Cistus albidus is one of the approximately 20 species of the Cistus genus. The genus’ name is derived from the ancient Greek term kistos. It is supposed that the name alludes to the woody capsule fruits. Evergreen in its Mediterranean homeland and between 50 and 250 centimeters tall, this shrub is called albidus, not because of the colour of its flowers, but because its leaves are finely covered with white hair (trichomes).
- phytochemistry
- pharmacology
- polyphenols
- terpenes
- traditional uses
1. Botanical Characteristics

Vegetative Development
2. Phytochemical Constituents
2.1. Terpenes
2.1.1. Mono- and Sesquiterpenes from the Essential Oils
2.1.2. Phenylpropanoids from the Essential Oils
2.1.3. Diterpenes
2.1.4. Tetraterpenes
2.2. Phenolic Compounds
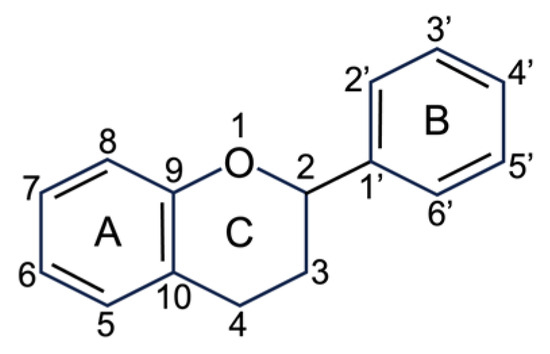
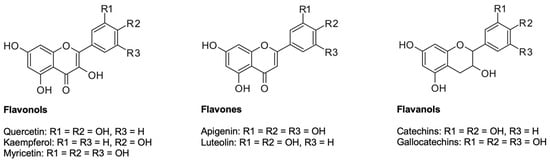
2.2.1. Flavonoids
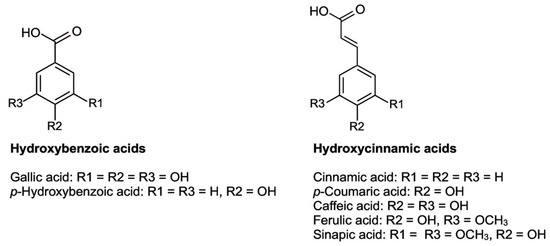
2.2.2. Phenolic Acids
2.3. Carbonylic Compounds
2.4. Phytohormones and Vitamin E
2.5. Alkanes
2.6. Other Compounds: Fatty and Carboxylic Acids
3. Preparation Methods of the
C. albidus
Extracts
Studies of the pharmacological properties of traditional medicines based on C. albidus preparations are related to the presence of terpenoids and polyphenols. In order to understand the use that has been given to C. albidus traditionally, it is necessary to first review the preparation methods used in popular medicine of this plant due to their influence on the pharmacological effect. 6.1. Traditional Preparations For the different applications, traditionally, only the aerial parts of C. albidus were harvested, mainly the leaves, but also flowers, flower buds, and to a lesser extent stems. The traditional preparation of C. albidus varies from an infusion to a prolonged decoction, while the dose usually used is around 3 g per 100 mL of water, taking a cup (150 mL) two or three times a day [10]. Within traditional preparations, decoction is the most used technique. It consists of boiling the plant material for a certain period of time and letting it rest afterwards. This method is primarily suitable for thermostable and water-soluble phytochemicals. During decoction, several compounds undergo chemical modifications. For example, catechins undergo epimerization, which is a change in their configuration relative to one of their stereogenic centres. Epimers, specifically epicatechins and epigallocatechins, have been shown to have important health benefits. It has been found that this epimerization occurs more readily in water with alkaline pH values than in purified water [260]. In addition, it has been shown that at temperatures greater than 98ºC, epimerization occurs faster than its degradation [261], so it can be deduced that the traditional preparation of C. albidus is the most effective way to extract catechins and their epimers. However, for green tea, the levels of epicatechin, epicatechin gallate, epigallocatechin, and epigallocatechin gallate were reported to increase only during the first 3 to 5 min of preparation (infusion at 85ºC), and the proportion of these flavonoids decreased as time increased. In contrast, another study found that levels of catechin, gallocatechin, and gallocatechin gallate increased continuously with the length of preparation time [262]. Taking these results into account, the pharmacological activities referred to in traditional use could be optimized by limiting the decoction time. Nonetheless, thermolabile compounds are lost in the decoction process. As a result, monoterpenes should not be contained in the resulting extract. Sesquiterpenes, however, would not be affected by extracting temperatures around 100 degrees but by low solubility in water due to their lipophilic character. It can therefore be assumed that terpenes play a minor role in the traditional decoction of plant material. On the other side, probably in order to use the entire compound spectrum of the plant, based on both terpenes and polyphenols, the dried and crushed leaves sometimes were used directly (orally) [9,263–266]. This usage ensures that the resulting medicine is rich in polyphenols terpenes and other volatiles. However, a loss of several terpenes and oxidative reactions could be induced by the drying process, as reported for other species as Cannabis sativa [267,268].
