Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Lian-Xin Peng and Version 2 by Jason Zhu.
Plant-derived exosome-like nanovesicles (PELNs) are bilayer membrane-enclosed nanovesicles secreted by plant cells, serving as carriers of various substances such as proteins, RNA, and metabolites. The mounting evidence suggests that PELN plays a crucial role in transmembrane signaling, nutrient transportation, apoptosis, and regulation of gut microbiota composition. This makes it a promising “dark nutrient” for plants to modulate human physiology and pathogenesis.
- plant-derived exosomal nanovesicles
- structural composition
- functional features
1. Absorption and Distribution of PELN in the Body
Plants serve as a vital source of food and medicine for humans, and their exosomes can enter the body through various pathways. Aimaletdinov et al. [1][92] conducted a systematic analysis of EV distribution in animals with different dietary patterns. The mode of administration and the composition of extracellular vesicles (EVs) are critical determinants for their biodistribution. Similar observations have been reported for plant-derived EVs, where ginseng-derived vesicles were found to accumulate primarily in the liver and spleen following intravenous or intraperitoneal injection, while oral administration resulted in preferential distribution within the gastrogut tract [2][66]. This may be attributed to direct transport via systemic circulation with injections versus recirculation through digestive barriers with oral delivery. The oral route is the most commonly used administration method, and there are significant variations in the distribution of PELN within the body following oral administration from different sources. For instance, Berger et al. [3][64] reported that PELN derived from orange juice is exclusively present in the intestine, while Zhuang et al. [4][80] observed that a significant proportion of ginger-derived PELN accumulates in the liver and exhibits a prolonged half-life of over 12 h. These examples illustrate that the distribution of PELN is influenced by its structural composition [5][14], size, charge [6][93] and other factors, as well as its ability to traverse gut barriers. However, there is still a dearth of systematic research on the in vivo distribution behavior and mechanism of PELN, and the relevant reports on human vesicles can serve as a valuable reference [7][51]. Furthermore, nasal administration of oat-derived PELN (OELN) has demonstrated its ability to traverse the blood–brain barrier and be absorbed by microglia through the β-glucan-HPAC hippocampal calcin pathway, thereby exerting biofunctional activity [8][7]. Additionally, garlic-derived PELN appears to be assimilated by brain microglia, suggesting that apart from crossing the gut barrier successfully, PELN may also effectively penetrate the blood–brain barrier [9][76].
2. Reconstructing Gut Microbiota
Gut microbiota are intricately associated with the body’s immune response, nutritional uptake, metabolic processes, and other physiological functions. They play a pivotal role in regulating energy metabolism, maintaining gut barrier function, and activating mucosal immunity [10][94]. Targeting gut microbiota through dietary interventions represents a potent approach to achieving personalized nutrition. While research has predominantly focused on exploiting plant exosomes as delivery systems for targeted drug development to cells, there is a dearth of comprehensive investigation into the delivery and subsequent response of gut microbiota [11][95]. The targeted delivery capabilities of PELN have the potential to precisely regulate the composition of gut microbiota. The study revealed that PELN was capable of influencing the abundance of Bacteroides and Lactobacillus in the gut microbiota. The study conducted by Teng et al. [5][14] revealed, based on 16sRNA analysis, that ginger-derived ELN is able to be absorbed by the gut microbiota, and ginger-derived GELN has the potential to enhance the abundance of Lactobacillus and Bacteroides while reducing the prevalence of Clostridium. More reliably, their clinical data also support such a conclusion. It is well known that PELN carries miRNAs that regulate gene expression, and they screened out a common probiotic, Lactobacillus rhamnosus (LGG), and through further bioinformatics analysis, the mdo-miR-7267-3P carried by PELN increased tryptophan-I3A of LGG by promoting the expression of single oxygenase ycnE, and I3A increased the expression of the anti-inflammatory factor IL-22 through the AHR pathway. In addition, they also found that the ath-miR167a-5p carried by PELN downregulated the expression of the LGG fimbriae gene spac, thereby preventing the accumulation of LGG, further elucidating the potential role of GELN-LGG in inflammatory bowel disease. Another study of lemon-derived nanovesicles (LELN) also found that LELN promoted the growth of Lactobacillus enterotica [12][75].
