Nanotechnology is opening new therapeutic possibilities of fighting against COVID-19 by enabling new prevention, diagnosis, drug delivery, and treatment methods. Nanomedicine is known as the branch of medicine involved in preventing and curing various diseases using nanoscale materials, such as biocompatible nanoparticles and nanorobots, for various applications, including diagnosis, delivery, and sensing. In addition, nanomedicines have exhibited important features, such as efficient transport through fine capillary blood vessels and lymphatic endothelium, longer circulation duration and blood concentration, higher binding capacity to biomolecules such as endogenous compounds including proteins, higher accumulation in target tissues, reduced inflammatory or immune responses, and oxidative stress in tissues.
- COVID-19
- SARS-CoV-2
- nanotechnology
- detection
- treatment
1. Introduction
2. Nanotechnology-Based Approaches in Preclinical and Clinical Studies: In Vitro and In Vivo
2.1. Nano-Based Approaches in Pre-Clinical Studies
2.1. Nano-Based Approaches in Pre-Clinical Studies
COVID-19 immune-based preclinical therapeutic approaches such as virus-binding molecules; inhibitors of specific enzymes involved in viral replication and transcription; small-molecule inhibitors of helicase, proteases, or other proteins critical for the virus survival; host cell protease; endocytosis inhibitors; and siRNA inhibitors are all potential therapeutic options for SARS-CoV-2 [3]. In addition, the effects induced by monoclonal antibodies (mAb) in COVID-19 patients may also improve the development of vaccines and increasingly specific diagnostics [4]. Moreover, every single one of these tools needs to be assessed regarding clinical efficacy and safety before treating infected patients.2.2. Nano-Based Approaches in Clinical Studies