4. Therapeutical Uses
4.1 Traditional Uses
Plant resources have always been an integral part of human society throughout history. Until the middle of the last century, traditional medicines provided an alternative and inexpensive source of primary health care for the rural population. However, with access to synthetic drugs, a large number of medicinal plants became obsolete, the memory of which in the population, after only two generations, is being lost. One of these medicinal plants is C. albidus, which has been used in traditional folk medicine for a variety of illnesses [10,286–288], especially for the treatment of fever, diarrhea and other gastrointestinal illnesses [8], skin diseases, rheumatism, and various inflammatory diseases [182]. For the sake of completeness, it is mentioned here that C. albidus has also been used as a tanning agent [181], as an insect repellent, and as a substitute for tobacco, highly appreciated, moreover, for its hypotensive effect [49,288,289]. The decoction of leaves was traditionally used in the Spanish Levant as a tranquilizer, in the Baixa Plana as a sedative [10], and as a remedy against Parkinson’s symptoms in Mallorca [290,291]. To relieve toothache, mouthwashes were made with a decoction of its leaves and flowers. A sip of the resulting liquid, once cold, was kept in the mouth for some time [266,292–294]. In addition, the decoction of the aerial parts was used as an external antiseptic, for wounds and skin infections [293–296]. In the Spanish Basque Country, several uses were reported. For example, decoction was applied for the treatment of ulcers and for the treatment of gangrene, and fresh leaves were used directly on the wound for disinfection [297]. In the Mediterranean region, the decoction of the aerial parts (leaves, stems, and flowers) has been used to regulate blood pressure [298,299]. It has also been a frequent remedy for hemorrhoids and to treat bruises and varicose veins [290]. The decoction of flowers and leaves has also been popularly used as an analgesic for oral infections [293] and for hepatoprotection in Granada and Mallorca [290,298]. The decoction of the fresh aerial parts, including the flowers, was used as a remedy against colds and flu infections, and against bronchitis [9,290,299] and whooping cough [10]. In the Spanish peninsula, C. albidus decoction has also been used as a remedy for osteoarthritis in the province of Jaen [266] and for rheumatism in the Valencian community and the Province of Jaen [292,300]. In addition, it was used as an external antiseptic for wound healing and skin infections in the provinces of Castellon, Mallorca, and Almería [10,290,293], and in Morocco [263,265]. In Sardinia (Italy), a traditional use is reported in poultices and ointments, which were applied directly to the wound [301]. In cases of gastrointestinal infections, in Almería (Spain), an infusion of dried leaves was prepared to reduce abdominal pain [293,298]. Against colic, in Castilla-La Mancha and Murcia (Spain) an infusion of young and tender shoots was administered, but it was also supplied by oral ingestion of the powder of dry leaves for treatment [9]. The dried leaf powder also served as an antidiarrheal in Jaen [266]. Infusions of fresh flowers and leaves have been used as an antiseptic for the urinary tract in Murcia [302] and also as an anti-inflammatory for orchitis in Valencia [303].
References
- Guzmán, B.; Vargas, P. Long-Distance Colonization of the Western Mediterranean by Cistus ladanifer (Cistaceae) despite the Absence of Special Dispersal Mechanisms. J. Biogeogr. 2009, 36, 954–968.
- Müller, M.; Siles, L.; Cela, J.; Munné-Bosch, S. Perennially Young: Seed Production and Quality in Controlled and Natural Populations of Cistus albidus Reveal Compensatory Mechanisms That Prevent Senescence in Terms of Seed Yield and Viability. J. Exp. Bot. 2014, 65, 287–297.
- Pérez-Llorca, M.; Casadesús, A.; Müller, M.; Munné-Bosch, S. Leaf Orientation as Part of the Leaf Developmental Program in the Semi-Deciduous Shrub, Cistus albidus L.: Diurnal, Positional, and Photoprotective Effects During Winter. Front. Plant Sci. 2019, 10, 767.
