This project focuses on utilizing mathematical Markov chain modeling as a stochastic process to analyze the stages of Acute Respiratory Distress Syndrome (ARDS). ARDS, characterized by a spectrum of severity ranging from floors to death, presents a complex clinical challenge. By employing Markov chain modeling, we aim to provide a structured framework for understanding the dynamic progression of ARDS.
Our approach involves constructing a Markov chain that represents the transition of patients through various stages of ARDS, including floors, mild, moderate, severe, and ultimately death. Each stage is associated with specific clinical characteristics and outcomes, forming the basis of our modeling framework.
In addition to describing the natural progression of ARDS, our project involves reviewing current clinical guidelines for managing the condition. We propose to examine the impact of each guideline on patient outcomes and the transition through different ARDS stages. By systematically analyzing the effects of various interventions and treatment strategies, we aim to provide insights into optimizing patient care and improving outcomes in ARDS management.
Ultimately, this project serves as a comprehensive exploration of ARDS progression, providing healthcare professionals with a valuable framework for thinking about the condition. By integrating mathematical modeling with clinical guidelines, we seek to enhance our understanding of ARDS and contribute to more effective treatment approaches tailored to individual patient needs.
- Critical Care
- Markov Chain
- Acute Respiratory Distress Syndrome
1. Modeling Acute Respiratory Distress Syndrome (ARDS) with a stochastic modeling process that utilizes transition diagrams and first step analyses
In terms of ARDS this means
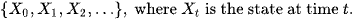
Where ARDS is defined via stages as below
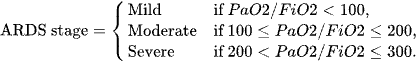
The Markov Chains have a crucial property
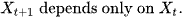
1.1. Questions of Interest
- Starting from Mild Acute Respiratory Distress Syndrome, what is the probability of decompensating to moderate or severe or death, what is the probability of downgrading to the floors
- Starting from Moderate Acute Respiratory Distress Syndrome, what is the probability of decompensating to severe or death, what is the probability of downgrading to the floors or improving to mild ARDS
- Starting from Severe Acute Respiratory Distress Syndrome, what is the probability of decompensating to death, what is the probability of downgrading to the floors or improving to mild or moderate ARDS
- For patients on the floor, what is the probability of decompensating to mild, moderate, or severe ARDS or death
- For patients that are dead it is a closed loop
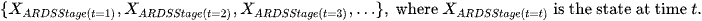
Definition: The state of a Markov chain at time T is the value of
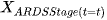
if
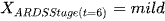
we say that at time 6 they are in Mild ARDS
Definition: The state space of a Markov Chain S, is the set of values that each
can take. For example,
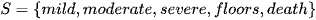
Let S have size N = 5
Definition: A trajectory of a Markov chain is a particular set of values for
For example if in our ARDS case
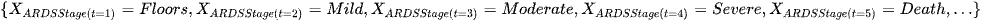
Then the trajectory up to time t = 5 is Floors -> Mild -> Moderate -> Severe -> Death
Trajectory just means Path Markov Property
The basic property of a Markov Chain is that the only the most recent point in the trajectory affects that happens next.

P(Xt+1) depends on P(Xt) but does not depend on Xt-1, ...X2, X1, X0.
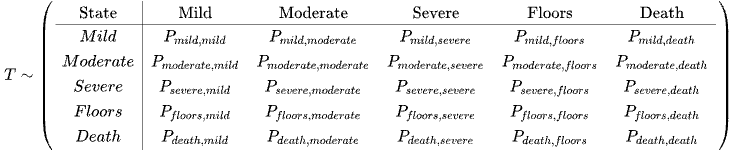
The Transition Matrix
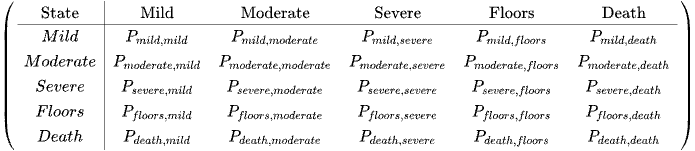
The row represents Now from or from X(t)
The columns represent Next or To (Xt+1)
Entry (i,j) is the conditional probability that next = j given that now = i: the probability of going from state i to state j
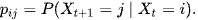
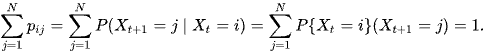
The transition matrix P list all possible states in the state space S.
P is a square matrix (NxN), because Xt+1 and Xt both take values in the same state space S (of size N) The rows of P each sum to 1
Let's define the distribution of X at a certain time
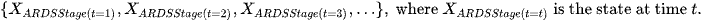
Where the state space is as below:
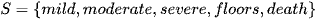
What we need to determine the probability distribution of each time point.
