Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Stefano Bacci and Version 4 by Stefano Bacci.
Photobiomodulation (PBM), formerly known as low-level laser light therapy (LLLT), is a safe phototherapy technique that uses wavelengths of the visible light spectrum which includes red light (RL, 620–700 nm) and near-infrared (NIR, 700–1440 nm). This treatment modality has been used in dermatology, both in clinical settings and at home. PBM involves the use of various light sources [2], including low-level lasers (LLL) and light-emitting diodes (LED), to deliver therapeutic light. Reviews on the matter have already been conducted.
- photobiomodulation
- dermatology
- laser
- photodynamic therapy
- LED
1. Introduction
Endre Mester, a Hungarian physician, first created the idea of photobiomodulation (PBM) in 1967 while researching the effects of laser light exposure on the growth of cancer cells in a mouse model. Mester discovered an unanticipated acceleration of hair regeneration during this investigation [1][28]. Naturally, since its inception, light therapy has undergone tremendous changes and has grown in scope. PBM has demonstrated efficacious outcomes in the management of non-healing wounds, scars, ulcers, musculoskeletal disorders, persistent pain, analgesia, and immunological regulation [2][29].
Increasingly, research indicates that particular electromagnetic radiation wavelengths, from visible to infrared, can produce photophysical and photochemical effects that can alter important biological processes in a variety of eukaryotic organs, including humans. Hence, non-ionizing light sources in the visible and infrared range, such as broadband lights, low-level lasers, and Light-Emitting Diodes (LEDs), are used in treatment to activate endogenous chromophores and stimulate biological functions in a non-thermal and, most importantly, non-cytotoxic manner [3][4][30,31].
The main characteristic of PBM is the direct interaction of continuous wave light at specific wavelengths directly with the tissue, i.e., with the endogenous chromophores.
However, due to a lack of understanding of the photo-physics and radiometric parameters that affect PBM’s accuracy and reliability, as well as a lack of knowledge about how PBM achieves its molecular effects, there is still disagreement about its practical application.
2. Blue LED Therapy
Blue LED technology was created in the early 1990s and has since found many uses in the biochemical and biological domains [5][6][32,33]. In fact, blue LED technology offers the possibility of a straightforward and affordable source for focusing on specific biological components, given that a number of biological molecules and chromophores exhibit a high absorption rate in the UV/blue area of the spectrum [7][34]. One use for it is the process of wound healing.
Blue light is absorbed mainly by the heme group, which can be found in the hemoglobin and in the cytochromes. These widely distributed biological elements have the capacity to trigger one or more intracellular signaling pathways following radiation exposure, which can alter the healing process [8][35]. Protoporphyrin IX is a chromophore that increases the sensitivity of cytochrome C and cytochrome C oxidase to blue light in the mitochondrial electron transport chain [8][9][10][11][35,36,37,38].
Cytochrome C and cytochrome C oxidase, once activated by blue light, strengthen the process of cellular respiration by interacting with the final two complexes of the chain and adjusting the ATP production [11][12][38,39]. For this reason, mitochondria represent a target organelle for blue light radiation. Mitochondria are involved in redox signaling and in maintaining the balance of reactive oxygen species (ROS), which is essential for several vital functions such as calcium homeostasis. In a preliminary study, Magni et al. [13][40] demonstrated that under blue light exposure, ROS are stimulated dose-dependently and that mitochondria are subject to morphological changes.
According to André-Lévigne et al. [14][41] and Dunnill et al. [15][42], flavins, which stimulate the synthesis of ROS and play a role in the signal transduction mechanism in numerous cellular pathways involved in tissue repair, can also be used to explain the therapeutic action of blue light. The shift from this phase to the proliferative one is facilitated by the modulation of ROS, which causes a controlled increase in inflammatory functions [16][17][43,44]. The macrophage phenotypic transition from M1 to M2 may also be responsible for this [2][3][29,30].
Compared to wounds that are not treated with blue light, wounds treated with it exhibit a quicker healing process as well as improved dermal collagen deposition and morphology [7][18][19][20][21][22][34,45,46,47,48,49]. Additionally, the modulation of the inflammatory response is better in wounds that have been treated. His process can be attributed to photochemical effects: nitric oxide release and fibroblast activation brought on by blue light promote re-epithelialization [8][23][24][35,50,51]. According to Fraccalvieri et al. [12][39] and Orlandi et al. [25][52], blue light helps injured tissue by regulating its energy supply, which is particularly important during the phases of proliferation and remodeling. It also establishes tissue repair, with a decrease in scar tissue and the likelihood of developing keloids, as well as the stimulation of angiogenesis [25][26][52,53].
