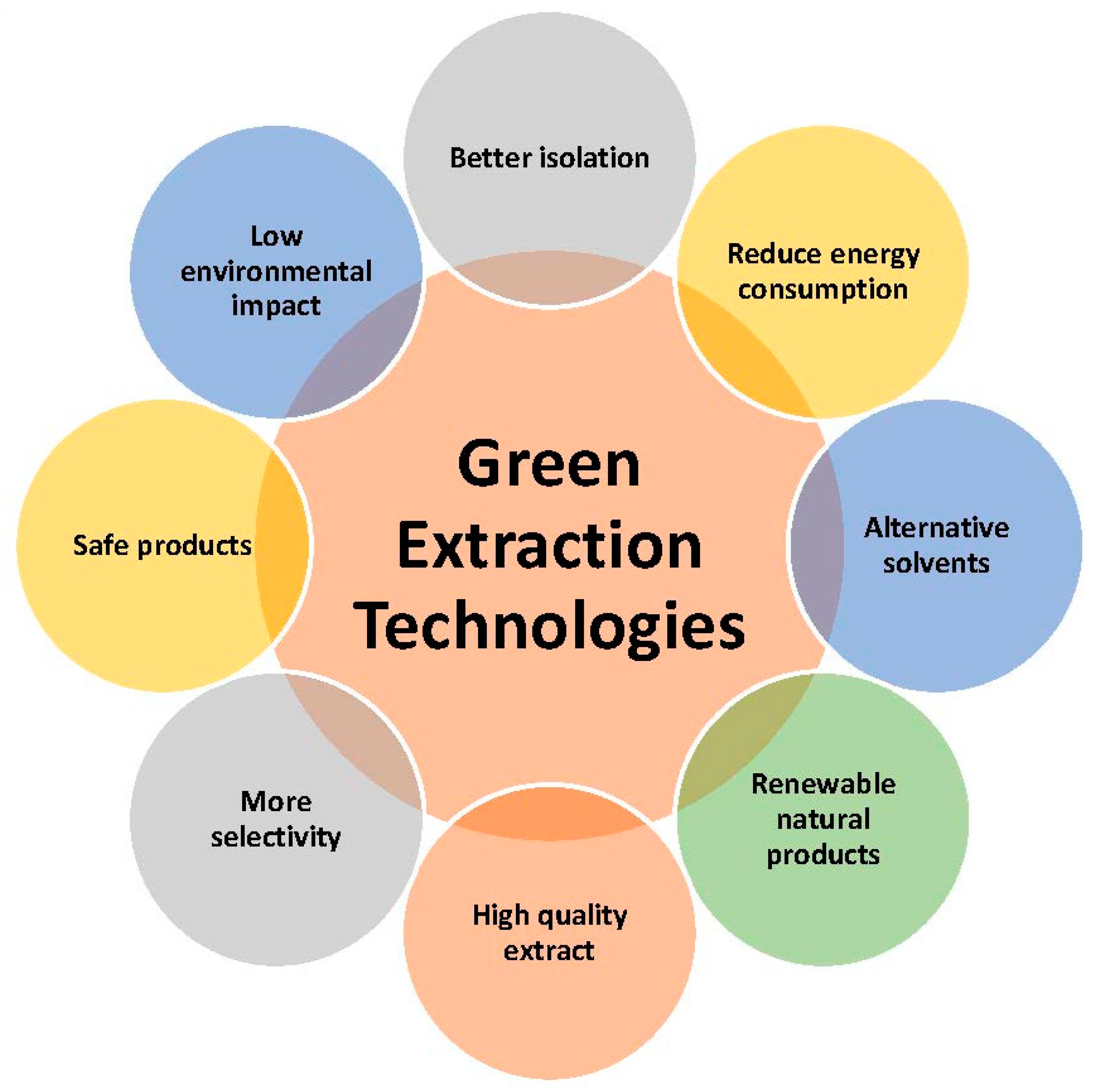
Some functional foods contain biologically active compounds (BAC) that can be derived from various biological sources (fruits, vegetables, medicinal plants, wastes, and by-products). Global food markets demand foods from plant materials that are “safe”, “fresh”, “natural”, and with “nutritional value” while processed in sustainable ways. Functional foods commonly incorporate some plant extract(s) rich with BACs produced by conventional extraction. This approach implies negative thermal influences on extraction yield and quality with a large expenditure of organic solvents and energy. On the other hand, sustainable extractions, such as microwave-assisted extraction (MAE), ultrasound-assisted extraction (UAE), high-pressure assisted extraction (HPAE), high voltage electric discharges assisted extraction (HVED), pulsed electric fields assisted extraction (PEF), supercritical fluids extraction (SFE), and others are aligned with the “green” concepts and able to provide raw materials on industrial scale with optimal expenditure of energy and chemicals.