- Pérez-Llorca, M.; Casadesús, A.; Munné-Bosch, S.; Müller, M. Contrasting Patterns of Hormonal and Photoprotective Isoprenoids in Response to Stress in Cistus albidus during a Mediterranean Winter. Planta 2019, 250, 1409–1422.
- Munné-Bosch, S.; Jubany-Marí, T.; Alegre, L. Enhanced Photo- and Antioxidative Protection, and Hydrogen Peroxide Accumulation in Drought-Stressed Cistus clusii and Cistus albidus Plants. Tree Physiol. 2003, 23, 1–12.
- Casadesús, A.; Bouchikh, R.; Pérez-Llorca, M.; Munné-Bosch, S. Linking Jasmonates with Vitamin E Accumulation in Plants: A Case Study in the Mediterranean Shrub Cistus albidus L. Planta 2021, 253, 36.
- Brossa, R.; Pintó-Marijuan, M.; Francisco, R.; López-Carbonell, M.; Chaves, M.M.; Alegre, L. Redox Proteomics and Physiological Responses in Cistus albidus Shrubs Subjected to Long-Term Summer Drought Followed by Recovery. Planta 2015, 241, 803–822.
- Roy, J.; Sonie, L. Germination and Population Dynamics of Cistus Species in Relation to Fire. J. Appl. Ecol. 1992, 29, 647.
- Casadesús, A.; Bouchikh, R.; Munné-Bosch, S. Contrasting Seasonal Abiotic Stress and Herbivory Incidence in Cistus albidus L. Plants Growing in Their Natural Habitat on a Mediterranean Mountain. J. Arid Environ. 2022, 206, 104842.
- Cabezudo, B.; Pérez Latorre, A.V.; Navarro, T.; Nieto Caldera, J.M. Estudios Fenomorfológicos En La Vegetación Del Sur de España. II. Alcornocales Mesomediterráneos. (Montes de Málaga, Málaga). Acta Bot. Malacit. 1993, 18, 179–188.
- Blasco, S.; Mateu, I. Flowering and Fruiting Phenology and Breeding System of Cistus albidus L. Acta Bot. Gallica 1995, 142, 245–251.
- Hernández, I.; Miret, J.A.; Van Der Kelen, K.; Rombaut, D.; Van Breusegem, F.; Munné-Bosch, S. Zeatin Modulates Flower Bud Development and Tocopherol Levels in Cistus albidus (L.) Plants as They Age. Plant Biol. 2015, 17, 90–96.
- Siles, L.; Müller, M.; Cela, J.; Hernández, I.; Alegre, L.; Munné-Bosch, S. Marked Differences in Seed Dormancy in Two Populations of the Mediterranean Shrub, Cistus albidus L. Plant Ecol. Divers. 2017, 10, 231–240.
- Rizzotto, M. Ricerche tassonomiche e corologiche sulle Cistaceae. 1: Il genere Cistus L. in Italia. Webbia 1979, 33, 343–378.
- Robles, C.; Dutoit, T.; Bonin, G. Inhibition Mechanisms and Successional Processes: A Case Study of Cistus albidus L. in Provence. Ecosyst. Sustain. Dev. 1998, 1, 437–446.
- Thanos, A.C.; Geroghiou, K.; Kadis, C.; Pantazi, C. Cistaceae: A plant family with hard seeds. Isr. J. Bot. 1993, 41, 251–263.
- Trabaud, L.; Oustric, J. Heat Requirements for Seed Germination of Three Cistus Species in the Garrigue of Southern France. Flora 1989, 183, 321–325.
- Trabaud, L.; Renard, P. Do light and litter influence the recruitment of cistus spp. Stands? Isr. J. Plant Sci. 1999, 47, 1–9.
- Baskin, J.M.; Baskin, C.C. A Classification System for Seed Dormancy. Seed Sci. Res. 2004, 14, 1–16.
- Barrajón-Catalán, E.; Fernández-Arroyo, S.; Roldán, C.; Guillén, E.; Saura, D.; Segura-Carretero, A.; Micol, V. A Systematic Study of the Polyphenolic Composition of Aqueous Extracts Deriving from Several Cistus Genus Species: Evolutionary Relationship: Polyphenolic Characterization of Cistus Aqueous Extracts. Phytochem. Anal. 2011, 22, 303–312.