Let's say X0, this will be the chance that in individual starts off from time 0 at each end point from being admitted from the emergency department. Pi is an Nx1 vector denoting the probability distribution of X0
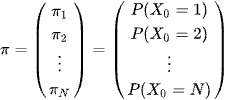
We will write
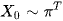
to denote the row vector of probabilities is given by the row vector pi row transposition
How do we calculate the probability of the current state? at the current time? using the partition rule below
1.2. Clinical Correlate
Let's say we are on our second timepoint t = 1 let's say moderate ARDS.
What is the probability they had the specific trajectory mild - > moderate ARDS.
Now we want to describe the probability distribution after time 0.
How do we get the states at time T?
Trajectory Probability Can characterize the trajectory of ARDS by probabilities.
- For example Transition of the trajectory from Severe -> Moderate -> Mild -> Floors
- Of great interest will be the probability of regressing from ARDS within one day or 7 days
- Do most patients deescalate in a predictable pattern? or is this more of a random disease where there will be a uniform distribution of trajectories?
Recall a trajectory is the sequence of values.
Because of the Markov Property (Memoryless) we can find the probability of any trajectory by multiplying together the starting proability and all subsequent single step properties
Let's envision a 7 day course (trajectory) of ARDS.
What is the probability of Mild -> Severe -> Moderate -> Mild -> Floors -> Severe -> Death?
Where the starting probability for Mild is as follows
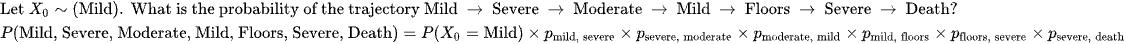
This is theoretical.
A Markov Chain maps the changes of the states in the aggregate population.
2. Review of Trials in Acute Respiratory Distress Syndrome (ARDS)
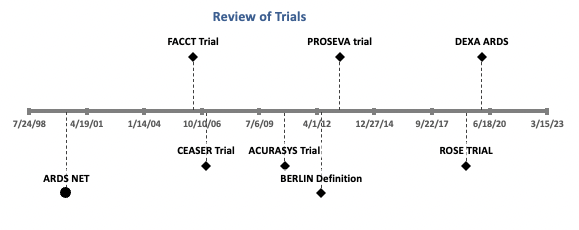
3. ARDS ARDS NET[1]
Summary of the ARDSNet ARMA Trial
The ARDSNet ARMA trial, conducted in 2000, was a landmark study in the field of mechanical ventilation for Acute Respiratory Distress Syndrome (ARDS). The trial aimed to evaluate the benefits of a ventilation strategy with lower tidal volumes compared to conventional higher tidal volumes.
Key findings of the ARMA trial include:
-
Reduction in Mortality: The trial demonstrated a significant 11% reduction in mortality with the use of low tidal volumes (4 to 8 mL/kg of predicted body weight) compared to higher tidal volumes conventionally used at the time.
-
Prevention of Ventilator-Induced Lung Injury: The trial aimed to prevent ventilator-induced lung injury, particularly barotrauma, by limiting plateau pressures. The low tidal volume arm adhered to a plateau pressure limit of ≤30 cm H₂O.
-
Benefits of Positive End-Expiratory Pressure (PEEP): The trial affirmed the utility of PEEP in ARDS management. PEEP was found to prevent lung injury associated with repeated opening and closing of distal airways and alveoli, improve homogeneity of the lung parenchyma, and enhance ventilation-perfusion matching.
Despite the clear benefits demonstrated by the ARMA trial, adherence to low tidal volume ventilation remains poor in clinical practice. While guidelines recommend tidal volumes of 6 mL/kg of predicted body weight and maintenance of the lowest possible plateau pressure, achieving these targets can be challenging.
Overall, the ARMA trial highlighted the importance of lung protective ventilation strategies, including the use of low tidal volumes and appropriate PEEP levels, in improving outcomes for patients with ARDS. Continued efforts to promote adherence to these strategies are essential to optimize patient care and reduce mortality in ARDS.
4. FACTT Trial
FACTT Trial[2]
In patients with Acute Lung Injury (ALI) or Acute Respiratory Distress Syndrome (ARDS) who are intubated and receiving positive pressure ventilation, the 2006 Fluids and Catheters Treatment Trial (FACTT) compared two fluid management strategies: liberal and conservative.
The liberal strategy targeted a higher central venous pressure (CVP) range of 10-14 mmHg, while the conservative strategy aimed for a lower CVP of less than 4 mmHg.
Key findings of the FACTT trial include:
-
Mortality: At 60 days, there was no significant difference in mortality between the two groups. Both the liberal and conservative fluid management strategies resulted in similar mortality rates.