- functional food
- extract
- biological active compounds
- innovative technology
1. Introduction
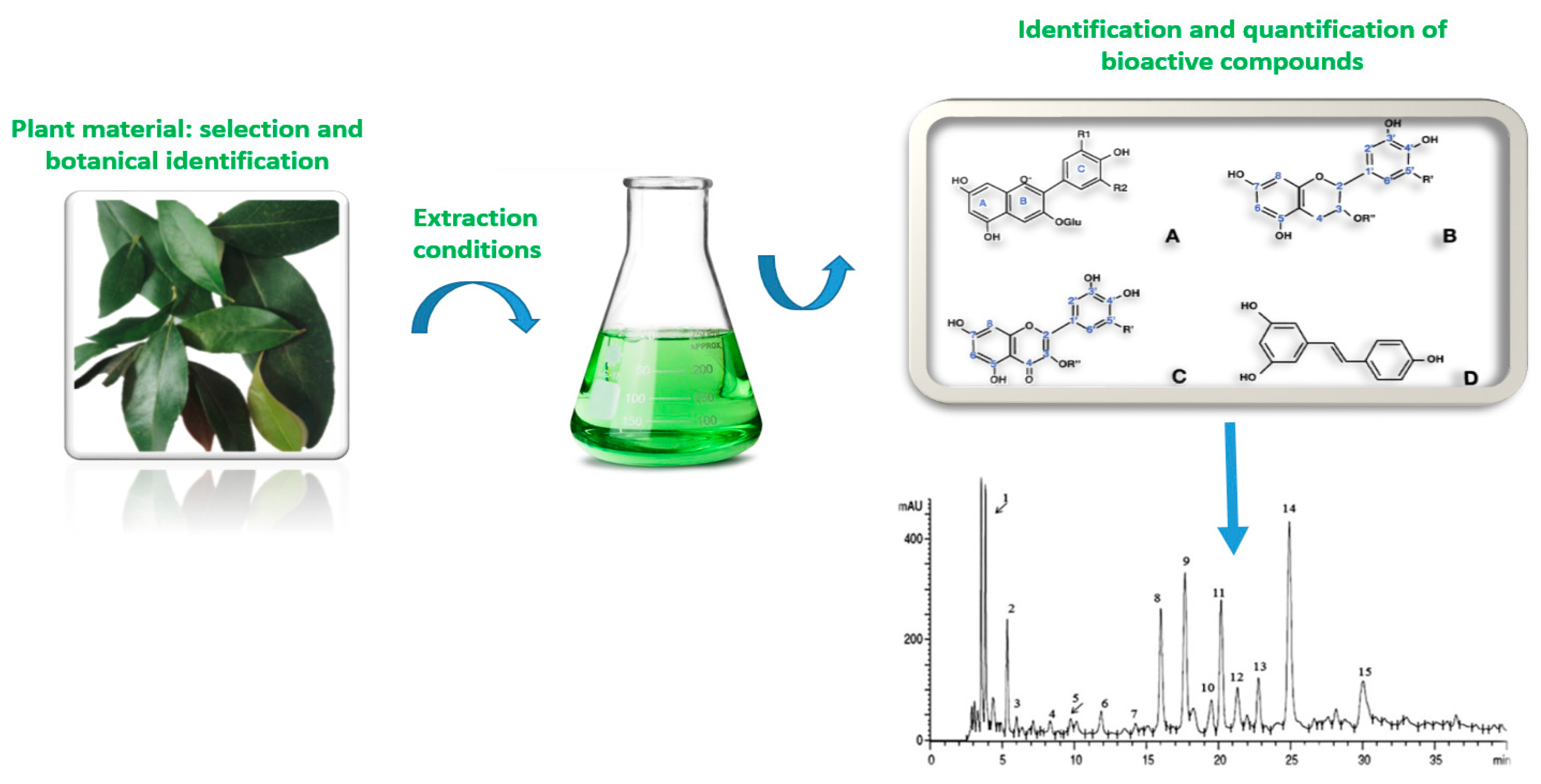
2. Health Benefits of Plant Materials
3. Innovative Extractions and Processing of BACs
References
- Granato, D.; Nunes, D.S.; Barba, F.J. An integrated strategy between food chemistry, biology, nutrition, pharmacology, and statistics in the development of functional foods: A proposal. Trends Food Sci. Technol. 2017, 62, 13–22.
