A dinuclear copper(II) complex of (
1
) (where
bipy
= 2,2′‑bipyridine,
bzt
= benzoate and
ox
= oxalate) was synthesised and characterised by diffractometric (powder and single-crystal XRD) and thermogravimetric (TG/DTG) analyses, spectroscopic techniques (IR, Raman, electron paramagnetic resonance spectroscopy (EPR) and electronic spectroscopy), magnetic measurements and density functional theory (DFT) calculations. The analysis of the crystal structure revealed that the oxalate ligand is in bis(bidentate) coordination mode between two copper(II) centres. The other four positions of the coordination environment of the copper(II) ion are occupied by one water molecule, a bidentate
bipy
and a monodentate
bzt
ligand. An inversion centre located on the
ox
ligand generates the other half of the dinuclear complex. Intermolecular hydrogen bonds and pi-pi for the organisation of the molecules in the solid state. Molar magnetic susceptibility and field dependence magnetisation studies evidenced a weak intramolecular–ferromagnetic interaction (
J
= +2.9 cm
‑1
) between the metal ions. The sign and magnitude of the calculated
J
value by density functional theory (DFT) are in agreement with the experimental data.
- dinuclear copper(II)
- ferromagnetic interaction
- magnetic properties
- noncovalent interaction
1. Introduction
Copper(II) complexes are interesting in coordination chemistry because of their vast applicability for bioinorganic purposes and synthesis of metallodrugs,[1][2] catalysis[3][4] and magnetism.[5][6] Concerning molecular magnetism, it is known that the magnetic interaction between two or more copper(II) centres is strongly dependent on the nature of the bridging ligand that works like a magnetic exchange pathway.[7][8] Among these ligands, the oxalate ion, ox2−, for example, is well known for its capability to create adequate magnetic exchange pathways for ferro- and antiferromagnetic interactions in oligonuclear copper(II) compounds.[9][10][11] The combination of oxalate and copper(II) ions leads to a large structural variety including different nuclearities such as mononuclear,[12][13] dinuclear,[14][15] trinuclear,[16][17] tetranuclear[17][18] and hexanuclear species[19][20] and coordination polymers.[14][21] This class of oxalate-bridged compounds is noteworthy in magnetic applications, as it may comprise many other transition metal ions such as MnII, FeII/III, CoII, NiII, CrII/III, VIV and RuII.[22][23][24][25]
2. Dinuclear Copper(II) Complexes
The dinuclear copper(II) complexes, which contain only one unpaired electron per metallic ion, are the most straightforward systems to investigate the through-ligand electron exchange mechanism from experimental and theoretical perspectives.[26][27][28] It is well known that the nature and strength of the magnetic interactions in dinuclear complexes including simple inorganic and extended organic bridging ligands such as hydroxo, azide, aromatic dicarboxylate, diamine, oxalate and related derivatives are highly dependent on the nature of the chelating terminal blocking ligands, which prevent complex polymerisation.[5] Furthermore, the variation in the spatial arrangement of terminal ligands may change the orbital overlap angle between the copper(II) and the bridging ligand, leading to different types of magnetic interactions.[29][30]