-
Ventilator Days: The conservative fluid management strategy was associated with a decrease in the number of days on mechanical ventilation compared to the liberal strategy. Patients in the conservative group spent fewer days requiring mechanical ventilation.
-
ICU Stay: Similarly, patients managed with the conservative fluid strategy had a reduced duration of stay in the intensive care unit (ICU) compared to those in the liberal fluid management group.
Overall, while mortality outcomes did not differ significantly between the two groups, the FACTT trial demonstrated that a conservative fluid management strategy in ALI/ARDS patients receiving positive pressure ventilation led to clinical benefits such as decreased ventilator days and reduced time spent in the ICU. These findings suggest that minimizing fluid administration and targeting lower CVP levels may contribute to improved outcomes and more efficient use of healthcare resources in this patient population.
5. CESAR TrialCESAR Trial[3]
he CESAR trial, conducted in the UK from July 2001 to August 2006, involved 180 patients with severe but reversible respiratory failure. The trial aimed to assess whether Extracorporeal Membrane Oxygenation (ECMO) treatment could improve 6-month survivability without severe disability compared to conventional ventilator management.
Patients were randomly assigned to two groups: ECMO (n=90) and Conventional Management (n=90), and were transferred to either a conventional treatment center or the ECMO center upon inclusion.
Primary Outcome:
- Survival without severe disability at 6 months: 63% in the ECMO group vs. 47% in the conventional management group (Relative Risk [RR] 0.69; 95% Confidence Interval [CI] 0.5-0.97; P=0.03).
Secondary Outcomes:
- Duration of stay in intensive care: 24 days in the ECMO group vs. 13 days in the conventional management group.
- Duration of stay in the hospital: 35 days in the ECMO group vs. 17 days in the conventional management group.
Adverse Events:
- One patient died during transfer to the ECMO center due to mechanical failure of the ambulance oxygen supply.
- Another patient experienced vessel perforation during ECMO cannulation, contributing to their eventual demise.
The CESAR trial concluded that ECMO treatment significantly increased 6-month survivability without severe disability compared to conventional ventilator management. However, it was associated with longer durations of stay in both intensive care and the hospital. The trial highlighted the importance of careful patient selection and management in ECMO therapy. Further research may be necessary to assess the cost-effectiveness of ECMO compared to conventional ventilator support.
6. ACURASYS Trial
ACURASYS Trial[4]
The ACURASYS trial conducted in 2010 demonstrated that the administration of the neuromuscular blocking agent (NMBA) cisatracurium within 48 hours of mechanical ventilation in patients with a PaO2:FiO2 ratio <150 significantly reduced 90-day mortality compared to placebo (31.6% vs. 40.7%). Additionally, it increased the number of ventilator-free days.
The benefits of cisatracurium in the ACURASYS trial were attributed to its ability to minimize ventilator-induced lung injury by preventing dyssynchrony. Other potential benefits included anti-inflammatory effects and decreased oxygen requirements due to muscle paralysis. However, NMBA use requires heavy sedation, which may lead to adverse effects.
Overall, the trial concluded that paralysis with cisatracurium for 48 hours in patients with early severe ARDS improved 90-day survival and increased ventilator-free days.
7. BERLIN DefinitionBERLIN Definition[5]
The 2012 Berlin Criteria for Acute Respiratory Distress Syndrome (ARDS) provide a standardized framework for diagnosing and categorizing the severity of this condition. The criteria consist of three main components: required criteria, risk factor assessment, and severity assessment.
Required Criteria:
Timing: ARDS onset within 1 week of a known clinical insult or the development of new/worsening respiratory symptoms.
Chest X-ray Findings: Bilateral opacities on chest X-ray not fully explained by effusions, lobar/lung collapse, or nodules.
Respiratory Failure: Respiratory failure not fully explained by cardiac failure or fluid overload.
Risk Factor Assessment:
Either a known risk factor for ARDS (e.g., pneumonia, trauma, sepsis, pancreatitis) is present, or objective assessment (e.g., echocardiography) excludes hydrostatic edema.
Severity Assessment:
Mild ARDS: PaO₂/FiO₂ ratio >200 to ≤300 mmHg with Positive End-Expiratory Pressure (PEEP) or Continuous Positive Airway Pressure (CPAP) ≥5 cm H₂O.
Moderate ARDS: PaO₂/FiO₂ ratio >100 to ≤200 mmHg with PEEP ≥5 cm H₂O.
Severe ARDS: PaO₂/FiO₂ ratio ≤100 mmHg with PEEP ≥5 cm H₂O.