- Musina, O.; Putnik, P.; Koubaa, M.; Barba, F.J.; Greiner, R.; Granato, D.; Roohinejad, S. Application of modern computer algebra systems in food formulations and development: A case study. Trends Food Sci. Technol. 2017, 64, 48–59.
- Čukelj, N.; Putnik, P.; Novotni, D.; Ajredini, S.; Voučko, B.; Duška, Ć. Market potential of lignans and omega-3 functional cookies. Br. Food J. 2016, 118, 2420–2433.
- Putnik, P.; Bursać Kovačević, D.; Režek Jambrak, A.; Barba, F.; Cravotto, G.; Binello, A.; Lorenzo, J.; Shpigelman, A. Innovative “green” and novel strategies for the extraction of bioactive added value compounds from citrus wastes-A Review. Molecules 2017, 22, 680.
- Putnik, P.; Bursać Kovačević, D.; Herceg, K.; Levaj, B. Influence of respiration on predictive microbial growth of Aerobic mesophilic bacteria and Enterobacteriaceae in fresh-cut apples packaged under modified atmosphere. J. Food Saf. 2016, 37, e12284.
- Putnik, P.; Bursać Kovačević, D.; Herceg, K.; Pavkov, I.; Zorić, Z.; Levaj, B. Effects of modified atmosphere, anti-browning treatments and ultrasound on the polyphenolic stability, antioxidant capacity and microbial growth in fresh-cut apples. J. Food Process Eng. 2016, 41, e12539.
- Putnik, P.; Bursać Kovačevć, D. Fresh-cut apples spoilage and predictive microbial growth under modified atmosphere packaging. In Food Safety and Protection; Rai, R., Aswathanarayan, J.B., Eds.; CRC Press Press: Boca Raton, FL, USA, 2017; p. 728.
- Putnik, P.; Bursać Kovačević, D.; Herceg, K.; Levaj, B. Influence of cultivar, anti-browning solutions, packaging gasses, and advanced technology on browning in fresh-cut apples during storage. J. Food Process Eng. 2017, 40, e12400.
- Putnik, P.; Bursać Kovačević, D.; Herceg, K.; Levaj, B. Influence of antibrowning solutions, air exposure, and ultrasound on color changes in fresh-cut apples during storage. J. Food Process. Preserv. 2017, 41, e13288.
- Putnik, P.; Bursać Kovačević, D.; Herceg, K.; Roohinejad, S.; Greiner, R.; Bekhit, A.E.-D.A.; Levaj, B. Modelling the shelf-life of minimally-processed fresh-cut apples packaged in a modified atmosphere using food quality parameters. Food Control 2017, 81, 55–64.
- Putnik, P.; Roohinejad, S.; Greiner, R.; Granato, D.; Bekhit, A.E.-D.A.; Bursać Kovačević, D. Prediction and modeling of microbial growth in minimally processed fresh-cut apples packaged in a modified atmosphere: A review. Food Control 2017, 80, 411–419.
- Barba, F.J.; Putnik, P.; Bursać Kovačević, D.; Poojary, M.M.; Roohinejad, S.; Lorenzo, J.M.; Koubaa, M. Impact of conventional and non-conventional processing on prickly pear (Opuntia spp.) and their derived products: From preservation of beverages to valorization of by-products. Trends Food Sci. Technol. 2017, 67, 260–270.