Regarding oxalate-bridged copper(II) compounds, the ferromagnetic or antiferromagnetic behaviour is determined by the overlap between the two magnetic orbitals. Poor overlap through oxalate leads to a weak antiferromagnetic interaction. On the other hand, if the overlap is zero, one could expect a ferromagnetic coupling.[8] Steric hindrance arising from the volume of the ancillary ligands leads to different orientations of the magnetic orbitals concerning the bridge plane, hence generating a different magnitude and nature of the magnetic interaction among the copper(II) centres.[17]
Although a variety of oxalate (ox2−)-bridged copper(II) complexes can be found in the literature, just a few contain more than one bulky ligand besides the oxalate bridge. A detailed search carried out in the Cambridge Structural Database (CSD)[31] for oxalate-bridged copper(II) complexes with ox2– in bis-bidentate coordination mode displayed a total of 423 results, of which 86 correspond to dinuclear complexes that possess two additional ligands besides ox2–. Out of 86 structures, 66 contain pentacoordinate metal ions bound to only one type of organic ligand such as pyridine,[1] 2,2′-bipyridine,[11][32] phenanthroline,[33][34] ethylenediamine,[35] pyrazole[36][37] and imidazole[38] and their derivatives, the coordination sphere being completed by simple inorganic ions such as nitrate,[39] chloride,[33] hydroxide[40] and perchlorate,[1] or by solvent molecules such as water,[6] methanol,[34] dimethylformamide,[41] tetrahydrofuran[42] or acetonitrile.[43] In the 20 remaining structures, all of which have been reported more recently, the metal ions are hexacoordinate and the chemical composition of the complexes, regarding the presence of at least one organic ligand, solvent molecules and simple inorganic ions, is similar to that observed for the pentacoordinate species.[8][11][14][15][44][45] Exceptions are found when tetradentate ligands direct hexa-coordination, decreasing the need for other molecular entities to complete the coordination sphere of the copper(II) ion.[46][47] Therefore, such systems show high structural and electronic versatility, which can be modulated by an appropriate choice of ligands other than the ox2- bridge.
Herein the synthesis of a new heteroleptic oxalate-bridged copper(II) dinuclear complex [{Cu(bipy)(bzt)(OH2)}2(μ-ox)] (1) (where bipy = 2,2′-bipyridine, bzt = benzoate and ox = oxalate) is reported, in which the coordination environment of the metal ion is described as distorted octahedral with the unusual coordination of two relatively bulky ligands, a bidentate 2,2′‑bipyridine and a monodentate benzoate, in addition to one water molecule. Structural correlations on how the orientation of the magnetic orbitals of the CuII ions is affected by the crystal field in 1 and the nature of magnetic interactions were investigated. We have also carried out theoretical calculations on the electronic structure and the electron paramagnetic resonance spectroscopy (EPR) spectra to bring light into its magnetic properties.
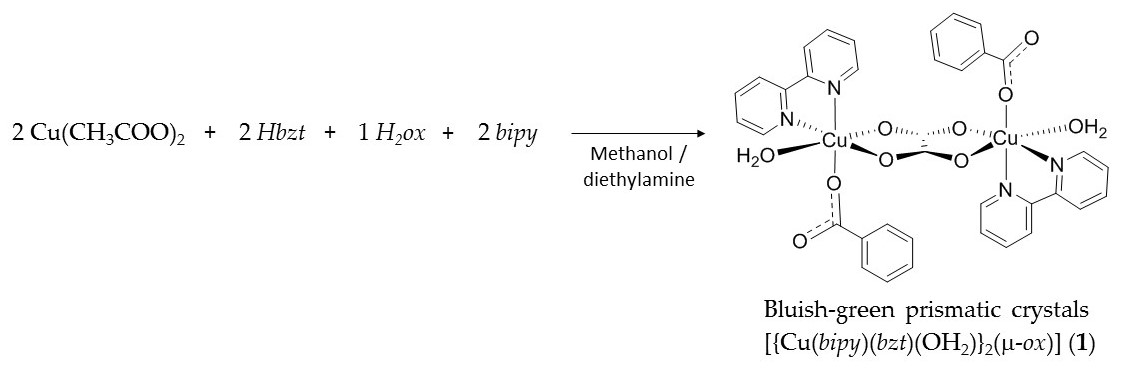
The reaction between copper(II) acetate monohydrate, benzoic acid, 2,2′-bipyridine and oxalic acid dihydrate in methanol gave the bluish-green prismatic shape crystals of [{Cu(bipy)(bzt)(OH2)}2(μ‑ox)] (1) in high yield, ca 90%, from a reproducible synthetic methodology (Scheme 1). Powder X-ray diffraction (PXRD) analysis revealed good correspondence between the simulated and experimental diffraction patterns.
