Atopic dermatitis (AD) is a chronic and relapsing inflammatory cutaneous disease. The role of host defense and microbial virulence factors in Staphylococcus aureus skin colonization, infection, and inflammation perpetuation in AD remains an area of current research focus. Extracellular vesicles (EV) mediate cell-to-cell communication by transporting and delivering bioactive molecules, such as nucleic acids, proteins, and enzymes, to recipient cells. Staphylococcus aureus spontaneously secretes extracellular vesicles (SA-derived EVs), which spread throughout the skin layers. Research has shown that SA-derived EVs from AD patients can trigger cytokine secretion in keratinocytes, shape the recruitment of neutrophils and monocytes, and induce inflammatory AD-type lesions in mouse models, in addition to their role as exogenous worsening factors for the disease.
- Staphylococcus aureus
- extracellular vesicles
- atopic dermatitis
- pathogenesis
- α-hemolysin
1. Introduction
2. Role of SA-Derived EVs in AD
In 2011, Hong et al. [24][23] conducted the first study to propose the role of S. aureus-derived EV in AD pathophysiology. The reseauthorchers reported that applying SA-derived EVs in tape-stripping mouse skin resulted in skin inflammation similar to that observed in AD patients, displaying a profile encompassed by (i) thickened epidermis, (ii) dermal infiltration of polymorphonuclear cells, (iii) an increase in Th1/Th17 inflammatory cytokines, and (iv) elevated serum IgE [24][23]. Importantly, these modifications were observed without the presence of live bacterial cells [24][23]. S. aureus colonization and infection evoke an immune response from the host by secreting pathogenic molecules and toxins (reviewed in Seiti Yamada Yoshikawa et al., 2019) [46][24]. Among these, wα-hemolysin is emphasize α-hemolysind, a cytotoxic protein secreted by S. aureus which induces cell death [47][25] on different cell types [48[26][27],49], including keratinocytes [23][28]. S. aureus from colonized AD skin releases greater amounts of α-hemolysin compared to S. aureus from the skin of healthy individuals. Furthermore, there are reports suggesting S. aureus α-hemolysin production is linked to AD severity [25][29]. An in vitro analysis revealed that EVs-associated α-hemolysin was more cytotoxic, and more effective to induce HaCaT keratinocytes death than soluble α-hemolysin, producing necrosis and inducing epidermal thickening and eosinophilic inflammation in the dermis [25[29][30],34], which could be explained by their better delivery to the host cell when encapsulated inside EVs. In addition, despite in vivo studies that indicate that soluble and EV-associated α-hemolysin induced skin barrier disruption and epidermal hyperplasia, an EV-associated toxin was the responsible for the induction of dermal infiltration [25][29]. Some S. aureus strains, such as ATCC14458 [24][23], a related cytotoxin producer, can release cytotoxins into the lumen of the EVs and induce their secretion to the extracellular space. Besides the assistance in the killing of the host cells by transferring these cytotoxic factors inside EVs, which favors their integration into the target cell cytoplasm [25][29], their association also shields the toxins from neutralization by the host immune system [24,25,50][23][29][31]. Mutually, in vitro and in vivo studies indicate that SA-derived EVs can upregulate pro-inflammatory mediators that elicit the Th17 response with augmented production of IgE, triggering AD-like inflammation [21,24][21][23]. Although AD is a disease ruled by type 2 immune responses (Th2, IL-4 and IL-13), IL-17 contributes to the worsening of the symptoms by enhancing IL-4 production from Th2 cells [51][32]. Moreover, SA-derived EVs induce the secretion of CXCL8 and TNF-α by primary human keratinocytes, the