- Putnik, P.; Barba, F.J.; Lorenzo, J.M.; Gabrić, D.; Shpigelman, A.; Cravotto, G.; Bursać Kovačević, D. An integrated approach to mandarin processing: Food safety and nutritional quality, consumer preference and nutrient bioaccessibility. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1345–1358.
- Lorenzo Rodriguez, J.M.; Munekata, P.; Putnik, P.; Bursać Kovačević, D.; Muchenje, V.; Barba, F. Sources, Chemistry and Biological Potential of Ellagitannins and Ellagic Acid Derivatives. In Studies in Natural Product Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2018.
- Montesano, D.; Rocchetti, G.; Putnik, P.; Lucini, L. Bioactive profile of pumpkin: An overview on terpenoids and their health-promoting properties. Curr. Opin. Food Sci. 2018, 22, 81–87.
- Poojary, M.M.; Putnik, P.; Kovačević, D.B.; Barba, F.J.; Lorenzo, J.M.; Dias, D.A.; Shpigelman, A. Stability and extraction of bioactive sulfur compounds from Allium genus processed by traditional and innovative technologies. J. Food Compos. Anal. 2017, 61, 28–39.
- Vinceković, M.; Viskić, M.; Jurić, S.; Giacometti, J.; Bursać Kovačević, D.; Putnik, P.; Donsì, F.; Barba, F.J.; Režek Jambrak, A. Innovative technologies for encapsulation of Mediterranean plants extracts. Trends Food Sci. Technol. 2017, 69, 1–12.
- Lorenzo, J.M.; Pateiro, M.; Domínguez, R.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Shpigelman, A.; Granato, D.; Franco, D. Berries extracts as natural antioxidants in meat products: A review. Food Res. Int. 2018, 106, 1095–1104.
- Kovačević, D.B.; Barba, F.J.; Granato, D.; Galanakis, C.M.; Herceg, Z.; Dragović-Uzelac, V.; Putnik, P. Pressurized Hot Water Extraction (PHWE) for the green recovery of bioactive compounds and steviol glycosides from Stevia rebaudiana Bertoni Leaves. Food Chem. 2018, 254, 150–157.
- Kovačević, D.B.; Putnik, P.; Pedisić, S.; Ježek, D.; Karlovic, S.; Dragovic-Uzelac, V. High hydrostatic pressure extraction of flavonoids from freeze-dried red grape skin as winemaking by-product. Ann. Nutr. Metab. 2015, 67, 521–522.
- Dragović-Uzelac, V.; Putnik, P.; Zorić, Z.; Ježek, D.; Karlovic, S.; Bursać Kovačević, D. Winery by-products: Anthocyanins recovery from red grape skin by high hydrostatic pressure extraction (HHPE). Ann. Nutr. Metab. 2015, 67, 522–523.
- Putnik, P.; Bursać Kovačević, D.; Ježek, D.; Šustić, I.; Zorić, Z.; Dragović-Uzelac, V. High-pressure recovery of anthocyanins from grape skin pomace (Vitis vinifera cv. Teran) at moderate temperature. J. Food Process. Preserv. 2017, 42, e13342.
- Putnik, P.; Barba, F.J.; Španić, I.; Zorić, Z.; Dragović-Uzelac, V.; Bursać Kovačević, D. Green extraction approach for the recovery of polyphenols from Croatian olive leaves (Olea europea). Food Bioprod. Process. 2017, 106, 19–28.
- Huang, H.W.; Hsu, C.P.; Yang, B.B.; Wang, C.Y. Advances in the extraction of natural ingredients by high pressure extraction technology. Trends Food Sci. Technol. 2013, 33, 54–62.
- Gabrić, D.; Barba, F.; Roohinejad, S.; Gharibzahedi, S.M.T.; Radojčin, M.; Putnik, P.; Bursać Kovačević, D. Pulsed electric fields as an alternative to thermal processing for preservation of nutritive and physicochemical properties of beverages: A review. J. Food Process Eng. 2018, 41, e12638.