In animal models of chronic ulcers, blue light treatment has also been shown to have a proangiogenic effect; this suggests that blue light therapy may be helpful throughout the whole tissue repair process [27][54]. In addition, blue light has been shown to have anti-inflammatory cytokine release, bacterial load reduction, and granulation stimulation [13][23][24][28][40,50,51,55]. The safety and efficacy of blue light in treating skin lesions, Inflammatory acne [29][30][56,57], burns [24][51], psoriasis [31][32][33][34][58,59,60,61], eczema [35][62], and diabetic ulcers are among the conditions that blue light is reported to be safe and effective for treating. Despite the evidence presented above, relatively few methodologically rigorous experiences have been conducted in daily clinical practice focused on chronic wounds [12][39].
In an in vitro research context, Rossi et al. [19][46] investigated the impact of blue LED light on the proliferation and metabolism of human fibroblasts derived from healthy skin cocultured with keratinocytes. As described in their article, the authors suggested using blue LED light to modulate the metabolism and growth of human fibroblasts.
Furthermore, human fibroblasts isolated from keloids and perilesional tissues were subjected to blue LED light irradiation, which was examined by Magni et al. [18][45]. The authors utilized various experimental techniques to demonstrate that blue LED light can modulate cell proliferation and metabolism in a dose-dependent manner and that these effects persist for at least 48 h after treatment.
Moreover, in keloid-derived fibroblasts and perilesional fibroblasts, the highest radiation doses decrease cell viability 24 and 48 h after treatment, respectively. In order to treat hypertrophic scars and keloids, the authors concluded that blue light irradiation was a novel and minimally invasive treatment option.
3. Photodynamic Therapy
Today, photodynamic therapy (PDT), which was first developed by Von Toppeiner and Jesionek in partnership [36][63], is extensively utilized to treat a wide range of illnesses [37][64]. The application of this therapy in dermatology spans the spectrum from treating bacterial, fungal, viral, immunological, or inflammatory illnesses to managing chronic wounds, including photorejuvenation in cosmetology [38][65].
The application of photosensitizers activated by a specific wavelength of light energy is used in this therapy: the topical use of 5 Aminolaevulinic Acid (ALA) has represented a breakthrough in PDT in the dermatological field because it is easily absorbed by the skin [39][40][66,67].
The molecular mechanism of action of PDT is complex. When triplet oxygen (3O2) is present, the photo-sensitizer can enter the tissue. The main purpose of PDT is to have a selective photokilling effect on a pathologic target while promoting healing in healthy tissue. In order to reach this goal, a photosensitizer is used and included in the target tissue. The photosensitizer has an absorption peak at a specific wavelength: when it is exposed to a light source (usually a laser) emitting at this wavelength, it generates Reactive Oxygen Species (ROS) that are very unstable. The generation of ROS can vary depending on the energy input and the characteristics of the photosensitizers [27][54]. While less noticeable levels of ROS may promote tissue proliferation and/or regeneration, high levels may cause a photokilling effect. This approach has been usually proposed in the treatment of cancerous cells or in the inactivation of multidrug-resistant pathogens [41][68].
PDT is used in medicine to treat a wide range of pathologies, both oncological and non-oncological [42][43][69,70]. As almost all the therapies are based on the use of light sources, its advantages include minimal invasiveness, ease of use in an outpatient setting, and a strong track record of short- and long-term safety.
Intravenous injection of photo-sensitizer can cause damage at the vascular level. Hypoxic damage can be beneficial in treating neoplasms, but it can also be detrimental in treating chronic non-neoplastic wounds because it exacerbates the hypoxic state, which is a major factor in noxa.
Without a single, perfect photo-sensitizer, the selection process should focus on molecules that have a proven track record of improving skin ulcer healing and can be applied topically. Two reviews [41][44][68,71] have recently examined this vast topic. Photo-sensitizers for chronic ulcers have been tested using various chemical categories, primarily in preclinical settings and a few pilot studies. These chemical categories include phenothiazines (methylene blue and toluidine blue), xanthenic dyes (rose bengal), and tetrapyrrolic macrocycles and analogs (porphyrins, chlorines, and phthalocyanines).
