Google Scholar] [CrossRef]
- Antoniak, K.; Studzińska-Sroka, E.; Szymański, M.; Dudek-Makuch, M.; Cielecka-Piontek, J.; Korybalska, K. Antiangiogenic, Anti-Inflammatory and Antioxidant Properties of Bidens tripartite Herb, Galium verum Herb and Rumex hydrolapathum Root. Molecules 2023, 28, 4966. [Google Scholar] [CrossRef]
- Semenescu, A.-D.; Moacă, E.-A.; Iftode, A.; Dehelean, C.-A.; Tchiakpe-Antal, D.-S.; Vlase, L.; Rotunjanu, S.; Muntean, D.; Chiriac, S.D.; Chioibaş, R. Recent Updates Regarding the Antiproliferative Activity of Galium verum Extracts on A375 Human Malignant Melanoma Cell Line. Life 2024, 14, 112. [Google Scholar] [CrossRef]
- Vuletic, M.; Jakovljevic, V.; Zivanovic, S.; Papic, M.; Papic, M.; Mladenovic, R.; Zivkovic, V.; Srejovic, I.; Jeremic, J.; Andjic, M.; et al. The Evaluation of Healing Properties of Galium verum-Based Oral Gel in Aphthous Stomatitis in Rats. Molecules 2022, 27, 4680. [Google Scholar] [CrossRef]
- Gurnule, T.R.; Gautam, S.P.; Awari, D.M.; Kediya, A.S.; Gandhare, B.R. Review: Bioactive Components, Phytochemical Screening, Traditional Claims and Pharmacological Applications of Galium verum. IJRAR 2024, 11, 743. [Google Scholar]
- Zaichikova, S.G.; Bokov, D.O.; Kiselevskii, M.V.; Antsyshkina, A.M.; Bondar, A.A.; Prostodusheva, T.V.; Shchepochkina, O.Y.; Gegechkori, V.I. Determination of The Chemical Composition of Lady’s Bedstraw (Galium verum L.) Herb Extract By GC-MS. Pharmacogn. J. 2020, 12, 857–863. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Galium verum—A Review. Indo Am. J. Pharm. Res. 2018, 5, 2142–2149. [Google Scholar]
- Casanova, L.; Ceriani, F.; Messinese, E.; Paterlini, L.; Beretta, S.; Bolzoni, F.M.; Brenna, A.; Diamanti, M.V.; Ormellese, M.; Pedeferri, M. Recent Advances in the Use of Green Corrosion Inhibitors to Prevent Chloride-Induced Corrosion in Reinforced Concrete. Materials 2023, 16, 7462. [Google Scholar] [CrossRef]
- Valdez-Salas, B.; Vazquez-Delgado, R.; Salvador-Carlos, J.; Beltran-Partida, E.; Salinas-Martinez, R.; Cheng, N.; Curiel-Alvarez, M. Azadirachta indica Leaf Extract as Green Corrosion Inhibitor for Reinforced Concrete Structures: Corrosion Effectiveness against Commercial Corrosion Inhibitors and Concrete Integrity. Materials 2021, 14, 3326. [Google Scholar] [CrossRef] [PubMed]
- Radha, K.; Patel, D.; Nithya, V.V.; Saravanan, D. Experimental study on corrosion inhibition of mild steel using Mukia maderaspatana leaves extract as green inhibitor. Surf. Sci. Tech. 2024, 2, 27. [Google Scholar] [CrossRef]
- Badea, G.E.; Fodor, A.; Petrehele, A.I.G.; Maior, I.; Toderaș, M.; Morgovan, C.M. Evaluation of Phosphopolyoxometalates with Mixed Addenda (Mo, W, V) as Corrosion Inhibitors for Steels. Materials 2023, 16, 7600. [Google Scholar] [CrossRef]
- El-Hashemy, M.A.; Almehmadi, A.M. Evaluation of Glebionis coronaria L. flower extract as a novel green inhibitor for mild steel corrosion in acidic environment. Biomass Convers. Biorefinery 2024, 15, 1121–1137. [Google Scholar] [CrossRef]
- Pahuja, P.; Yadav, M.; Dhanda, M.; Arora, R.; Ahlawat, S.; Satija, A.; Jhanjhariya, N.; Kumar, S.; Lata, S. An eco-friendly corrosion inhibitor Cuscuta reflexa extract and PEG400 for mild steel inhibition in acidic medium: Computational and experimental investigations. Environ. Sci. Pollut. Res. 2023, 30, 98701–98717. [Google Scholar] [CrossRef]
- Ramezanzadeh, M.; Bahlakeh, G.; Sanaei, Z.; Ramezanzadeh, B. Studying the Urtica dioica leaves extract inhibition effect on the mild steel corrosion in 1 M HCl solution: Complementary experimental, ab initio quantum mechanics, Monte Carlo and molecular dynamics studies. J. Mol. Liq. 2018, 272, 120–136. [Google Scholar] [CrossRef]
- Alimohammadi, M.; Ghaderi, M.; Ahmad Ramasani, S.A.; Mahdavian, M. Falcaria vulgaris leaves extract as an eco-friendly corrosion inhibitor for mild steel in hydrochloric acid media. Sci. Rep. 2023, 13, 3737. [Google Scholar] [CrossRef]
- Pastore, C.; Dal Santo, S.; Zenoni, S.; Movahed, N.; Allegro, G.; Valentini, G.; Filippetti, I.; Tornielli, G.B. Whole Plant Temperature Manipulation Affects Flavonoid Metabolism and the Transcriptome of Grapevine Berries. Front. Plant Sci. 2017, 8, 929. [Google Scholar] [CrossRef]
- Sulaimon, A.A.; Murungi, P.I.; Tackie-Otoo, B.N.; Nwankwo, P.C.; Bustam, M.A. Analysis of natural okra extracts as corrosion inhibitors for mild steel in acidic medium. Environ. Sci. Pollut. Res. 2023, 30, 119309–119328. [Google Scholar] [CrossRef]