- Koubaa, M.; Barba, F.; Bursać Kovačević, D.; Putnik, P.; Santos, M.S.; Queirós, R.P.; Moreira, S.A.; Duarte, R.R.; Saraiva, J.A. Pulsed electric field processing of different fruit juices. In Fruit Juices, 1st ed.; Rajauria, G., Tiwari, B., Eds.; Academic Press: Cambridge, MA, USA, 2017; p. 760.
- Herrero, M.; Plaza, M.; Cifuentes, A.; Ibáñez, E. Green processes for the extraction of bioactives from Rosemary: Chemical and functional characterization via ultra-performance liquid chromatography-tandem mass spectrometry and in-vitro assays. J. Chromatogr. A 2010, 1217, 2512–2520.
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Review of green food processing techniques. Preservation, transformation, and extraction. Innov. Food Sci. Emerg. 2017, 41, 357–377.
- Granato, D.; Putnik, P.; Kovačević, D.B.; Santos, J.S.; Calado, V.; Rocha, R.S.; Cruz, A.G.D.; Jarvis, B.; Rodionova, O.Y.; Pomerantsev, A. Trends in Chemometrics: Food Authentication, Microbiology, and Effects of Processing. Compr. Rev. Food Sci. Food Saf. 2018, 17, 663–677.
- Alberti, A.; Zielinski, A.A.F.; Zardo, D.M.; Demiate, I.M.; Nogueira, A.; Mafra, L.I. Optimisation of the extraction of phenolic compounds from apples using response surface methodology. Food Chem. 2014, 149, 151–158.
- Chavez-Santoscoy, R.A.; Gutierrez-Uribe, J.A.; Serna-Saldívar, S.O. Phenolic Composition, Antioxidant Capacity and In Vitro Cancer Cell Cytotoxicity of Nine Prickly Pear (Opuntia spp.) Juices. Plant Food Hum. Nutr. 2009, 64, 146–152.
- Fernandez-Lopez, J.A.; Almela, L.; Obon, J.M.; Castellar, R. Determination of Antioxidant Constituents in Cactus Pear Fruits. Plant Food Hum. Nutr. 2010, 65, 253–259.
- Jiménez-Aguilar, D.M.; López-Martínez, J.M.; Hernández-Brenes, C.; Gutiérrez-Uribe, J.A.; Welti-Chanes, J. Dietary fiber, phytochemical composition and antioxidant activity of Mexican commercial varieties of cactus pear. J. Food Compos. Anal. 2015, 41, 66–73.
- Moussa-Ayoub, T.E.; El-Samahy, S.K.; Rohn, S.; Kroh, L.W. Flavonols, betacyanins content and antioxidant activity of cactus Opuntia macrorhiza fruits. Food Res. Int. 2011, 44, 2169–2174.
- Stintzing, F.C.; Herbach, K.M.; Mosshammer, M.R.; Carle, R.; Yi, W.; Sellappan, S.; Akoh, C.C.; Bunch, R.; Felker, P. Color, Betalain Pattern, and Antioxidant Properties of Cactus Pear (Opuntia spp.) Clones. J. Agric. Food Chem. 2005, 53, 442–451.
- Chang, S.-F.; Hsieh, C.-L.; Yen, G.-C. The protective effect of Opuntia dillenii Haw fruit against low-density lipoprotein peroxidation and its active compounds. Food Chem. 2008, 106, 569–575.
- Ennouri, M.; Fetoui, H.; Hammami, M.; Bourret, E.; Attia, H.; Zeghal, N. Effects of diet supplementation with cactus pear seeds and oil on serum and liver lipid parameters in rats. Food Chem. 2007, 101, 248–253.
- Galati, E.M.; Mondello, M.R.; Giuffrida, D.; Dugo, G.; Miceli, N.; Pergolizzi, S.; Taviano, M.F. Chemical Characterization and Biological Effects of Sicilian Opuntia ficus indica (L.) Mill. Fruit Juice: Antioxidant and Antiulcerogenic Activity. J. Agric. Food Chem. 2003, 51, 4903–4908.
- Szajdek, A.; Borowska, E.J. Bioactive Compounds and Health-Promoting Properties of Berry Fruits: A Review. Plant Food Hum. Nutr. 2008, 63, 147–156.