The selection of the light source is also very important. In clinical practice, PDT is performed using laser or LED (Light Emitting Diodes) sources. Lasers are strictly monochromatic, thus enabling an excellent matching with the photosensitizer absorption curve, high fluences and a spatially narrow beam. On the other hand, LED sources usually present a large emission spectrum but are more affordable, and their wide angular emission can cover larger tissue areas. The wavelength controls how well light can pass through tissue. Specifically, lengths between 600 and 800 nm are thought to have sufficient skin penetration to be utilized in clinical settings. Fundamentally, red is the color that penetrates the skin the most, followed by green and blue [45][72].
Given that the technique relies on a tissue’s interaction with light energy, it is clear that the effectiveness is directly correlated with the total amount of energy applied per treated area’s volume. A number of parameters, including power, irradiance, energy density, irradiation time, and release of light mode (simple or fractional), can be used to express this [46][73]. Energy density, which is expressed as J/cm and is derived from the measurement of time (in seconds) and irradiance (in W/cm2), is the most widely used format for reporting a PDT treatment schedule [47][74].
According to research on wound healing, particularly chronic wounds, PDT can cause an acute inflammatory response that is primarily related to immune system activation [48][75].
This is supported by the description of how PDT not only causes new fibroblasts (effector cells) to diversify but also fosters close relationships between these cell types and mast cells, which are positive for TNF-α and Fibroblast Growth Factor (FGF) in their granules. This was reported by Corsi et al. [49][76].
Thus, these results support the hypothesis that mast cells could transmit signals for the same fibroblast recruitment and differentiation following therapy [49][76]. When it comes to mast cells, they proliferate and degranulate during the course of treatment. Their increase could have resulted from nearby cells migrating, precursors already present in the tissue differentiating, or precursors entering the tissue and differentiating into mast cells.
As a result, these cell types would be both attracted and stimulated to release their granules into the dermis in reaction to the therapy. During therapy, the venules, or the vessels of the sub-papillary plexus, appear to be a preferred location for cell infiltration and clustering [49][76].
The presence of TNF-alpha, GM-CSF, and TGF-β in mast cells after PDT treatment provides additional evidence of immune system activation. TNF-alpha plays a crucial role in differentiating dendritic cells, including plasmacytoid cells, which interact with regulatory T-type lymphocytes. GM-CSF is also involved in this process. TGF-beta is essential for the differentiation of macrophages.
According to Grandi et al. [40][67], there is no doubt that the induction of TGF-beta is connected to the subsequent decrease in wound volume. In fact, TGF-beta seems to affect the epithelial-mesenchymal transition—which permits keratinocyte migration from the margins toward the wound bed—at different phases of ulcer healing. Additionally, this cytokine can stimulate myofibroblast differentiation as a component of the processes observed in scar reshaping [50][77].
Additional research has demonstrated that PDT significantly affects neutrophil activation, which may account for the rise in pro-inflammatory cytokines following treatment. Lipid mediators are produced in tandem with the acute phase of inflammation resolution and the restoration of tissue homeostasis). These mediators have been linked to anti-inflammatory and immunomodulatory properties, such as the inhibition of leukocyte chemotaxis, the blockade of TNF-alpha and IL-6 production, and the induction of increased IL-10 expression [51][52][53][54][78,79,80,81]. As a result, thwe researchers ccan draw the conclusion that PDT significantly affects the immune system, having both immunostimulatory and immunosuppressive effects. It also likely influences the type of cell death that is induced.
According to Steinmann [55][82], the nervous system has the ability to control the immune system’s activity; ulcer healing is another example of this close relationship. Actually, results from experiments indicate that neurogenic stimuli significantly impact wound healing following injury and that delayed wound healing following skin nerve resection is seen in animal models [56][57][83,84].
Studies have demonstrated that following PDT treatment, there is an increase in neuronal populations belonging to the autonomous nervous system, which is found in the dermis. These neuronal populations contain the typical nerve mediators involved in ulcer healing (CGRP, NGF, NKA, NPY, SP, PGP 9.5, and VIP). Additionally, after a single irradiation, the proportion of mast cells that contain and secrete VIP and NGF rises. Given that mast cell degranulation is stimulated by both VIP and NGF, these results appear to be consistent with the previously documented rise in mast cell degranulation index following PDT treatment, indicating that neurogenic stimuli may play a role in this phenomenon.