Scheme 1. Synthetic methodology carried out between Cu(CH3COO)2·H2O, Hbzt, H2ox·2H2O and bipy to produce complex 1. Where bipy = 2,2′-bipyridine, bzt = benzoate and ox = oxalate.
3. Conclusions
Conclusions: A new dinuclear copper(II) complex was synthesised and characterised. The crystal structure showed that [[{Cu(bipy)(bzt)(OH2)}2(μ-ox)] (1) is centrosymmetric and contains two rather bulky ligands, benzoate and 2,2′-bipyridine, while the majority of the complexes of this class contain just one bulky ligand. The magnetic interaction predicted by EPR was confirmed by magnetic measurements and indicated ferromagnetic coupling between the metal centres. The determined J value was supported by DFT calculations. Therefore, the present study highlights the importance of the oxalate bridge as a magnetic-exchange pathway, and how bulky ancillary ligands can affect the magnetic response.
Methods, results and discussions could be seen in the published article, DOI: 10.3390/molecules25081898
References
- Ke-Bin Huang; Zhen-Feng Chen; Yan-Cheng Liu; Xiao-Li Xie; Hong Liang; Dihydroisoquinoline copper(ii) complexes: crystal structures, cytotoxicity, and action mechanism. RSC Advances 2014, 5, 81313-81323, 10.1039/c5ra15789g.
- Mohammad Usman; Farukh Arjmand; Rais Ahmad Khan; Ali Alsalme; Musheer Ahmad; Mousumi Sen Bishwas; Sartaj Tabassum; Tetranuclear cubane Cu4O4 complexes as prospective anticancer agents: Design, synthesis, structural elucidation, magnetism, computational and cytotoxicity studies. Inorganica Chimica Acta 2018, 473, 121-132, 10.1016/j.ica.2017.12.039.
- Ria Sanyal; Priyanka Kundu; Grigory Zhigulin; Bipinbihari Ghosh; Elena Rychagova; Sergey Yu. Ketkov; Shyamal Kumar Chattopadhyay; Ennio Zangrando; Debasis Das; Catecholase activity of Mannich-based dinuclear Cu II complexes with theoretical modeling: new insight into the solvent role in the catalytic cycle. New J. Chem. 2015, 40, 6623-6635, 10.1039/C6NJ00105J.
- Arfa Parween; Sumita Naskar; Antonio J. Mota; Arturo Espinosa Ferao; Shyamal Kumar Chattopadhyay; Eric Rivière; William Lewis; Subhendu Naskar; C i -Symmetry, [2 × 2] grid, square copper complex with the N 4 ,N 5 -bis(4-fluorophenyl)-1H-imidazole-4,5-dicarboxamide ligand: structure, catecholase activity, magnetic properties and DFT calculations. New J. Chem. 2016, 41, 11750-11758, 10.1039/C7NJ01667K.
- Isabel Castro; María Luisa Calatayud; Consuelo Yuste; María Castellano; Rafael Ruiz; Joan Cano; Juan Faus; Michel Verdaguer; Francisco Lloret; Dinuclear copper(II) complexes as testing ground for molecular magnetism theory. Polyhedron 2019, 169, 66-77, 10.1016/j.poly.2019.04.023.
- Debajit Dutta; Hiren Nath; Antonio Frontera; Manjit Kumar Bhattacharyya; A novel oxalato bridged supramolecular ternary complex of Cu(II) involving energetically significant π-hole interaction: Experimental and theoretical studies. Inorganica Chimica Acta 2019, 487, 354-361, 10.1016/j.ica.2018.12.037.
- Marı́a Luisa Calatayud; Isabel Castro; Jorunn Sletten; Francesc Lloret; Miguel Julve; Syntheses, crystal structures and magnetic properties of chromato-, sulfato-, and oxalato-bridged dinuclear copper(II) complexes. Inorganica Chimica Acta 2000, 300, 846-854, 10.1016/s0020-1693(99)00590-3.