- Nishimura, T.; Egusa, A.S.; Nagao, A.; Odahara, T.; Sugise, T.; Mizoguchi, N.; Nosho, Y. Phytosterols in onion contribute to a sensation of lingering of aroma, a koku attribute. Food Chem. 2016, 192, 724–728.
- Prakash, D.; Singh, B.N.; Upadhyay, G. Antioxidant and free radical scavenging activities of phenols from onion (Allium cepa). Food Chem. 2007, 102, 1389–1393.
- Corzo-Martinez, M.; Corzo, N.; Villamiel, M. Biological properties of onions and garlic. Trends Food Sci. Technol. 2007, 18, 609–625.
- Bisen, P.S.; Emerald, M. Nutritional and Therapeutic Potential of Garlic and Onion (Allium sp.). Curr. Nutr. Food Sci. 2016, 12, 190–199.
- Santas, J.; Carbo, R.; Gordon, M.; Almajano, M. Comparison of the antioxidant activity of two Spanish onion varieties. Food Chem. 2008, 107, 1210–1216.
- Nile, S.H.; Nile, A.S.; Keum, Y.S.; Sharma, K. Utilization of quercetin and quercetin glycosides from onion (Allium cepa L.) solid waste as an antioxidant, urease and xanthine oxidase inhibitors. Food Chem. 2017, 235, 119–126.
- Wang, B.-S.; Huang, G.-J.; Lu, Y.-H.; Chang, L.-W. Anti-inflammatory effects of an aqueous extract of Welsh onion green leaves in mice. Food Chem. 2013, 138, 751–756.
- Ribeiro-Santos, R.; Carvalho-Costa, D.; Cavaleiro, C.; Costa, H.S.; Albuquerque, T.G.; Castilho, M.C.; Ramos, F.; Melo, N.R.; Sanches-Silva, A. A novel insight on an ancient aromatic plant: The rosemary (Rosmarinus officinalis L.). Trends Food Sci. Technol. 2015, 45, 355–368.
- Sueishi, Y.; Sue, M.; Masamoto, H. Seasonal variations of oxygen radical scavenging ability in rosemary leaf extract. Food Chem. 2018, 245, 270–274.
- Nieto, G. Biological activities of three essential oils of the Lamiaceae family. Medicines 2017, 4, 63.
- Berdahl, D.B.; McKeague, J. Rosemary and sage extracts as antioxidants for food preservation. In Handbook of Antioxidants for Food Preservation; Woodhead Publishing: Cambridge, UK, 2015; Volume 276, pp. 177–217.
- Abu-Darwish, M.S.; Cabral, C.; Ferreira, I.V.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Al-bdour, T.H.; Salgueiro, L. Essential oil of common sage (Salvia officinalis L.) from Jordan: Assessment of safety in mammalian cells and its antifungal and anti-inflammatory potential. BioMed Res. Int. 2013, 2013, 538940.
- Kolac, U.K.; Ustuner, M.C.; Tekin, N.; Ustuner, D.; Colak, E.; Entok, E. The anti-inflammatory and antioxidant effects of Salvia officinalis on lipopolysaccharide-induced inflammation in rats. J. Med. Food 2017, 20, 1193–1200.
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170.
- Wu, J.; Ge, S.; Liu, H.; Wang, S.; Chen, S.; Wang, J.; Li, J.; Zhang, Q. Properties and antimicrobial activity of silver carp (Hypophthalmichthys molitrix) skin gelatin-chitosan films incorporated with oregano essential oil for fish preservation. Food Packag. Shelf Life 2014, 2, 7–16.
- Tohidi, B.; Rahimmalek, M.; Arzani, A. Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem. 2017, 220, 153–161.
- Moghimi, R.; Ghaderi, L.; Rafati, H.; Aliahmadi, A.; McClements, D.J. Superior antibacterial activity of nanoemulsion of Thymus daenensis essential oil against E. coli. Food Chem. 2016, 194, 410–415.