2.2. Nano-Based Approaches in Clinical Studies
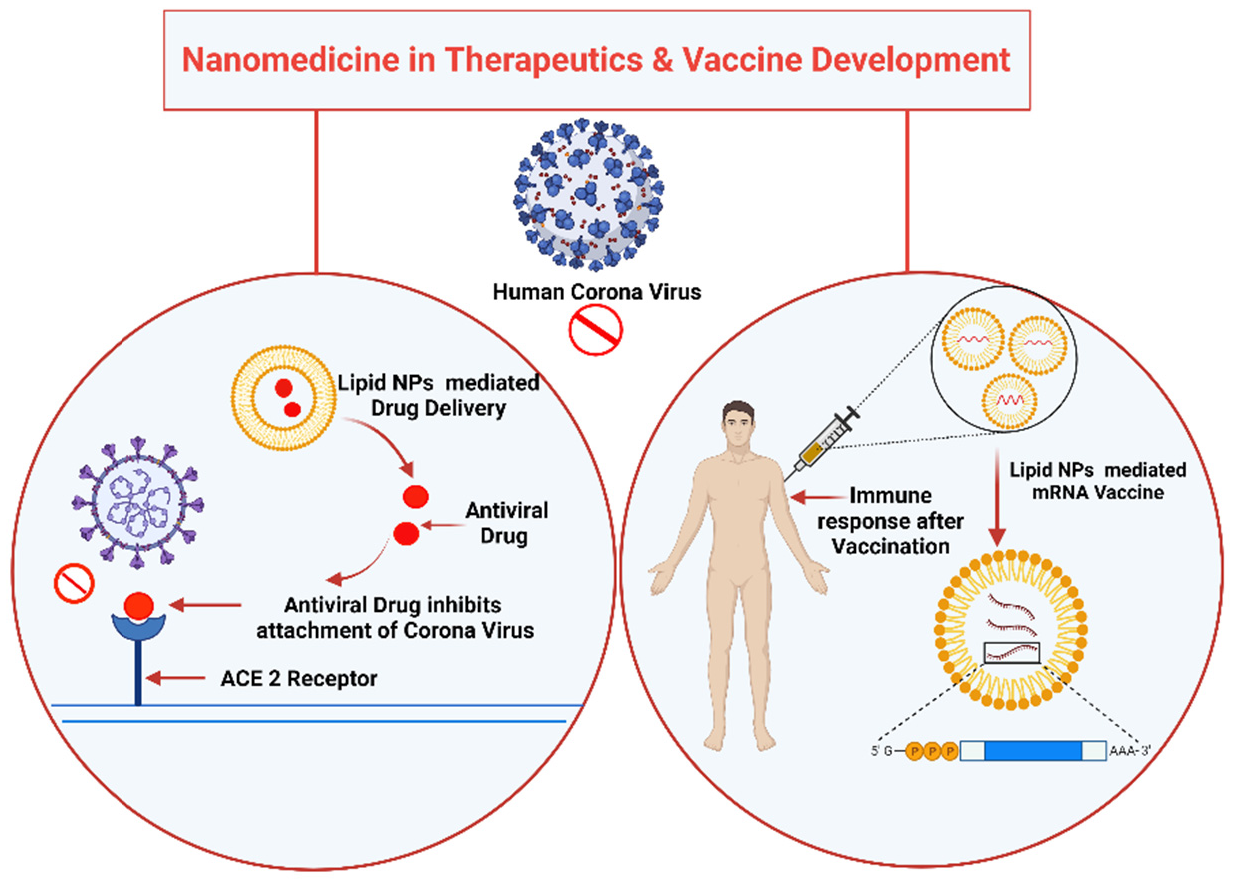

3. Future Perspectives to Tackle COVID-19 Using Nanotechnology
COVID-19 has introduced the scientific community to a global challenge it has perhaps never had to face before. However, it has also taught scientists and the population that this kind of situation could occur again. Therefore, cutting-edge tools, notably nanotechnology, should be solidly developed to tackle SARS-CoV2 infection. Nanoparticle-based medicine is a very effective tool with the potential to reduce the burden of illness. Nanoparticles that are much smaller than a micrometer have received exceptional attention in managing COVID-19 disease caused by SARS-CoV2 due to their distinctive properties (suitable size, simple preparation, minimal cost, effortless modification, etc.). Nanotechnology-based approaches for combating COVID-19 include the innovation of tools for speedy, precise, and sensitive diagnosis of SARS-CoV2 infection, production of efficient disinfectants, efficient delivery of mRNA-based vaccines into human cells, and delivery of antiviral drugs into the host. Nanotechnology is being geared up for implementation in the fight against SARS-CoV2 infection in a wide range of areas, as shown in Figure 2.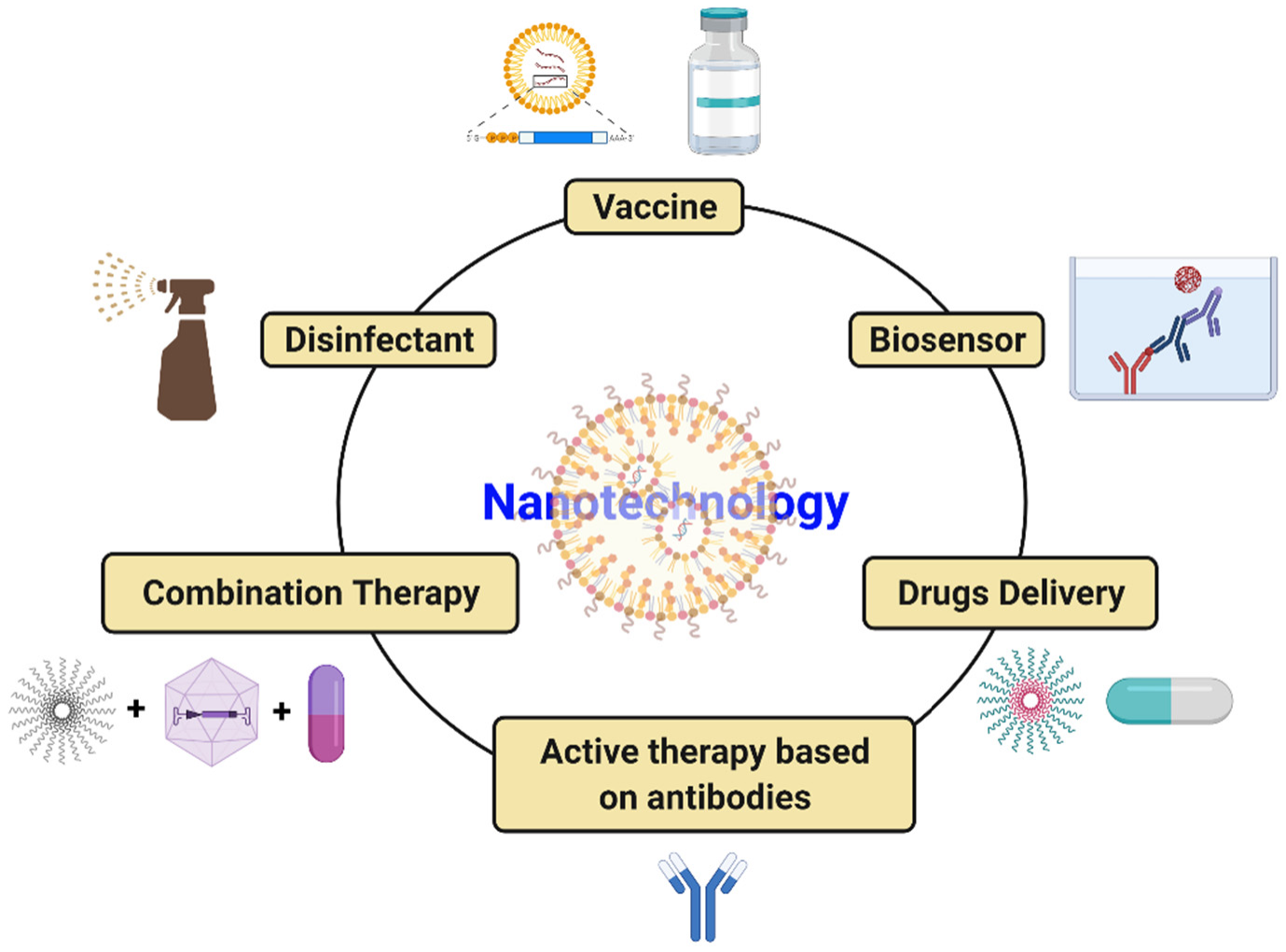

References
- Tang, Z.; Zhang, X.; Shu, Y.; Guo, M.; Zhang, H.; Tao, W. Insights from nanotechnology in COVID-19 treatment. Nano Today 2020, 36, 101019.