- Danielle Cangussu; Humberto O. Stumpf; Harry Adams; James A. Thomas; Francesc Lloret; Miguel Julve; Oxalate, squarate and croconate complexes with bis(2-pyrimidylcarbonyl)amidatecopper(II): synthesis, crystal structures and magnetic properties. Inorganica Chimica Acta 2005, 358, 2292-2302, 10.1016/j.ica.2004.11.046.
- Ai-Li Zheng; Zhan-Feng Ju; Wei Li; Jie Zhang; Ferromagnetic dinuclear copper(II) complex based on bridging oxalate and bipyridinium ligands. Inorganic Chemistry Communications 2006, 9, 489-492, 10.1016/j.inoche.2006.02.010.
- Oscar Castillo; Antonio Luque; Pascual Román; Francesc Lloret; Miguel Julve; Syntheses, Crystal Structures, and Magnetic Properties of One-Dimensional Oxalato-Bridged Co(II), Ni(II), and Cu(II) Complexes withn-Aminopyridine (n= 2−4) as Terminal Ligand. Inorganic Chemistry 2001, 40, 5526-5535, 10.1021/ic0103401.
- Miguel Julve; A. N. Gleizes; Lise Marie Chamoreau; Eliseo Ruiz; Michel Verdaguer; Antiferromagnetic Interactions in Copper(II) µ-Oxalato Dinuclear Complexes: The Role of the Counterion. European Journal of Inorganic Chemistry 2017, 2018, 509-516, 10.1002/ejic.201700935.
- Lu-Yi Zheng; Yan-Hui Chi; Yuan Liang; Ethan Cottrill; Ning Pan; Jing-Min Shi; Green and mild oxidation: from acetate anion to oxalate anion. Journal of Coordination Chemistry 2018, 71, 3947-3954, 10.1080/00958972.2018.1543870.
- Conor Brennan; Jorunn Sletten; Brita Vangdal; D. Paul Rillema; Francesc Lloret; Miguel Julve; Syntheses, crystal structures and magnetic properties of new oxalato-, croconato- and squarato-containing copper(ii) complexesElectronic supplementary information (ESI) available: Stereoview of the structure of compound 2 (Fig. S1) and X-ray data as described in the text. See http://www.rsc.org/suppdata/nj/b3/b301212n/. New J. Chem. 2002, 27, 1775-1783, 10.1039/b301212n.
- Elena Melnic; Victor Ch Kravtsov; Karl Kramer; Jan Van Leusen; Silvio Decurtins; Shi-Xia Liu; Paul Kögerler; Svetlana G. Baca; Versatility of copper(II) coordination compounds with 2,3-bis(2-pyridyl)pyrazine mediated by temperature, solvents and anions choice. Solid State Sciences 2018, 82, 1-12, 10.1016/j.solidstatesciences.2018.05.008.
- Hamid Golchoubian; Razieh Samimi; Solvato- and thermochromism study in oxalato-bridged dinuclear copper(II) complexes of bidentate diamine ligands. Polyhedron 2017, 128, 68-75, 10.1016/j.poly.2017.02.042.
- A. Timothy Royappa; Andrew D. Royappa; Raphael F. Moral; Arnold L. Rheingold; Robert J. Papoular; Deke M. Blum; Tien Q. Duong; Jacob R. Stepherson; Oliver D. Vu; Banghao Chen; et al.Matthew R. SuchomelJames A. GolenGilles AndréNikolaos KourkoumelisAndrew D. MercerAllegra M. PekarekDylan C. Kelly Copper(I) oxalate complexes: Synthesis, structures and surprises. Polyhedron 2016, 119, 563-574, 10.1016/j.poly.2016.09.043.
- Anna Świtlicka; Barbara Machura; Jerzy Mrozinski; Božena Kalińska; Rafał Kruszyński; Mateusz Penkala; Effect of N-donor ancillary ligands on structural and magnetic properties of oxalate copper(ii) complexes. New J. Chem. 2013, 38, 1611-1626, 10.1039/c3nj01541f.