In light of this, thwe researchers ccan presume that an increase in NGF and VIP release following therapy results in mast cell activity and that these mediators can activate dermal neurons and nerve fibers [54][58][59][81,85,86]. Corsi et al. [49][76] suggest that increased TGF-beta, cellular infiltrate response, and increased ECM secretion by fibroblasts may all be related to the activation of nerve fibers.
Due to its gaseous nature and relatively short half-life, NO is the smallest known signaling molecule that can cross membranes freely. It has recently been added to the list of mediators involved in wound healing [60][87]. In fact, the presence of bacterial antigens, apoptotic bodies, or inflammatory cytokines increases the expression of the enzyme, which suggests this molecule is derived from the NOS enzyme complex, where the inducible isoform is overregulated during stressful situations.
The inflammatory phase of wound repair, which is characterized by the promotion of vasodilation and antibacterial activity, has been theorized to be facilitated by iNOS [54][61][62][81,88,89]. Experimental preliminary results show increased expression of iNOS in chronic wounds treated with photodynamic therapy. In contrast to granulocytes to M2-type macrophages, vessels, and neurons where iNOS expression increases, mast cells have a higher degranulation index and contain iNOS; however, the proportion of these cells containing this mediator decreases following treatment [63][90].
However, research is currently being done in the lab to determine how different cell types respond to PDT in terms of iNOS secretion and subsequent wound healing.
4. LED vs. Low-Level Laser Light Therapy Comparison
The treatment known as PBM has been labeled by various terms. The term “photobiomodulation” is more widely accepted among authors as it refers to the general mechanism, whereas using the term LLLT may confuse the reader into thinking that PBM can only be done with lasers [64][91].
PBM is used to refer to the interaction of light sources with a target modulatory action on specific biological reactions or pathways. The term LLLT arises from the discovery of the photobiomodulatory effects of lasers on the periphery of treated lesions. Despite the fact that this term has been widely used, PBM effects on the skin can be obtained not only by applying a laser at low energies with that intention. LED lights or non-coherent sources, without seeking selective photothermolysis, can also be used for PBM [65][92] (Figure 1).
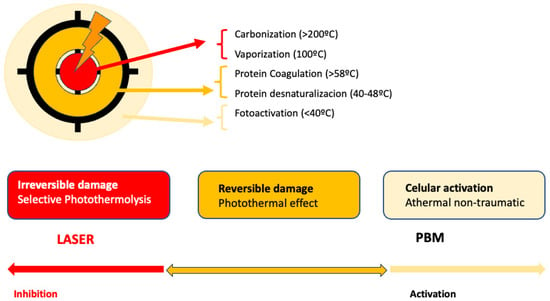
Figure 1. Represents the action of lasers in dermatology: In the center, selective photothermolysis occurs with the selective destruction of the chromophore and necrosis of the target tissue. In the surrounding tissue, PMB effects occur, corresponding to the dissipation of laser energy or lower doses.
Consequently, there are two main ways to apply PBM in dermatology. One method involves using LED lights, while the other utilizes low-dose lasers below the selected target threshold. Both light sources differ in some key aspects. Laser light is coherent and exact, whereas LED light is non-coherent in a bandwidth of 1–2 nm [64][91]. The application methods also vary. Despite using low doses [66][93], lasers deliver high energy in a short time, resulting in short sessions administered by an expert dermatologist [67][94]. Laser devices are more expensive. LEDs, on the other hand, are simple, more affordable devices that do not require specialized handling. LEDs apply energy over a longer duration compared to lasers [65][92]. For PBM applications, both devices require repeated sessions, with protocols not clearly established, typically ranging from once per week to multiple times per week [68][2]. Most of the published works on PBM utilize lasers as light sources, representing up to 90 percent of the more than 3000 published works [64][91]. This could lead to the wrong assumption that PBM is only achieved with lasers. However, numerous studies currently support the idea that LEDs are another valid option for applying PBM. Table 1 shows the differences between laser and LED devices when used as light sources in PBM.
Table 1.