Liu et al. [13][57] found through bioinformatics tools that some miRNAs in PELN derived from Tartary buckwheat can target functional genes in the physiological processes related to Escherichia coli and Lactobacillus rhamnosus, thereby significantly promoting the growth of Escherichia coli and Lactobacillus rhamnosus, and by co-fermenting Tartary buckwheat exosomes with fecal microorganisms, it was found that Tartary buckwheat exosomes can change the composition of gut microbiota and promote the production of short-chain fatty acids. Currently, the understanding of the interaction between PELN and gut microbiota is limited. Further research is needed to investigate how PELN is selectively absorbed by gut microbiota and delivers active molecules to specific microorganisms in order to intervene in the mechanism of action of gut microbiota. The use of bioinformatics tools can help narrow down the scope of research, which will facilitate a better understanding of the mechanism behind PELN’s actions on gut microbiota.
3. Ameliorating Inflammation
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder of the gastrogut tract, encompassing ulcerative colitis (UC), Crohn’s disease (CD), and undetermined colitis (IC). As the primary point of entry for dietary components into the body, Peyer’s patches in gut-associated lymphoid tissue play a crucial role in mitigating gut inflammation through diverse mechanisms (Figure 12B). Ju et al. [14][9] administered grape-derived nanovesicles (GELN) via gavage and observed that GELN facilitated the self-renewal of gut stem cells through the Wnt/β-catenin pathway, which is known to suppress inflammatory bowel disease. Specifically, Lgr5-EGFPhi gut stem cells internalize GELN via pinocytosis, facilitating the entry of GELN into the cell and subsequent nuclear accumulation of β-catenin, thereby initiating activation of the Wnt signaling pathway. This subsequently triggers the Tcf4 transcriptional mechanism, resulting in the upregulation of a cascade of growth-promoting genes such as Sox2, Nanog, OCT4, KLF4, c-Myc and EGFR. Zhang et al. [15][6] demonstrated that GELNs exhibited a preferential affinity for the mouse colon and effectively stimulated the proliferation of gut epithelial cells through oral administration of GELN. The administration of GELN was found to result in reductions in apolipoprotein (Lcn-2) levels in mice with DSS-induced IBD, accompanied by a decrease in spleen weight and an increase in colon length. Further protein analysis revealed a significant reduction in colonic myeloperoxidase (MPO), an indicator of neutrophil infiltration, while E-cadherin, which plays a crucial role in epithelial cell–cell adhesion and tissue structure maintenance, exhibited a significant increase. The authors’ conclusion was further supported by the observed changes in inflammatory factors, which provided additional evidence for the beneficial effects of GELN on IBD. Specifically, GELN was found to enhance the proliferation of gut epithelial cells and mitigate detrimental factors. In a study conducted by Deng et al. [16][10], it was discovered that nanovesicles derived from broccoli are involved in the upregulation of AMPK protease expression, resulting in reduced phosphorylation due to downregulation of rapamycin target (MTOR)/S6 kinase (S6k). This ultimately leads to increased tolerance of gut cells towards dendritic cells (DCs). By characterizing inflammatory factors such as IFN-g, interleukin, and TNF-α, they observed that enhanced tolerance towards dendritic cells effectively suppresses colitis.
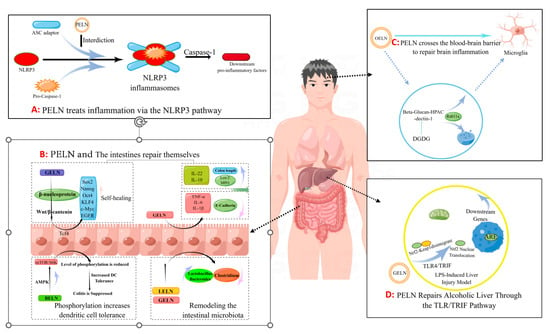
Figure 12.
The pathway by which PELN regulates inflammation (by Figdraw).