- Yuji Miyazato; Eiji Asato; Tohru Wada; Masaaki Ohba; Synthesis and Characterization of a Di-µ-oxalato Tetracopper(II) Complex with Tetranucleating Macrocyclic Ligand. Bulletin of the Chemical Society of Japan 2016, 89, 430-436, 10.1246/bcsj.20150338.
- Consuelo Yuste; Laura Cañadillas-Delgado; Ana Labrador; Fernando S. Delgado; Catalina Ruiz-Perez; Francesc Lloret; Miguel Julve; Low-Dimensional Copper(II) Complexes with the Trinucleating Ligand 2,4,6-Tris(di-2-pyridylamine)-1,3,5-triazine: Synthesis, Crystal Structures, and Magnetic Properties†. Inorganic Chemistry 2009, 48, 6630-6640, 10.1021/ic900599g.
- Ramon Vicente; Albert Escuer; Xavier Solans; Mercè Font-Bardia; Synthesis, magnetic behaviour and structural characterization of the alternating hexanuclear copper(II) compound [Cu6(tmen)6(µ-N3)2(µ-C2O4)3(H2O)2][ClO4]4·2H2O (tmen = Me2NCH2CH2NMe2). J. Chem. Soc., Dalton Trans. 1995, 1995, 1835-1838, 10.1039/dt9960001835.
- Douglas Hideki Nakahata; Raphael De Paiva; Wilton R. Lustri; Camila M. Ribeiro; Fernando Pavan; Gisele G. Da Silva; Ana Lúcia Tasca Gois Ruiz; João E. De Carvalho; Pedro Corbi; Sulfonamide-containing copper(II) metallonucleases: Correlations with in vitro antimycobacterial and antiproliferative activities. Journal of Inorganic Biochemistry 2018, 187, 85-96, 10.1016/j.jinorgbio.2018.07.011.
- Catalin Maxim; S. Ferlay; Cyrille Train; Binuclear heterometallic M(III)–Mn(II) (M = Fe, Cr) oxalate-bridged complexes associated with a bisamidinium dication: a structural and magnetic study. New J. Chem. 2010, 35, 1254, 10.1039/c0nj00976h.
- Jorgen Glerup; Patricia A. Goodson; Derek J. Hodgson; Kirsten Michelsen; Magnetic Exchange through Oxalate Bridges: Synthesis and Characterization of (.mu.-Oxalato)dimetal(II) Complexes of Manganese, Iron, Cobalt, Nickel, Copper, and Zinc. Inorganic Chemistry 1995, 34, 6255-6264, 10.1021/ic00129a009.
- Bharat Baruah; Vladimir Golub; Charles J. O’Connor; Animesh Chakravorty; Synthesis of Oxalato-Bridged (Oxo)vanadium(IV) Dimers Using L-Ascorbic Acid as Oxalate Precursor: Structure and Magnetism of Two Systems. European Journal of Inorganic Chemistry 2003, 2003, 2299-2303, 10.1002/ejic.200300013.
- Ioannis Bratsos; Barbara Serli; Ennio Zangrando; Nikos Katsaros; Enzo Alessio; Replacement of Chlorides with Dicarboxylate Ligands in Anticancer Active Ru(II)-DMSO Compounds: A New Strategy That Might Lead to Improved Activity. Inorganic Chemistry 2007, 46, 975-992, 10.1021/ic0613964.
- Carlo Adamo; Vincenzo Barone; Alessandro Bencini; Federico Totti; Ilaria Ciofini; On the Calculation and Modeling of Magnetic Exchange Interactions in Weakly Bonded Systems: The Case of the Ferromagnetic Copper(II) μ2-Azido Bridged Complexes. Inorganic Chemistry 1999, 38, 1996-2004, 10.1021/ic9812306.
- Carmen J. Calzado; Jesús Cabrero; Jean-Paul Malrieu; Rosa Caballol; Analysis of the magnetic coupling in binuclear complexes. I. Physics of the coupling. The Journal of Chemical Physics 2002, 116, 2728-2747, 10.1063/1.1430740.