- Köksal, E.; Bursal, E.; Gülçin, İ.; Korkmaz, M.; Çağlayan, C.; Gören, A.C.; Alwasel, S.H. Antioxidant activity and polyphenol content of Turkish thyme (Thymus vulgaris) monitored by liquid chromatography and tandem mass spectrometry. Int. J. Food Prop. 2016, 20, 514–525.
- Pacifico, S.; Piccolella, S.; Papale, F.; Nocera, P.; Lettieri, A.; Catauro, M. A polyphenol complex from Thymus vulgaris L. plants cultivated in the Campania Region (Italy): New perspectives against neuroblastoma. J. Funct. Foods 2016, 20, 253–266.
- Forbes-Hernandez, T.Y.; Giampieri, F.; Gasparrini, M.; Mazzoni, L.; Quiles, J.L.; Alvarez-Suarez, J.M.; Battino, M. The effects of bioactive compounds from plant foods on mitochondrial function: A focus on apoptotic mechanisms. Food Chem. Toxicol. 2014, 68, 154–182.
- Angulo, J.; Mahecha, L.; Yepes, S.A.; Yepes, A.M.; Bustamante, G.; Jaramillo, H.; Valencia, E.; Villamil, T.; Gallo, J. Quantitative and nutritional characterization of fruit and vegetable waste from marketplace: A potential use as bovine feedstuff? J. Environ. Manag. 2012, 95, S203–S209.
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting citrus wastes into value-added products: Economic and environmently friendly approaches. Nutrition 2017, 34, 29–46.
- Aneja, K.R.; Dhiman, R.; Aggarwal, N.K.; Aneja, A. Emerging Preservation Techniques for Controlling Spoilage and Pathogenic Microorganisms in Fruit Juices. Int. J. Microbiol. 2014, 2014, 758942.
- Yu, H.; Yang, G.; Sato, M.; Yamaguchi, T.; Nakano, T.; Xi, Y. Antioxidant activities of aqueous extract from Stevia rebaudiana stem waste to inhibit fish oil oxidation and identification of its phenolic compounds. Food Chem. 2017, 232, 379–386.
- Criado, M.; Barba, F.; Frigola, A.; Rodrigo, D. Effect of Stevia rebaudiana on oxidative enzyme activity and its correlation with antioxidant capacity and bioactive compounds. Food Bioprocess Technol. 2014, 7, 1518–1525.
- Koubaa, M.; Roselló-Soto, E.; Šic Žlabur, J.; Režek Jambrak, A.; Brnčić, M.; Grimi, N.; Boussetta, N.; Barba, F.J. Current and new insights in the sustainable and green recovery of nutritionally valuable compounds from Stevia rebaudiana Bertoni. J. Agric. Food Chem. 2015, 63, 6835–6846.
- Ferrazzano, G.; Cantile, T.; Alcidi, B.; Coda, M.; Ingenito, A.; Zarrelli, A.; Di Fabio, G.; Pollio, A. Is Stevia rebaudiana Bertoni a non cariogenic sweetener? A Review. Molecules 2016, 21, 38.
- Dimitrios, B. Sources of natural phenolic antioxidants. Trends Food Sci. Technol. 2006, 17, 505–512.
- Ferreira, I.C.F.R.; Barros, L.; Soares, M.E.; Bastos, M.L.; Pereira, J.A. Antioxidant activity and phenolic contents of Olea europaea L. leaves sprayed with different copper formulations. Food Chem. 2007, 103, 188–195.
- Liakopoulos, G.; Stavrianakou, S.; Karabourniotis, G. Trichome layers versus dehaired lamina of Olea europaea leaves: Differences in flavonoid distribution, UV-absorbing capacity, and wax yield. Environ. Exp. Bot. 2006, 55, 294–304.
- Brahmi, F.; Mechri, B.; Dabbou, S.; Dhibi, M.; Hammami, M. The efficacy of phenolics compounds with different polarities as antioxidants from olive leaves depending on seasonal variations. Ind. Crops Prod. 2012, 38, 146–152.
- Bulotta, S.; Celano, M.; Lepore, S.M.; Montalcini, T.; Pujia, A.; Russo, D. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: Focus on protection against cardiovascular and metabolic diseases. J. Transl. Med. 2014, 12, 219.