- Pourmohseni, M.; Rashidi, A.; Karimkhani, M. Preparation of corrosion inhibitor from natural plant for mild stil immersed in an acidic environmental: Experimental and theoretical study. Sci. Rep. 2024, 14, 7937. [Google Scholar] [CrossRef] [PubMed]
- Berrissoul, A.; Dafali, A.; Benhiba, F.; Outada, H.; Warad, I.; Dikici, B.; Zarrouk, A. Experimental and theoretical insights into Artemisia Stems aqueous extract as a sustainable and eco–friendly corrosion inhibitor for mild steel in 1 M HCl environment. Environ. Sci. Pollut. Res. 2024, 31, 36643–36662. [Google Scholar] [CrossRef] [PubMed]
- Latos-Brozio, M.; Masek, A.; Piotrowska, M. Effect of enzymatic polymerization on the thermal stability of flavonoids. J. Therm. Anal. Calorim. 2023, 148, 5357–5374. [Google Scholar] [CrossRef]
- Kim, J.M.; Kang, J.Y.; Park, S.K.; Han, H.J.; Lee, K.-Y.; Kim, A.-N.; Kim, J.C.; Choi, S.-G.; Heo, H.J. Effect of storage temperature on the antioxidant activity and catechins stability of Matcha (Camellia sinensis). Food Sci. Biotechnol. 2020, 29, 1261–1271. [Google Scholar] [CrossRef]
- Sun, X.; Tian, H.; Zou, F.; Li, W.; Qiang, Y.; Hou, B. Turning Waste into Treasure: Invasive Plant Ambrosia trifida L. Leaves as a High-Efficiency Inhibitor for Steel in Simulated Pickling Solutions. Materials 2024, 17, 3758. [Google Scholar] [CrossRef]
- Haldhar, R.; Vanaraj, R.; Dagdag, O.; Berisha, A.; Kim, S.-C. Convolvulus microphyllus Extract as a Green, Effective, and Affordable Corrosion Inhibitor: Theoretical Calculations and Experimental Studies. Coatings 2023, 13, 860. [Google Scholar] [CrossRef]
- Daoudi, W.; El Aatiaoui, A.; Dagdag, O.; Zaidi, K.; Haldhar, R.; Kim, S.-C.; Oussaid, A.; Aouinti, A.; Berisha, A.; Benhiba, F.; et al. Anti-Corrosion Coating Formation by a Biopolymeric Extract of Artemisia herba-alba Plant: Experimental and Theoretical Investigations. Coatings 2023, 13, 611. [Google Scholar] [CrossRef]
- de Souza Morais, W.R.; da Silva, J.S.; Queiroz, N.M.P.; de Paiva e Silva Zanta, C.L.; Ribeiro, A.S.; Tonholo, J. Green Corrosion Inhibitors Based on Plant Extracts for Metals and Alloys in Corrosive Environment: A Technological and Scientific Prospection. Appl. Sci. 2023, 13, 7482. [Google Scholar] [CrossRef]
- Abd-El-Nabey, B.A.; Abd-El-Khalek, D.E.; El-Housseiny, S.; Mohamed, M.E. Plant extracts as corrosion and scale inhibitors: A review. Int. J. Corros. Scale Inhib. 2020, 9, 1287–1328. [Google Scholar] [CrossRef]
- Badawi, A.K.; Fahim, I.S. Acritical review on green corrosion inhibitors based on plant extracts: Advances potential presence in the market. Int. J. Corros. Scale Inhib. 2021, 10, 1385–1406. [Google Scholar] [CrossRef]
GV-extract proved to be a new corrosion inhibitor, with specific advantages : spontaneous flora, widespread on globe, sustainability, cost-effectiveness, high inhibitor efficiency, reduced health risks.
Electrochemical, analytical and statistical techniques from this study establish the mechanism of corrosion and the chemical species involved, but is not exhaustive, further investigations will be continued, more powerful methods should be used to provide additional information, to analyze in detail the interaction of the inhibitor with the metal surface.
References
- Badea, G.E.; Stănăşel, O.; Bassyouni, M.; Toderaș, M.; Petrehel, A.I.G.; Ionaș, C.D. An investigation on the antioxidant capabilities, chemical analysis and potential green applications of the yellow bedstraw (galium verum) ethanol extract. 2024.
- de Souza Morais, W.R.; da Silva, J.S.; Queiroz, N.M.P.; de Paiva e Silva Zanta, C.L.; Ribeiro, A.S.; Tonholo, J. Green Corrosion Inhibitors Based on Plant Extracts for Metals and Alloys in Corrosive Environment: A Technological and Scientific Prospection. Appl. Sci. 2023, 13, 7482.
- Abd-El-Nabey, B.A.; Abd-El-Khalek, D.E.; El-Housseiny, S.; Mohamed, M.E. Plant extracts as corrosion and scale inhibitors: A review. Int. J. Corros. Scale Inhib. 2020, 9, 1287–1328.
- Badawi, A.K.; Fahim, I.S. Acritical review on green corrosion inhibitors based on plant extracts: Advances potential presence in the market. Int. J. Corros. Scale Inhib. 2021, 10, 1385–1406.
- Rajni, R. Industrial applications of green chemistry: Status, Challenges and Prospects. SN Appl. Sci. 2020, 2, 263.