recruitment of neutrophils, and the formation of neutrophil extracellular traps, leading to better S. aureus skin colonization. The stimulation of CXCL8 is TLR2- and NFκB-dependent, and the induction level positively correlates with the membrane lipid and protein A in a similar quantity as those from pathogenic S. aureus [33]. SA-derived EVs can mediate inflammatory responses in AD pathogenesis; Kim et al. [30][34] examined the effect of these EVs on human dermal microvascular endothelial cells (HDMECs). HDMECs treated with SA-derived EVs increased the expression of cell adhesion molecules such as E-selectin, VCAM1, and ICAM1, and IL-6 with improved recruitment of monocytes in a TLR-4/NFκB-dependent signaling pathway [30][34]. All these findings together suggest that SA-derived EVs are key molecules in AD pathogenesis and are potential therapeutic targets. Figure 1 illustrates the role of SA-derived EVs in AD and some key biological effects are summarized in Table 1.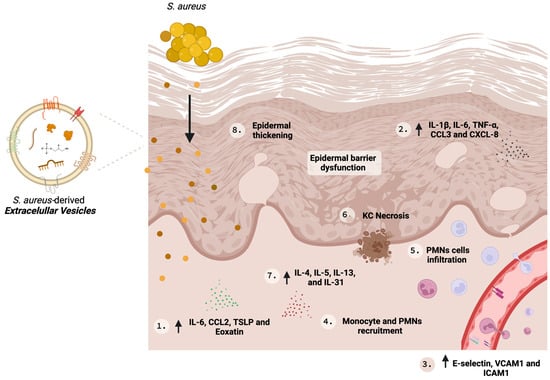
| S. aureus Strain | Study Type | Experimental Model | Inflammatory Effector Molecules Upregulated |
Histological Features |
Others Observed Effects |
Ref. |
|---|---|---|---|---|---|---|
| ATCC14458 | In vivo | EV were applied by tape stripping mouse skin | IL-4, IL-5, IL-17, IFN-γ | Infiltration of polymorphonuclear cells and epidermal thickness | - | [24][23] |
| 03ST17 | In vivo | Topical application of EVs into DFE induced lesions on AD-like mouse model; | IL-13, IL-31, CXCL8, CCL2 and CCL3 | Infiltration of polymorphonuclear cells and epidermal thickness | Severe eczematous dermatitis, swelling, redness, bullae, and eschar formation | [27][35] |
| USA300 | In vitro | Primary human keratinocytes | CXCL8 and TNF-α | - | Recruitment of neutrophils and induction of NETs | [33] |
| ATCC 6538 | In vitro | Immortalized human dermal microvascular endothelial cells | E-selectin, VCAM1, ICAM1 and IL-6 | - | Recruitment of monocytes | [30][34] |
| ATCC14458 and from AD patients | In vitro | Immortalized human keratinocytes | IL-1β and IL-6 | - | Cytotoxic effect associated with EV-α-Hemolysin | [25][29] |
| ATCC14458 | In vitro | Primary mouse dermal fibroblasts | IL-6, TSLP, CCL2 and Eotaxin | - | - | [24][23] |
| 03ST17 | In vitro | Immortalized human keratinocytes | IL-6 | - | - | [27][35] |
References
- Sacotte, R.; Silverberg, J.I. Epidemiology of adult atopic dermatitis. Clin. Dermatol. 2018, 36, 595–605.
- Asher, M.I.; Montefort, S.; Björkstén, B.; Lai, C.K.; Strachan, D.P.; Weiland, S.K.; Williams, H. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 2006, 368, 733–743.
- Bylund, S.; Kobyletzki, L.B.; Svalstedt, M.; Svensson, A. Prevalence and Incidence of Atopic Dermatitis: A Systematic Review. Acta Derm. Venereol. 2020, 100, adv00160.
- Blicharz, L.; Szymanek-Majchrzak, K.; Mlynarczyk, G.; Czuwara, J.; Waskiel-Burnat, A.; Goldust, M.; Samochocki, Z.; Rudnicka, L. Multilocus-sequence typing reveals clonality of Staphylococcus aureus in atopic dermatitis. Clin. Exp. Dermatol. 2023, 48, 1341–1346.
- Orfali, R.L.; Yoshikawa, F.S.Y.; Oliveira, L.; Pereira, N.Z.; de Lima, J.F.; Ramos YÁ, L.; Duarte, A.; Sato, M.N.; Aoki, V. Staphylococcal enterotoxins modulate the effector CD4+ T cell response by reshaping the gene expression profile in adults with atopic dermatitis. Sci. Rep. 2019, 9, 13082.
- Orfali, R.L.; da Silva Oliveira, L.M.; de Lima, J.F.; de Carvalho, G.C.; Ramos, Y.A.L.; Pereira, N.Z.; Pereira, N.V.; Zaniboni, M.C.; Sotto, M.N.; da Silva Duarte, A.J.; et al. Staphylococcus aureus enterotoxins modulate IL-22-secreting cells in adults with atopic dermatitis. Sci. Rep. 2018, 8, 6665.
- Garcovich, S.; Maurelli, M.; Gisondi, P.; Peris, K.; Yosipovitch, G.; Girolomoni, G. Pruritus as a Distinctive Feature of Type 2 Inflammation. Vaccines 2021, 9, 303.