Additionally, Zhuang et al. [4][80] discovered that 6-gingerol in GELN induces Nrf2 nuclear translocation through modulation of the TLR4/TRIF pathway, leading to dissociation from Keap1 and subsequent translocation into the nucleus. This results in the formation of a heterodimer with Maf and further activation of ARE-mediated downstream gene expression, ultimately promoting the repair process for alcoholic liver injury (Figure 12D). The GELN compound has demonstrated the ability to specifically target NLRP3 inflammasomes, leading to a significant improvement in inflammation [17][73]. Similar findings have been reported in the HELN study on honey sources. Although HELN does not affect the levels of sensory protein NLRP3, adaptor protein ASC, and enzyme procaspase-1, it appears to inhibit the oligomerization of ASC adaptor protein, thereby preventing the formation of the NLRP3 inflammasome platform [18][8]. Liu et al. [19][78] identified a similar mechanism in shiitake mushrooms, while De Robertis et al. [20][79] utilized high-throughput sequencing of blueberry-derived nanovesicles combined with bioinformatics to predict the target genes of three miRNAs and found that they may regulate ROS levels through the TNF-α pathway (Figure 12A). Additionally, the mechanism of PELN’s action on brain inflammation has also attracted scholarly attention. Xu et al. [8][7] discovered that OELN can be internalized by cells through the β-glucan-hippocampal calcin (HPAC) pathway, while OLEN’s DGDG inhibits brain inflammation by blocking the activity of β-glucan and dectin-1, thereby facilitating its internalization and recycling without being directed towards the lysosomal pathway. Moreover, Sundaram et al. [9][76] found that garlic-derived nanovesicles (GELN) can be taken up by microglia via PA-mediated transmembrane action and interact with brain acid soluble protein 1 to form a competitive inhibitor for CaM binding, consequently suppressing c-MYC expression and ameliorating LPS-induced brain inflammation (Figure 12C). In addition, Teng et al. [21][81] found that miRNAs carried by ginger-derived ELN can inhibit the key proteins Nsp12 and Nsp13 of new coronary pneumonia.
4. Tumor Inhibition
The primary challenge in utilizing PELN as a potential therapeutic modality for cross-regional regulation lies in assessing its cytotoxicity, which necessitates an initial evaluation of its impact on normal cellular physiological activity and subsequent examination of its ability to inhibit abnormal cells. Extensive experimentation has demonstrated that PELN derived from cabbage, blueberry, strawberry, grapefruit, bitter melon, carrot, camellia, oats, and other sources does not exert any adverse effects on normal cell lines such as HaCaT, A375, HDF, and ADMSC; instead, it promotes cell proliferation. Moreover, PENL exhibits inhibitory properties against abnormal cell lines including A549, S W480, and LAMA84 [22][11].
Studies have demonstrated that diverse sources of PELN exert a favorable impact on human tumor cells through distinct mechanisms of action. For instance, ELN derived from bitter melon effectively suppresses the invasion and migration of the U251 human glioma cell line by modulating the P13K/AKT pathway [23][84] (Figure 23A). Furthermore, ginseng-derived ELN impedes the migration, invasion, cloning, and adhesion abilities of A549 and H1299 lung cancer cell lines by downregulating thymidine phosphorylase mRNA expression in the pentose phosphate pathway [24][86] (Figure 23B). Moreover, in studies investigating colon cancer, lemon-derived ELN has been shown to inhibit colon cancer through distinct mechanisms. Raimondo et al. [25][83] demonstrated that LELN suppresses cancer cell growth by attenuating ERK/p38-MAPK phosphorylation levels, thereby impairing signaling pathways. In addition, Raimondo et al. [22][11] revealed that LELN induces the Caspase cascade via the TRAIL pathway, ultimately mediating apoptosis in colon cancer cells (Figure 23C). Furthermore, in multiple melanoma studies, ELN derived from grapefruit exhibited a reduction in cancer cell viability by downregulating the phosphorylation level of ERK/AKT, thereby impairing its signaling [26][12], Cao et al. [2][66] discovered that ginseng-derived nanovesicles (GELN) promoted M2-to-M1 polarization through the TLR-4/MyD88 pathway. They demonstrated that this pathway is associated with GELN’s lipids or proteins rather than nucleic acids or other contents (Figure 23D).
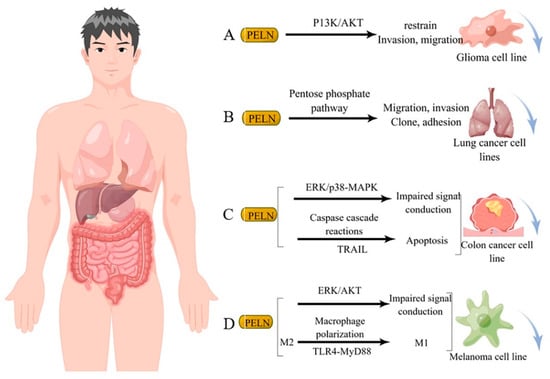
Figure 23. Potential mechanism underlying tumor suppression by PELN. (A): PELN and glioma; (B): PELN and lung cancer; (C): PELN and colon cancer; (D): PELN and melanoma. (by Figdraw).