- Polunin, O.; Schauer, T.; Everard, B. Pflanzen Europas; BLV-Bestimmungsbuch; BLV(-Verl. Ges.): München, Germany, 1971; ISBN 978-3-405-10929-5.
- Ormeño, E.; Baldy, V.; Ballini, C.; Fernandez, C. Production and Diversity of Volatile Terpenes from Plants on Calcareous and Siliceous Soils: Effect of Soil Nutrients. J. Chem. Ecol. 2008, 34, 1219–1229.
- Robles, C.; Garzino, S. Essential Oil Composition of Cistus albidus Leaves. Phytochemistry 1998, 48, 1341–1345.
- Castells, E.; Peñuelas, J. Is There a Feedback between N Availability in Siliceous and Calcareous Soils and Cistus albidus Leaf Chemical Composition? Oecologia 2003, 136, 183–192.
- El Mamoun, I.; Mouna, F.; Mohammed, A.; Najib, B.; Zine-El Abidine, T.; Abdelkarim, G.; Didier, B.; Laurent, L.; Abdelaziz, S. Zinc, Lead, and Cadmium Tolerance and Accumulation in Cistus libanotis, Cistus albidus and Cistus salviifolius: Perspectives on Phytoremediation. Remediat. J. 2020, 30, 73–80.
- Lukas, B.; Jovanovic, D.; Schmiderer, C.; Kostas, S.; Kanellis, A.; Gómez Navarro, J.; Aytaç, Z.; Koç, A.; Sözen, E.; Novak, J. Intraspecific Genetic Diversity of Cistus creticus L. and Evolutionary Relationships to Cistus albidus L. (Cistaceae): Meeting of the Generations? Plants 2021, 10, 1619.
- ISO Standard No. 9235:2013; International Organization for Standardization Aromatic Natural Raw Materials. ISO: Geneva, Switzerland, 2013.
- Bechlaghem, K.; Allali, H.; Benmehdi, H.; Aissaoui, N.; Flamini, G. Chemical Analysis of the Essential Oils of Three Cistus Species Growing in North- West of Algeria. Agric. Conspec. Sci. 2019, 84, 283–293.
- Llusià, J.; Peñuelas, J.; Ogaya, R.; Alessio, G. Annual and Seasonal Changes in Foliar Terpene Content and Emission Rates in Cistus albidus L. Submitted to Soil Drought in Prades Forest (Catalonia, NE Spain). Acta Physiol. Plant. 2010, 32, 387–394.
- Palá-Paúl, J.; Velasco-Negueruela, A.; Pérez-Alonso, M.J.; Sanz, J. Seasonal Variation in Chemical Composition of Cistus albidus L. from Spain. J. Essent. Oil Res. 2005, 17, 19–22.
- Maccioni, S.; Baldini, R.; Cioni, P.L.; Tebano, M.; Flamini, G. In Vivo Volatiles Emission and Essential Oils from Different Organs and Pollen Of Cistus albidus from Caprione (Eastern Liguria, Italy). Flavour Fragr. J. 2007, 22, 61–65.
- Morales-Soto, A.; Oruna-Concha, M.J.; Elmore, J.S.; Barrajón-Catalán, E.; Micol, V.; Roldán, C.; Segura-Carretero, A. Volatile Profile of Spanish Cistus Plants as Sources of Antimicrobials for Industrial Applications. Ind. Crops Prod. 2015, 74, 425–433.
- Ormeño, E.; Mévy, J.P.; Vila, B.; Bousquet-Mélou, A.; Greff, S.; Bonin, G.; Fernandez, C. Water Deficit Stress Induces Different Monoterpene and Sesquiterpene Emission Changes in Mediterranean Species. Relationship between Terpene Emissions and Plant Water Potential. Chemosphere 2007, 67, 276–284.
- Ormeño, E.; Bousquet-Mélou, A.; Mévy, J.-P.; Greff, S.; Robles, C.; Bonin, G.; Fernandez, C. Effect of Intraspecific Competition and Substrate Type on Terpene Emissions from Some Mediterranean Plant Species. J. Chem. Ecol. 2007, 33, 277–286.
- Paolini, J.; Tomi, P.; Bernardini, A.-F.; Bradesi, P.; Casanova, J.; Kaloustian, J. Detailed Analysis of the Essential Oil from Cistus albidus L. by Combination of GC/RI, GC/MS and 13C-NMR Spectroscopy. Nat. Prod. Res. 2008, 22, 1270–1278.
