Anti-SARS-CoV-2 vaccines have played a pivotal role in reducing the risk of developing severe illness from COVID-19, thus helping end the COVID-19 global public health emergency. Intriguingly, as SARS-CoV-2 variants emerged, individuals who were fully vaccinated did get infected in high numbers, and viral loads in vaccinated individuals were as high as those in the unvaccinated. However, vaccines undoubtedly offered protection against severe illness even without conferring immunity in the classical sense. The lessons learned from anti-SARS-CoV-2 vaccines are a call to the medical community to revisit and redefine the concept of vaccine effectiveness. This endeavor is likely to increase increase vaccine confidence, and thus bolster global health education efforts and preventive care.
- SARS-CoV-2
- immunity
- cytokines
- infectious diseases
1. Introduction
2. SARS-CoV-2—Killer Virus or Just a Trigger for Kitchen Sink Inflammation?
SARS-CoV-2, which causes COVID-19, is a non-segmented, single-stranded, positive-sense RNA virus from the β genus of coronaviruses [18][19][18,19]. SARS-CoV-2 infection was first identified in Wuhan, Hubei Province, China in December of 2019, and was initially characterized as virally-induced pneumonia by clinicians before it was finally isolated in bronchoalveolar lavage fluid from patients [19][20][19,20]. The clinical presentation of COVID-19 ranges from asymptomatic infection to mild respiratory symptoms to severe viral pneumonia [21]. While SARS-CoV-2 initially infects and compromises the respiratory system, it also induces multiorgan dysfunction and damage [22][23][24][22,23,24]. One of the main factors implicated in COVID-19 multiorgan failure is a massive release of proinflammatory cytokines—formally known as cytokine release syndrome (CRS) but better known by the colloquial term “cytokine storms” [25][26][25,26]. The inflammatory reaction in COVID-19 is due to the overactivation of multiple cellular subtypes in the human body [27]. Inhaled viral particles bind to epithelial cells in the nasal mucosa or travel down the nasopharyngeal tract to reach the more distal areas of the airway. The effect of the virus in eliciting an inflammatory response through different cell types is detailed below, and this gives us an idea of how the virus wreaks havoc on homeostasis in the host. Local and systemic inflammation followed by systemic disruption of homeostasis leads to multiorgan symptoms, multiorgan damage, and the high case fatality rate associated with SARS-CoV-2 infection (Figure 1).2.1. Epithelial System
The epithelial cells are the first line of defense against invading pathogens [28]. The viral particles encounter different kinds of epithelial cells as they travel from the nose and mouth, which are the most common points of entry, all the way down to the alveolar sacs—the sites of gaseous exchange (Figure 1) [29]. The nasal mucosa has different cell types, such as ciliated epithelial cells, mucous cells, and basal cells [30]. These cells express angiotensin converting enzyme-2 (ACE2) and the transmembrane protein serine protease-2 (TMPRSS2) on their plasma membranes [31][32][33][31,32,33]. ACE2, TMPRSS2, and other plasma membrane proteins aid in the docking of SARS-CoV-2 onto host cells and facilitate endocytosis of the virus [32]. Once the virus has entered host cells, the virus releases its genetic material into the cytoplasm and hijacks the host’s cellular machinery to produce more viral particles [34][35][34,35]—i.e., the cell is now infected with the virus. The release of viral particles from cells has been detected as early as one hour post-infection; however, 6–8 h post-infection is when significant viral load has been detected and infection of neighboring cells and loss of ciliated epithelium has been observed [36]. The loss of cilia on the epithelium results in reduced mucociliary clearance, which leads to increased local infection and progression of the disease [37][38][37,38]. This loss of clearance has been implicated in increased COVID-19 severity in individuals with pre-existing pulmonary inflammatory conditions like asthma [39], cystic fibrosis [40], and chronic obstructive pulmonary disease (COPD), where ciliary function is already impaired [41]. In nasal epithelial cells, there is a