Summary of published clinical trials on PMB and photorejuvenation and wrinkles treatment.
| Author/Year | Type of LED | Patients | Design of the Study | Protocol of Treatment | Results | ||||
|---|---|---|---|---|---|---|---|---|---|
| Weis 2005 [69] | Weis 2005 [95] | RL 590 nm | N = 90 | 8 treatments in 4 weeks 6 months follow-up |
0.1 J/cm | 2 | pulsing | 90% of patients reduced photoaging signs. Histological response: - 90% improve Collagen I - 4% decrease MMPI |
|
| Russell 2005 [70] | Russell 2005 [96] | RL 630 nm + NIR 830 nm |
N = 31 | 9 light treatments Flow up weeks 9 and 12 |
RL 126 J/cm | 2 | NIR 66 J/cm | 2 | 52% of patients reduced photoaging signs 81% of patients reported improvement in periocular wrinkles |
| Goldberg 2007 [71] | Goldberg 2007 [97] | RL 630 nm + NIR 830 nm |
N = 36 | 9 treatments in 12 weeks | RL 126 J/cm | 2 | NIR 66 J/cm | 2 | Significant improvement in softness, smoothness, and firmness |
| Yoon-Lee 2007 [72] | Yoon-Lee 2007 [98] | RL 630 nm + NIR 830 nm |
N = 112 | 4 Groups: NIR, RL, NIR + RL and placebo 8 sessions, 4 weeks, and 3 months follow-up |
RL 126 J/cm | 2 | NIR 66 J/cm | 2 | Both RL and NIR had effective and significant wrinkle reduction Skin elasticity better NIR and NIR + RL Melanin decrease RL |
| Baez 2007 [73] | Baez 2007 [99] | RL 630 nm + NIR 830 nm |
N = 30 | 9 sessions, 12 weeks | RL 126 J/cm | 2 | NIR 66 J/cm | 2 | 91% color improvement 82% smoothness improvement 25–50% investigator assessment improvement |
| Wunsch 2014 [74] | Wunsch 2014 [100] | RLT 611–650 nm ELT 570–850 nm |
N = 136 | 2 sessions per week 30 treatments 3 Groups: RLT, ELT, and placebo |
No difference between wavelengths Both treatments have significant differences in wrinkles |
||||
| Hee-Nam 2017 [75] | Hee-Nam 2017 [101] | RL 660 nm LED 411–777 nm |
N = 52 | 1 session/day 12 weeks 2 Groups: RL, LED |
5.17 J/cm | 2 | Both treatments significantly improve wrinkles | ||
| Rocha-Mota 2023 [76] | Rocha-Mota 2023 [102] | RL 660 nm AL 590 nm |
N = 137 Split-face |
10 sessions periocular 4 weeks |
3.8 J/cm | 2 | Significant periocular wrinkles, with RL 31.6% and 29.9% with AL. |
The use of light in a no-thermal effect is supported by the photon’s absorption of the cells’ receptors. The three main chromophores in the skin are melanin in the epidermis, hemoglobin in the dermis, and water in all the skin, with longer wavelengths achieving deeper penetration (Figure 2) (1). Red light (RL) targets melanin and hemoglobin, whereas NIR light targets water in the deeper layers of the epidermis [77][105] (Figure 3). Blue light (BL 400–500 nm) has been included in some devices, but it is considered very close to ultraviolet light with deleterious effects on the skin and without modulatory effects [78][106].
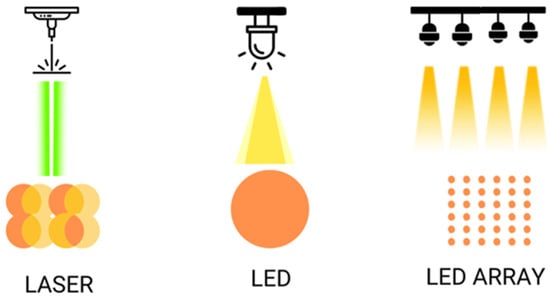
Figure 2.
Shows the different ways of illumination with laser and LED, simple or on arrays.
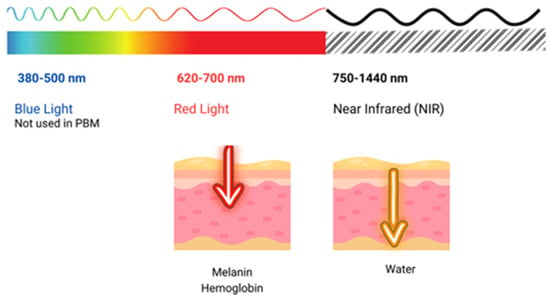
Figure 3.
Shows the penetration of light at different wavelengths throughout the visible and near-infrared (NIR) spectrum.