- Carmen J. Calzado; Jesús Cabrero; Jean-Paul Malrieu; Rosa Caballol; Analysis of the magnetic coupling in binuclear complexes. II. Derivation of valence effective Hamiltonians from ab initio CI and DFT calculations. The Journal of Chemical Physics 2002, 116, 3985-4000, 10.1063/1.1446024.
- Zhiqiang Xu; Laurence K. Thompson; David O. Miller; Dicopper(II) Complexes Bridged by Single N−N Bonds. Magnetic Exchange Dependence on the Rotation Angle between the Magnetic Planes. Inorganic Chemistry 1997, 36, 3985-3995, 10.1021/ic970235k.
- Laurence K. Thompson; Santokh S. Tandon; Francisco Lloret; Juan Cano; Miguel Julve; An Azide-Bridged Copper(II) Ferromagnetic Chain Compound Exhibiting Metamagnetic Behavior.. Inorganic Chemistry 1997, 36, 3301-3306, 10.1021/ic970023n.
- Colin Groom; Ian J. Bruno; Matthew Lightfoot; Suzanna C. Ward; The Cambridge Structural Database.. Acta Crystallographica Section B Structural Science, Crystal Engineering and Materials 2016, 72, 171-9, 10.1107/S2052520616003954.
- Marijana Jurić; Damir Pajić; Dijana Žilić; Boris Rakvin; Dalibor Milić; Pavica Planinic; Synthesis, crystal structures and magnetic properties of the oxalate-bridged single CuIICuII and cocrystallized CuIIZnII systems. Three species (CuCu, CuZn, ZnZn) in the crystalline lattice. Polyhedron 2015, 98, 26-34, 10.1016/j.poly.2015.05.034.
- Somen Goswami; Soumen Singha; Rajat Saha; Anupam Singha Roy; Manirul Islam; S. Kumar; A bi-nuclear Cu(II)-complex for selective epoxidation of alkenes: Crystal structure, thermal, photoluminescence and cyclic voltammetry. Inorganica Chimica Acta 2019, 486, 352-360, 10.1016/j.ica.2018.10.066.
- Willian X. C. Oliveira; Cynthia L. M. Pereira; Carlos Basílio Pinheiro; Francisco Lloret; Miguel Julve; Oxotris(oxalate)niobate(V): An oxalate delivery agent in the design of building blocks. Journal of Coordination Chemistry 2018, 71, 707-724, 10.1080/00958972.2018.1428313.
- Razieh Samimi; Hamid Golchoubian; Dinuclear copper(II) complexes with ethylenediamine derivative and bridging oxalato ligands: solvatochromism and density functional theory studies. Transition Metal Chemistry 2017, 42, 643-653, 10.1007/s11243-017-0170-8.
- M. Luisa Calatayud; Marta Orts-Arroyo; Miguel Julve; Francisco Lloret; Nadia Marino; Giovanni De Munno; Rafael Ruiz-García; Isabel Castro; Magneto-structural correlations in asymmetric oxalato-bridged dicopper(II) complexes with polymethyl-substituted pyrazole ligands. Journal of Coordination Chemistry 2018, 71, 657-674, 10.1080/00958972.2017.1421950.
- Samira Bahemmat; Bernhard Neumüller; Mitra Ghassemzadeh; One-Pot Synthesis of an Oxalato-Bridged CuIICoordination Polymer Containing an In Situ Produced Pyrazole Moiety: A Precursor for the Preparation of CuO Nanostructures. European Journal of Inorganic Chemistry 2015, 2015, 4116-4124, 10.1002/ejic.201500255.
- Chen Liu; Khalil A. Abboud; Crystal structures of μ-oxalato-bis-[azido-(hista-mine)-copper(II)] and μ-oxalato-bis-[(dicyan-amido)(hista-mine)-copper(II)].. Acta Crystallographica Section E Crystallographic Communications 2015, 71, 1379-83, 10.1107/S2056989015019908.