delayed or a muted interferon response that is observed after infection. The levels of type I (interferon alpha, beta) and type III (interferon lambda) interferons are lower in severe COVID-19 compared to mild cases [42][43][44][42,43,44]. As the virus moves down the airway, it encounters the bronchial epithelial cells, the mucus cells, and the club cells [30] (Figure 1). In the middle and lower airway, ciliated epithelial cells that become infected lose their cilia and have a denuded appearance, and the levels of proinflammatory signals in these cells correlate with the disease severity [45]. When the virus finally reaches the alveoli (the distal-most regions of the airway) where the epithelium, endothelium, and blood cells interface and enable gas exchange [46], it activates numerous signaling cascades in multiple cell types, which cause the most damage and destruction [47] (Figure 1). In alveoli, the virus primarily infects alveolar type 2 epithelial cells (a.k.a., AT2 cells or type 2 pneumocytes), potentially due to the abundance of ACE2 on their surface [48]. Infection of type 2 pneumocytes seems to be the initial step that triggers a domino of inflammatory signals [49]. It has been shown that alveolar type 1 cells can also be infected, but to a lesser extent [50]. The infection and damage of alveolar pneumocytes results in a cascade of physiological changes, beginning with an increased production of proinflammatory cytokines, such as interleukin (IL)-1 (IL-1), IL-6, IL-8, tumor necrosis factor-alpha (TNF-α), and elevated levels of C-reactive protein (CRP) and D-dimer [51][52][53][54][55][51,52,53,54,55]. These inflammatory cytokines result in recruitment of immune cells—mainly neutrophils and macrophages to loci of inflammation. The recruitment of immune cells further exacerbates the situation, as it results in the loss of barrier function of the underlying endothelial layer [56][57][56,57]. Alveolar macrophages, which are recruited to sites of damage have been shown to produce various chemokines, such as CCL2, CCL3, CCL7, CCL8, CCL13, CCL20, and cytokines, such as CXCL1, CXCL3, and CXCL10 [58][59][58,59]. Among these chemokines, CCL2 and CCL3 attract more monocytes and macrophages to the alveoli and induce CXCR1 gene expression in them; this promotes the production of tissue-damaging, proinflammatory reactive oxygen species [60]. The sum effect is largescale destruction of epithelial tissue, which promotes the recruitment of neutrophils that try to resolve the situation by forming neutrophil extracellular traps (NET) [61]. The heightened cellular signaling and subsequent tissue damage described above leads to alveolar flooding with the interstitial fluid and the blood, which leads to progressive hypoxia. Thus, the replication of the virus in the alveoli results in the progression towards acute respiratory distress syndrome (ARDS), triggering an imbalance between pro-coagulation and anticoagulation (i.e., pro-fibrinolysis) pathways, plus stimulating complement activation, damage to hyaline membranes, and the formation of clots in the small and large blood vessels [62][63][62,63].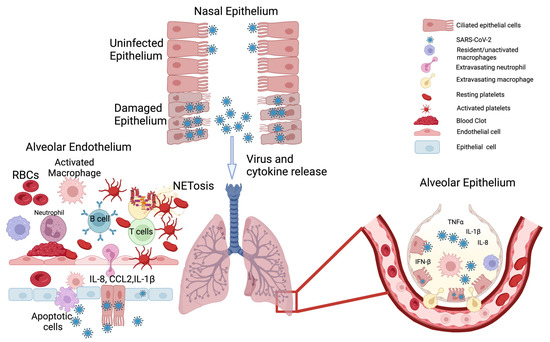
2.2. Endothelial System
The endothelial cells line the innermost layers of the blood vessels (adjacent to the lumen) and tightly regulate the transport of nutrients, gases, metabolic wastes, bioactive molecules, and cells [64]. The endothelial cells lining the smaller capillaries express ACE2 and TMPRSS2, which as mentioned earlier are necessary for the host cell binding and internalization of SARS-CoV-2 [65]. The virus, on entering the endothelial cells, replicates and then disseminates through the blood stream to organs other than the lungs. The endothelial cells respond to the plethora of cytokines/chemokines that are produced by the epithelial cells upon infection. The predominant chemical signals that affect endothelial barrier function are IL-6 and TNF-α. Activation of the endothelial cells by IL-6 or TNF-α results in the production of IL-8 (a major chemoattractant for neutrophils) and monocyte chemoattractant protein (MCP-1) and in the activation of the C5a complement [66]. Indeed, plasma IL-6 levels in patients with COVID-19 correlate with the disease severity [67]. The recruitment of overly activated immune