- Teuscher, E. Biogene Arzneimittel: Lehrbuch der Pharmazeutischen Biologie; 2020; ISBN 978-3-8047-3607-8. Available online: https://www.amazon.de/Biogene-Arzneimittel-Lehrbuch-Pharmazeutischen-Biologie/dp/3804736076 (accessed on 10 August 2023).
- Tomás-Menor, L.; Morales-Soto, A.; Barrajón-Catalán, E.; Roldán-Segura, C.; Segura-Carretero, A.; Micol, V. Correlation between the Antibacterial Activity and the Composition of Extracts Derived from Various Spanish Cistus Species. Food Chem. Toxicol. 2013, 55, 313–322.
- Fadel, H.; Kebbi, S.; Chalchat, J.-C.; Figueredo, G.; Chalard, P.; Benayache, F.; Ghedadba, N.; Benayache, S. Identification of Volatile Components and Antioxidant Assessment of the Aerial Part Extracts from an Algerian Cistus albidus L. of the Aures Region. J. New Technol. Mater. 2020, 10, 38–46.
- Mastino, P.M.; Marchetti, M.; Costa, J.; Usai, M. Comparison of Essential Oils from Cistus Species Growing in Sardinia. Nat. Prod. Res. 2017, 31, 299–307.
- Sticher, O.; Heilmann, J.; Zündorf, I.; Hänsel, R.; Steinegger, E. Pharmakognosie—Phytopharmazie; 10., völlig neu Bearbeitete Auflage.; Wissenschaftliche Verlagsgesellschaft: Stuttgart, Germany, 2015; ISBN 978-3-8047-3144-8.
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Lehrbuch der Lebensmittelchemie; Springer-Lehrbuch; Sechste, Vollständig Überarbeitete Auflage; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-540-73201-3.
- Mastino, P.; Marchetti, M.A.; Costa, J.; Juliano, C.; Usai, M. Analytical Profiling of Phenolic Compounds in Extracts of Three Cistus Species from Sardinia and Their Potential Antimicrobial and Antioxidant Activity. Chem. Biodivers. 2021, 18, e2100053.
- Qa’dan, F.; Petereit, F.; Nahrstedt, A. Prodelphinidin Trimers and Characterization of a Proanthocyanidin Oligomer from Cistus albidus. Pharmazie 2003, 58, 416–419.
- Wiesner, J.V. Die Rohstoffe des Pflanzenreiches; Verlag W. Engelmann: Leipzig, Berlin, Germany, 1921.
- Gonçalves, S.; Gomes, D.; Costa, P.; Romano, A. The Phenolic Content and Antioxidant Activity of Infusions from Mediterranean Medicinal Plants. Ind. Crops Prod. 2013, 43, 465–471.
- Legrum, W. Riechstoffe, Zwischen Gestank und Duft: Vorkommen, Eigenschaften und Anwendung von Riechstoffen und deren Gemischen; Studienbücher Chemie; 1. Aufl.; Vieweg + Teubner: Wiesbaden, Germany, 2011; ISBN 978-3-8348-1245-2.
- Fahlbusch, K.-G.; Hammerschmidt, F.-J.; Panten, J.; Pickenhagen, W.; Schatkowski, D.; Bauer, K.; Garbe, D.; Surburg, H. Flavors and Fragrances. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA, Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003; p. a11_141. ISBN 978-3-527-30673-2.
- Fenaroli, G.; Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 4th ed.; CRC Press: Boca Raton, FL, USA, 2002; ISBN 978-0-8493-0946-5.
- Pérez-Llorca, M.; Caselles, V.; Müller, M.; Munné-Bosch, S. The Threshold between Life and Death in Cistus albidus L. Seedlings: Mechanisms Underlying Drought Tolerance and Resilience. Tree Physiol. 2021, 41, 1861–1876.
- Oñate, M.; Munné-Bosch, S. Loss of Flower Bud Vigour in the Mediterranean Shrub, Cistus albidus L. at Advanced Developmental Stages. Plant Biol. 2010, 12, 475–483.
- Jubany-Mari, T.; Munne-Bosch, S.; Lopez-Carbonell, M.; Alegre, L. Hydrogen Peroxide Is Involved in the Acclimation of the Mediterranean Shrub, Cistus albidus L., to Summer Drought. J. Exp. Bot. 2008, 60, 107–120.