These criteria allow clinicians to systematically evaluate patients for ARDS, ensuring early recognition and appropriate management. By categorizing ARDS severity based on oxygenation status, the criteria aid in prognostication and guide therapeutic interventions
8. PROSEVA TrialPROSEVA Trial[6]
The 2013 PROSEVA trial demonstrated that prone positioning, compared to supine positioning, within 36 hours of mechanical ventilation in patients with a PaO2:FiO2 ratio <150 mm Hg, significantly reduced both 28-day (16.0% vs. 32.8%) and 90-day mortality.
Prone positioning involves safely moving the patient to alleviate lung compression behind cardiac and mediastinal structures, thereby improving ventilation-perfusion (VQ) matching in ARDS. The trial emphasized the importance of an experienced care team to prevent complications such as pressure ulcers and accidental extubation.
Early prone positioning for at least 12 hours a day should be considered in severe ARDS cases with a PaO2:FiO2 ratio <150. While the use of ECMO has increased, the efficacy of prone positioning appears to be more strongly supported by data, especially in patients with refractory hypoxemia.
Overall, the PROSEVA trial findings suggest that prone positioning can effectively reduce mortality in patients with severe ARDS, making it a valuable intervention in the management of this condition.
9. ROSE TrialROSE Trial[7]
The ROSE trial, conducted in 2019, aimed to investigate the efficacy of early continuous neuromuscular blockade with cisatracurium in patients with moderate to severe ARDS when used alongside current light sedation protocols. The trial challenged the findings of the ACURASYS trial, which suggested a mortality benefit with neuromuscular blockade.
However, the ROSE trial found no significant difference in 90-day mortality between patients treated with cisatracurium and deep sedation versus those treated with light sedation without neuromuscular blockade. These results have been controversial due to limited inclusion criteria and potential confounders, such as differences in sedation levels.
One proposed explanation for the disparity in mortality outcomes between the two trials is "reverse triggering," wherein deeply sedated patients may experience additional gas delivery and overinflation after a mechanically assisted breath, potentially disadvantaging the control group in the ACURASYS trial.