cells, a hallmark of COVID-19, can result in endothelial cell death, which promotes vascular leak and initiation of microfoci for clot formation [68][69][68,69]. Activated endothelial cells can release large amounts of von Willebrand factor (VWF) and factor VIII, which play an active role in clot formation [70]. In addition to stimulating the formation of fibrin, endothelial cells also secrete plasminogen activator inhibitor-1 (PAI-1), which inhibits clot dissolution [71]. During the initial stage of infection, the loss of contact with the surrounding cells results in the formation of circulating endothelial cells (CECs), which can travel from one tissue to another. During the course of infection, there is a loss of endothelial barrier integrity, which results in fluid accumulation in the alveolar and pleural spaces. This is a result of the upregulation of interleukin 2 receptor (IL-2R) on the endothelial cells and the increased release of IL-2 from the activated T cells [72]. Thus, direct viral infection or indirect activation of endothelial cells causes a loss of barrier function, which then results in fluid accumulation, increased extravasation of immune cells, widespread microthrombosis, diffuse fibrin deposits, and secretion of PAI-1 that prevents clot dissolution—all working together to increase the likelihood of thromboembolisms [73][74][73,74] (Figure 1).2.3. The Mismatch between Viral Load and Symptom Severity
We attempted to answer the question as to whether SARS-CoV-2 is a killer virus or just a trigger for “kitchen sink inflammation”. The related expressions “to throw everything but the kitchen sink” or “to throw the kitchen sink” imply doing everything possible to address a problem, regardless of whether the solutions are likely to be good or bad [75][76][75,76]. Due to the novelty of SARS-CoV-2 to the human immune system, when people were first infected, the immune response was indeed comparable to throwing an inflammatory kitchen sink at the viral invader, resulting in cell-signaling storms [25][26][47][55][77][78][79][80][81][82][83][25,26,47,55,77,78,79,80,81,82,83]. The fact that some individuals with high viral loads presented with minimal symptoms, and some individuals with severe symptoms had low viral loads suggest that, SARS-CoV-2 by itself is not a killer virus, but rather, it is a nonspecific and overly aggressive inflammatory response, which causes the most harm [84][85][86][87][88][84,85,86,87,88]. This is not to diminish the fact that SARS-CoV-2 infection has been implicated in the deaths of nearly seven million people, chronic illness in 10–20% of the survivors, and about 800 million cumulative cases in just over three years [89][90][89,90]. However, the available data point towards severe illness and deaths being linked to an inflammatory response, which overwhelms the host, rather than widespread virus-mediated killing of cells in the respiratory system and other vital systems of the body [88]. Interestingly, an overly aggressive immune response, rather than the pathogen itself, might underlie severe illness in various infectious diseases [91]. Therefore, public health agencies must invest resources in finding safe, effective, practical, and standardized therapies, which can work to combat a wide range of existing and novel pathogens. In hindsight, one of the simplest public health strategies to blunt the impact of SARS-CoV-2 would have been to assume that it would behave just like its predecessor SARS-CoV (implicated in the SARS outbreak of the early 2000s) and amplify the importance of commonsense preventative healthcare measures (e.g., hand hygiene, mask wearing, and avoiding unnecessary social activities) until safe and effective pharmacological measures were available [92][93][94][92,93,94]. The recognition of “self” from “non-self” is a fundamental aspect of immunity, and this function serves an important role in protecting the body from disease-causing elements [95][109]. Vaccination takes advantage of this by introducing a harmless version of the disease-causing pathogen, allowing the body to recognize and remember it. There are two phases of immunity that are activated upon infection or inoculation through vaccination: Innate immunity and adaptive immunity [96][97][98][110,111,112] (Figure 2).