- Yu, H.; Liu, P.; Tang, H.; Jing, J.; Lv, X.; Chen, L.; Jiang, L.; Xu, J.; Li, J. Oleuropein, a natural extract from plants, offers neuroprotection in focal cerebral ischemia/reperfusion injury in mice. Eur. J. Pharmacol. 2016, 775, 113–119.
- Murotomi, K.; Umeno, A.; Yasunaga, M.; Shichiri, M.; Ishida, N.; Koike, T.; Matsuo, T.; Abe, H.; Yoshida, Y.; Nakajima, Y. Oleuropein-rich diet attenuates hyperglycemia and impaired glucose tolerance in type 2 diabetes model mouse. J. Agric. Food Chem. 2015, 63, 6715–6722.
- Hur, W.; Kim, S.W.; Lee, Y.K.; Choi, J.E.; Hong, S.W.; Song, M.J.; Bae, S.H.; Park, T.; Um, S.-J.; Yoon, S.K. Oleuropein reduces free fatty acid-induced lipogenesis via lowered extracellular signal-regulated kinase activation in hepatocytes. Nutr. Res. 2012, 32, 778–786.
- Barbaro, B.; Toietta, G.; Maggio, R.; Arciello, M.; Tarocchi, M.; Galli, A.; Balsano, C. Effects of the olive-derived polyphenol oleuropein on human health. Int. J. Mol. Sci. 2014, 15, 18508–18524.
- Şahin, S.; Bilgin, M. Olive tree (Olea europaea L.) leaf as a waste by-product of table olive and olive oil industry: A review. J. Sci. Food Agric. 2018, 98, 1271–1279.
- Zhu, Z.; Wu, Q.; Di, X.; Li, S.; Barba, F.J.; Koubaa, M.; Roohinejad, S.; Xiong, X.; He, J. Multistage recovery process of seaweed pigments: Investigation of ultrasound assisted extraction and ultra-filtration performances. Food Bioprod. Process. 2017, 104, 40–47.
- Tongnuanchan, P.; Benjakul, S. Essential oils: Extraction, bioactivities, and their uses for food preservation. J. Food Sci. 2014, 79, R1231–R1249.
- Harbourne, N.; Marete, E.; Christophe, J.J.; O’Riordan, D. Conventional extraction techniques for phytochemicals. In Handbook of Plant Food Phytochemicals Sources, Stability and Extraction; Tiwari, B.K., Brunton, N.P., Brennan, C.S., Eds.; John Wiley & Sons, Ltd.: Oxford, UK, 2013; Volume 1, pp. 399–453.
- Rockenbach, I.I.; Rodrigues, E.; Gonzaga, L.V.; Caliari, V.; Genovese, M.I.; Goncalves, A.E.D.S.; Fett, R. Phenolic compounds content and antioxidant activity in pomace from selected red grapes (Vitis vinifera L. and Vitis labrusca L.) widely produced in Brazil. Food Chem. 2011, 127, 174–179.
- Maier, T.; Goppert, A.; Kammerer, D.R.; Schieber, A.; Carle, R. Optimization of a process for enzyme-assisted pigment extraction from grape (Vitis vinifera L.) pomace. Eur. Food Res. Technol. 2008, 227, 267–275.
- Barba, F.J.; Zhu, Z.; Koubaa, M.; de Souza Sant’Ana, A.; Orlien, V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends Food Sci. Technol. 2016, 49, 96–109.
- Rosello-Soto, E.; Parniakov, O.; Deng, Q.; Patras, A.; Koubaa, M.; Grimi, N.; Boussetta, N.; Tiwari, B.K.; Vorobiev, E.; Lebovka, N.; et al. Application of non-conventional extraction methods: Toward a sustainable and green production of valuable compounds from mushrooms. Food Eng. Rev. 2016, 8, 214–234.
- Mnayer, D.; Fabiano-Tixier, A.-S.; Petitcolas, E.; Ruiz, K.; Hamieh, T.; Chemat, F. Extraction of green absolute from thyme using ultrasound and sunflower oil. Resour.-Effic. Technol. 2017, 3, 12–21.
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523.