Additionally, patients in the cisatracurium group in the ROSE trial experienced serious cardiovascular events, further raising concerns about the use of neuromuscular blockade in this context. Overall, the trial suggests that early neuromuscular blockade does not improve mortality and may be associated with adverse outcomes, highlighting the need for cautious consideration of its use in ARDS management.
10. ARDSDEXA ARDS[8]
The DEXA-ARDS trial, conducted in 2020, investigated the efficacy of dexamethasone therapy in adult, mechanically ventilated patients with moderate to severe ARDS. The clinical question addressed whether the addition of dexamethasone, administered at a dose of 20mg daily for 5 days followed by 10mg daily for an additional 5 days, improves ventilator-free days at 28 days.
The bottom line of the trial indicates that the addition of dexamethasone therapy initiated within 24 hours of meeting ARDS criteria resulted in several favorable outcomes. These include an increase in ventilator-free days, a decrease in mortality rates, and a shorter duration of mechanical ventilation. Importantly, these benefits were achieved without significant increases in adverse events.
Specifically, the DEXA-ARDS trial demonstrated a 60-day mortality benefit in patients receiving dexamethasone compared to controls (21% vs. 36%). Furthermore, patients in the dexamethasone group experienced more ventilator-free days, indicating a reduced need for mechanical ventilation.
Overall, the findings of the DEXA-ARDS trial suggest that early initiation of dexamethasone therapy in patients with moderate to severe ARDS can lead to improved clinical outcomes and may represent a promising treatment strategy in ARDS management.
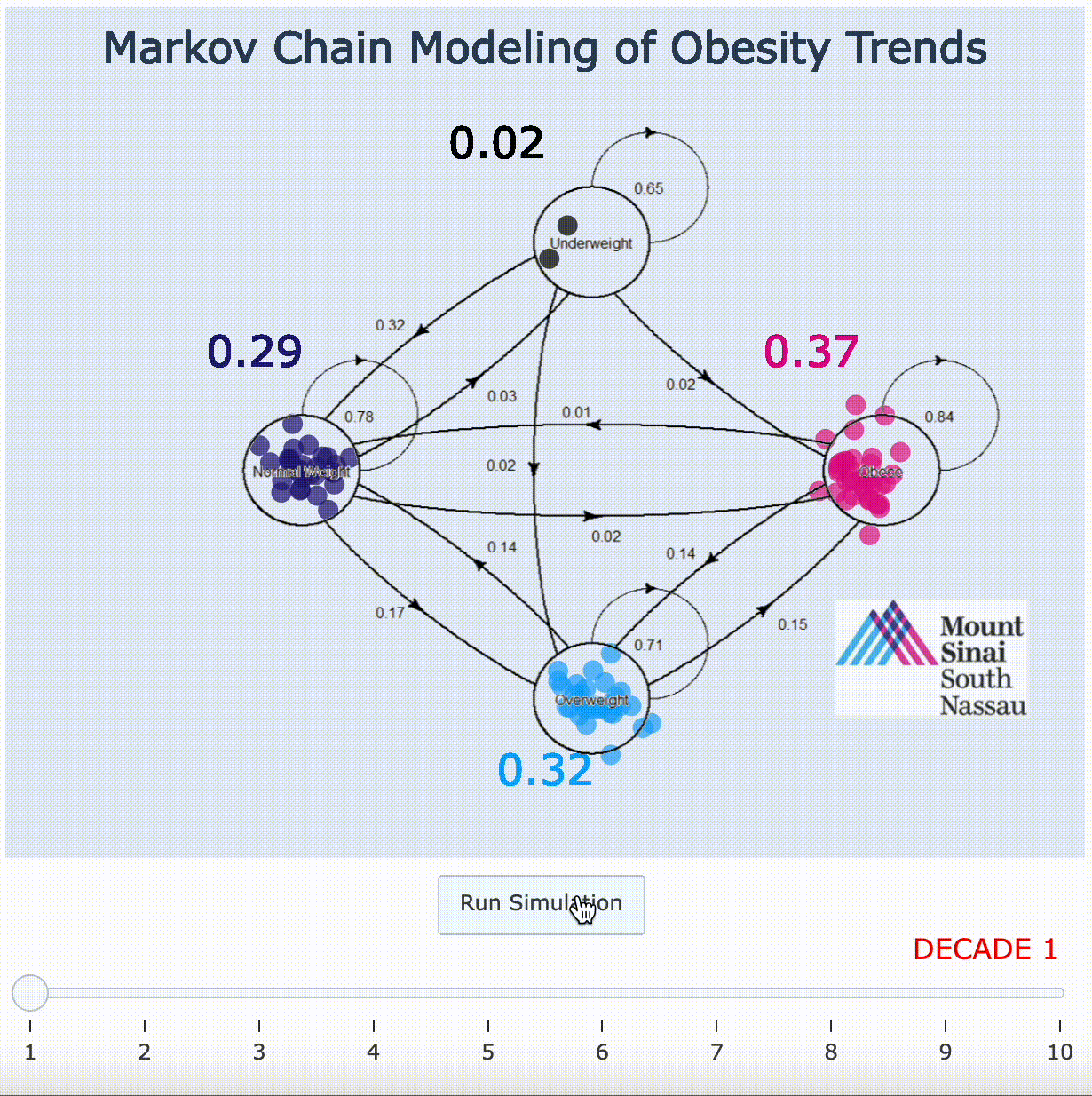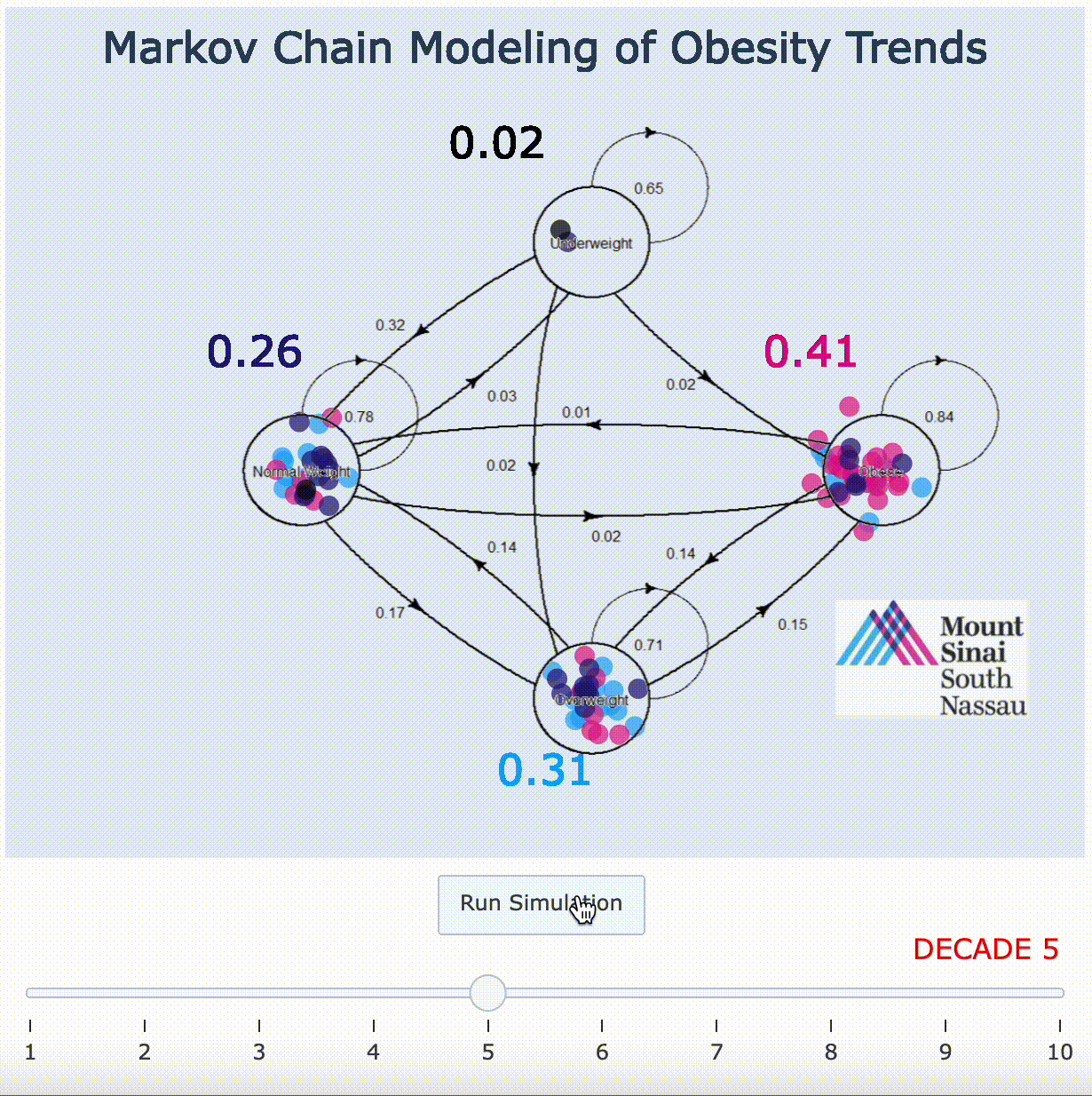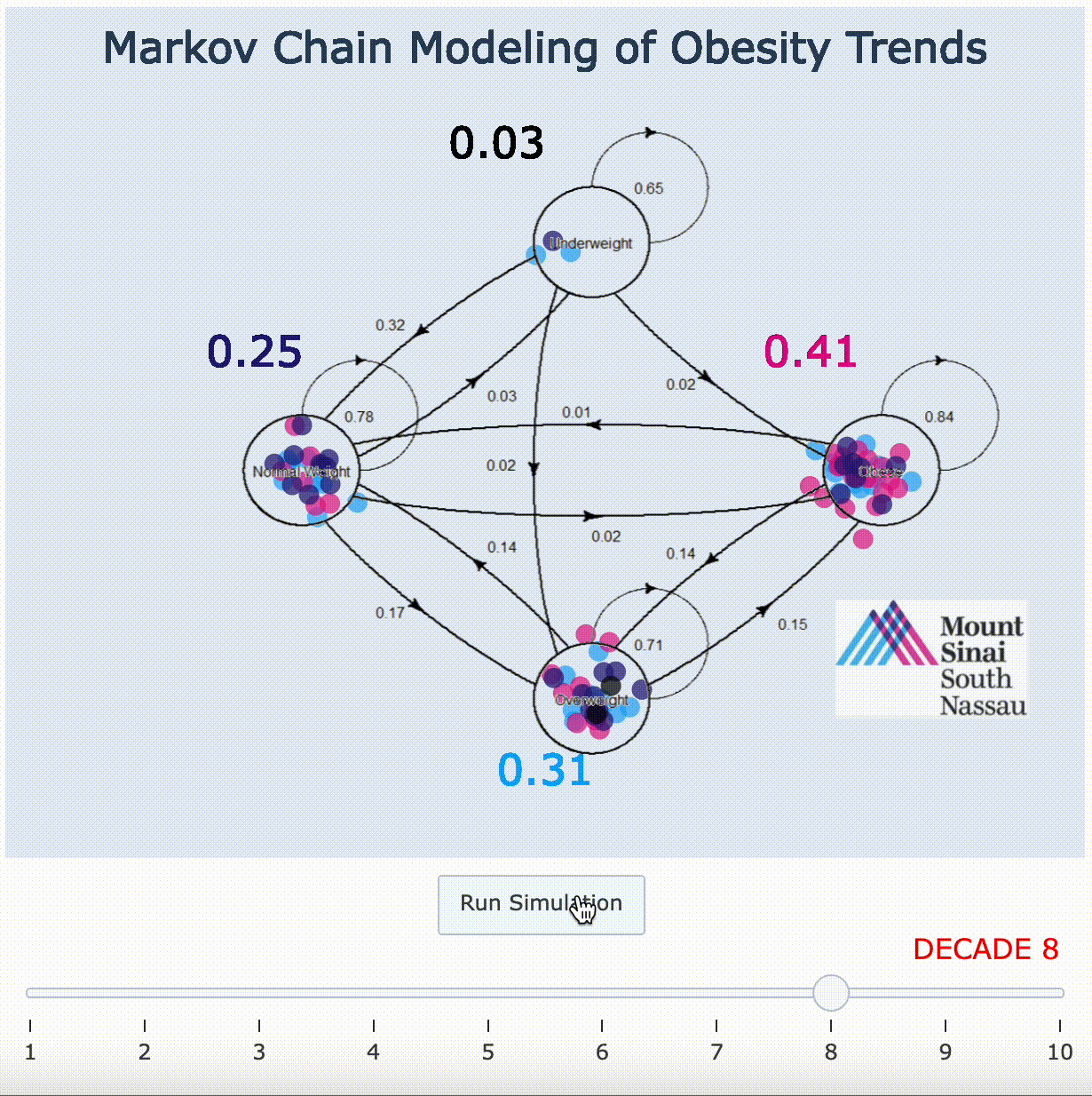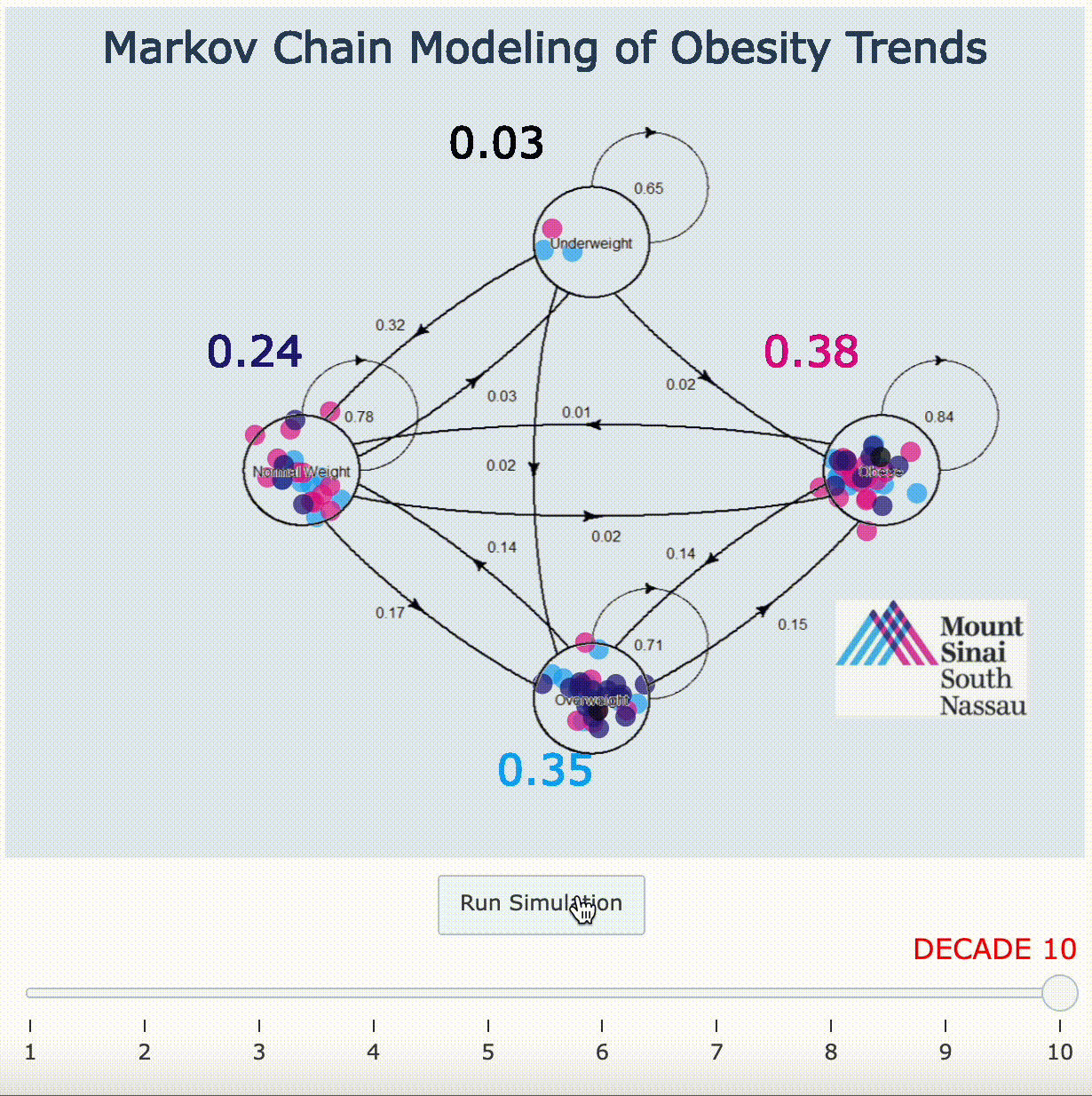
11. Markov Chain Adaptation From LANDMARK Obesity Papers[9][10]

References
- Acute Respiratory Distress Syndrome Network; Roy G Brower; Michael A Matthay; Alan Morris; David Schoenfeld; B Taylor Thompson; Arthur Wheeler; Ventilation with Lower Tidal Volumes as Compared with Traditional Tidal Volumes for Acute Lung Injury and the Acute Respiratory Distress Syndrome. New Engl. J. Med.. 2000, 342, 1301-1308.