- Kil Sik Min; Myunghyun Paik Suh; Self-Assembly, Structures, and Magnetic Properties of Ladder-Like Copper(II) Coordination Polymers. Journal of Solid State Chemistry 2000, 152, 183-190, 10.1006/jssc.2000.8682.
- Tuncer Hökelek; Canan Unaleroglu; Yüksel Mert; Crystal Structure of [Bis(N,N,N',N'-tetramethylethylenediamine)-O,O'-.MU.-O,O'-oxalato]dihydroxy Dicopper(II).. Analytical Sciences 1999, 16, 1235-1236, 10.2116/analsci.16.1235.
- Wei Huang; Takuji Ogawa; Spectral and structural studies of a new oxalato-bridged dinuclear copper(II) complex having two 3-(thiophen-2-yl)-1,10-phenanthroline ligands in a trans configuration. Inorganica Chimica Acta 2009, 362, 3877-3880, 10.1016/j.ica.2009.04.016.
- Pierre Thuéry; Increasing Complexity in the Uranyl Ion–Kemp’s Triacid System: From One- and Two-Dimensional Polymers to Uranyl–Copper(II) Dodeca- and Hexadecanuclear Species. Crystal Growth & Design 2014, 14, 2665-2676, 10.1021/cg500353k.
- Miao Du; Ya-Mei Guo; Shen-Tan Chen; Xian-He Bu; Joan Ribas; Crystal structures, spectra and magnetic properties of di-2-pyridylamine (dpa) CuII complexes [Cu(dpa)2(N3)2]·(H2O)2 and [Cu2(μ-ox)(dpa)2(CH3CN)2](ClO4)2. Inorganica Chimica Acta 2003, 346, 207-214, 10.1016/s0020-1693(02)01430-5.
- Sujittra Youngme; Gerard A Van Albada; Narongsak Chaichit; Pimprapun Gunnasoot; Palangpon Kongsaeree; Ilpo Mutikainen; Olivier Roubeau; Jan Reedijk; Urho Turpeinen; Synthesis, spectroscopic characterization, X-ray crystal structure and magnetic properties of oxalato-bridged copper(II) dinuclear complexes with di-2-pyridylamine. Inorganica Chimica Acta 2003, 353, 119-128, 10.1016/s0020-1693(03)00207-x.
- Oscar Castillo; Iñaki Muga; Antonio Luque; Juan M Gutierrez-Zorrilla; Jon Sertucha; Pablo Vitoria; Pascual Román; Synthesis, chemical characterization, X-ray crystal structure and magnetic properties of oxalato-bridged copper(II) binuclear complexes with 2,2′-bipyridine and diethylenetriamine as peripheral ligands. Polyhedron 1999, 18, 1235-1245, 10.1016/s0277-5387(98)00421-5.
- Alexey N. Gusev; Radovan Herchel; Eziz Bayjyyev; Galyna A. Nyshchimenko; Grigory G. Alexandrov; I.L. Eremenko; M. Hasegawa; Ivan Nemec; Zdenek Travnicek; Wolfgang Linert; et al. Versatile coordination modes of bis[5-(2-pyridine-2-yl)-1,2,4-triazole-3-yl]alkanes in Cu( ii ) complexes. Dalton Transactions 2013, 43, 7153-7165, 10.1039/C4DT00462K.
- Uttam R. Pokharel; Frank R. Fronczek; Andrew Maverick; Reduction of carbon dioxide to oxalate by a binuclear copper complex. Nature Communications 2014, 5, 5883, 10.1038/ncomms6883.
- Chen Liu; Khalil A. Abboud; Crystal structures of μ-oxalato-bis-[azido-(hista-mine)-copper(II)] and μ-oxalato-bis-[(dicyan-amido)(hista-mine)-copper(II)].. Acta Crystallographica Section E Crystallographic Communications 2015, 71, 1379-83, 10.1107/S2056989015019908.