- Rangayasami, A.; Kannan, K.; Murugesan, S.; Radhika, D.; Sadasivuni, K.K.; Reddy, K.R.; Raghu, A.V. Influence of nanotechnology to combat against COVID-19 for global health emergency: A review. Sens. Int. 2021, 2, 100079.
- Dhama, K.; Sharun, K.; Tiwari, R.; Dadar, M.; Malik, Y.S.; Singh, K.P.; Chaicumpa, W. COVID-19, an emerging coronavirus infection: Advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum. Vaccines Immunother. 2020, 16, 1232–1238.
- Marston, H.D.; Paules, C.I.; Fauci, A.S. Monoclonal Antibodies for Emerging Infectious Diseases-Borrowing from History. N. Engl. J. Med. 2018, 378, 1469–1472.
- Rab, S.; Afjal, A.; Javaid, M.; Haleem, A.; Vaishya, R. An update on the global vaccine development for coronavirus. Diabetes Metab. Syndr. 2020, 14, 2053–2055.
- Chen, Y.N.; Hsueh, Y.H.; Hsieh, C.T.; Tzou, D.Y.; Chang, P.L. Antiviral Activity of Graphene–Silver Nanocomposites against Non-Enveloped and Enveloped Viruses. Int. J. Environ. Res. Public Health 2016, 13, 430.
- Florindo, H.F.; Kleiner, R.; Vaskovich-Koubi, D.; Acúrcio, R.C.; Carreira, B.; Yeini, E.; Tiram, G.; Liubomirski, Y.; Satchi-Fainaro, R. Immune-mediated approaches against COVID-19. Nat. Nanotechnol. 2020, 15, 630–645.
- Shah, V.K.; Firmal, P.; Alam, A.; Ganguly, D.; Chattopadhyay, S. Overview of Immune Response During SARS-CoV-2 Infection: Lessons From the Past. Front. Immunol. 2020, 11, 1949.
- Fleischmann, R.; Genovese, M.C.; Lin, Y.; St John, G.; Van der Heijde, D.; Wang, S.; Gomez-Reino, J.J.; Maldonado-Cocco, J.A.; Stanislav, M.; Kivitz, A.J.; et al. Long-term safety of sarilumab in rheumatoid arthritis: An integrated analysis with up to 7 years’ follow-up. Rheumatology 2020, 59, 292–302.