- The National Heart; Lung; Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Comparison of Two Fluid-Management Strategies in Acute Lung Injury. New Engl. J. Med.. 2006, 354, 2564-2575.
- Giles J Peek; Miranda Mugford; Ravindranath Tiruvoipati; Andrew Wilson; Elizabeth Allen; Mariamma M Thalanany; Clare L Hibbert; Ann Truesdale; Felicity Clemens; Nicola Cooper; Richard K Firmin; Diana Elbourne; Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009, 374, 1351-1363.
- Laurent Papazian; Jean-Marie Forel; Arnaud Gacouin; Christine Penot-Ragon; Gilles Perrin; Anderson Loundou; Samir Jaber; Jean-Michel Arnal; Didier Perez; Jean-Marie Seghboyan; Jean-Michel Constantin; Pierre Courant; Jean-Yves Lefrant; Claude Guérin; Gwenaël Prat; Sophie Morange; Antoine Roch; Neuromuscular Blockers in Early Acute Respiratory Distress Syndrome. New Engl. J. Med.. 2010, 363, 1107-1116.
- Ards Definition Task Force; V M Ranieri; Gordon D Rubenfeld; B T Thompson; Niall D Ferguson; Ellen Caldwell; Eddy Fan; Luigi Camporota; Arthur S Slutsky; Acute Respiratory Distress Syndrome. JAMA. 2012, 307, 2526-2533.
- Claude Guérin; Jean Reignier; Jean-Christophe Richard; Pascal Beuret; Arnaud Gacouin; Thierry Boulain; Emmanuelle Mercier; Michel Badet; Alain Mercat; Olivier Baudin; Marc Clavel; Delphine Chatellier; Samir Jaber; Sylvène Rosselli; Jordi Mancebo; Michel Sirodot; Gilles Hilbert; Christian Bengler; Jack Richecoeur; Marc Gainnier; Frédérique Bayle; Gael Bourdin; Véronique Leray; Raphaele Girard; Loredana Baboi; Louis Ayzac; Prone Positioning in Severe Acute Respiratory Distress Syndrome. New Engl. J. Med.. 2013, 368, 2159-2168.
- The National Heart; Lung; Blood Institute PETAL Clinical Trials Network; Early Neuromuscular Blockade in the Acute Respiratory Distress Syndrome. New Engl. J. Med.. 2019, 380, 1997-2008.
- Jesús Villar; Carlos Ferrando; Domingo Martínez; Alfonso Ambrós; Tomás Muñoz; Juan A Soler; Gerardo Aguilar; Francisco Alba; Elena González-Higueras; Luís A Conesa; Carmen Martín-Rodríguez; Pablo Serna-Grande; Rosana Rivas; José Ferreres; Javier Belda; Lucía Capilla; Alec Tallet; Julián Álvarez; José M. Añón; María J. Asensio; Jesús Blanco; Marisa Blasco; Lucia Cachafeiro; Rafael del Campo; José A. Carbonell; Nieves Carbonell; Agustín Cariñena; Demetrio Carriedo; Mario Chico; Luís A. Conesa; Ruth Corpas; Javier Cuervo; Francisco J. Díaz-Domínguez; Cristina Domínguez-Antelo; Lorena Fernández; Rosa L. Fernández; Eneritz Gamboa; Raúl I. González-Luengo; Jesús M. González-Martín; Ramón Ortiz Díaz-Miguel; Raquel Pérez-González; Ana M. Prieto; Isidro Prieto; Leticia Rojas-Viguera; Miguel A. Romera; Jesús Sánchez-Ballesteros; José M. Segura; Ainhoa Serrano; Rosario Solano; Juan A. Soler; Marina Soro; Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir. Med.. 2020, 8, 267-276.
- Alexander A. Huang; Samuel Y. Huang; Stochastic modeling of obesity status in United States adults using Markov Chains: A nationally representative analysis of population health data from 2017–2020. Obes. Sci. Pr.. 2023, 9, 653-660.
- Alexander A. Huang; Samuel Y. Huang; Covariate dependent Markov chains constructed with gradient boost modeling can effectively generate long-term predictions of obesity trends. BMC Res. Notes. 2023, 16, 1-8.