- Steinhoff, M.; Ahmad, F.; Pandey, A.; Datsi, A.; AlHammadi, A.; Al-Khawaga, S.; Al-Malki, A.; Meng, J.; Alam, M.; Buddenkotte, J. Neuroimmune communication regulating pruritus in atopic dermatitis. J. Allergy Clin. Immunol. 2022, 149, 1875–1898.
- Agelopoulos, K.; Renkhold, L.; Wiegmann, H.; Dugas, M.; Suer, A.; Zeidler, C.; Schmelz, M.; Pereira, M.P.; Stander, S. Transcriptomic, Epigenomic, and Neuroanatomic Signatures Differ in Chronic Prurigo, Atopic Dermatitis, and Brachioradial Pruritus. J. Investig. Dermatol. 2023, 143, 264–272.e263.
- Simpson, E.L.; Tom, W.L.; Bushmakin, A.G.; Cappelleri, J.C.; Yosipovitch, G.; Stander, S.; Luger, T.; Sanders, P.; Gerber, R.A.; Myers, D.E. Relationship Among Treatment, Pruritus, Investigator’s Static Global Assessment, and Quality of Life in Patients with Atopic Dermatitis. Dermatol. Ther. 2021, 11, 587–598.
- Alexander, H.; Paller, A.S.; Traidl-Hoffmann, C.; Beck, L.A.; De Benedetto, A.; Dhar, S.; Girolomoni, G.; Irvine, A.D.; Spuls, P.; Su, J.; et al. The role of bacterial skin infections in atopic dermatitis: Expert statement and review from the International Eczema Council Skin Infection Group. Br. J. Dermatol. 2019, 182, 1331–1342.
- Kim, J.; Kim, B.E.; Leung, D.Y.M. Pathophysiology of atopic dermatitis: Clinical implications. Allergy Asthma Proc. 2019, 40, 84–92.
- Simpson, E.L.; Villarreal, M.; Jepson, B.; Rafaels, N.; David, G.; Hanifin, J.; Taylor, P.; Boguniewicz, M.; Yoshida, T.; De Benedetto, A.; et al. Patients with Atopic Dermatitis Colonized with Staphylococcus aureus Have a Distinct Phenotype and Endotype. J. Invest. Dermatol. 2018, 138, 2224–2233.
- Kim, J.; Kim, B.E.; Ahn, K.; Leung, D.Y.M. Interactions Between Atopic Dermatitis and Staphylococcus aureus Infection: Clinical Implications. Allergy Asthma Immunol. Res. 2019, 11, 593–603.
- Oliveira, D.; Borges, A.; Simoes, M. Staphylococcus aureus Toxins and Their Molecular Activity in Infectious Diseases. Toxins 2018, 10, 252.
- Vandenesch, F.; Lina, G.; Henry, T. Staphylococcus aureus hemolysins, bi-component leukocidins, and cytolytic peptides: A redundant arsenal of membrane-damaging virulence factors? Front. Cell Infect. Microbiol. 2012, 2, 12.
- Kim, J.H.; Lee, J.; Park, J.; Gho, Y.S. Gram-negative and Gram-positive bacterial extracellular vesicles. Semin. Cell Dev. Biol. 2015, 40, 97–104.
- Gill, S.; Catchpole, R.; Forterre, P. Extracellular membrane vesicles in the three domains of life and beyond. FEMS Microbiol. Rev. 2019, 43, 273–303.
- Ellen, A.F.; Albers, S.V.; Huibers, W.; Pitcher, A.; Hobel, C.F.; Schwarz, H.; Folea, M.; Schouten, S.; Boekema, E.J.; Poolman, B.; et al. Proteomic analysis of secreted membrane vesicles of archaeal Sulfolobus species reveals the presence of endosome sorting complex components. Extremophiles 2009, 13, 67–79.
- Dagnelie, M.A.; Corvec, S.; Khammari, A.; Dreno, B. Bacterial extracellular vesicles: A new way to decipher host-microbiota communications in inflammatory dermatoses. Exp. Dermatol. 2020, 29, 22–28.
- Bose, S.; Aggarwal, S.; Singh, D.V.; Acharya, N. Extracellular vesicles: An emerging platform in gram-positive bacteria. Microb. Cell 2020, 7, 312–322.
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell Vesicles 2018, 7, 1535750.
- Hong, S.W.; Kim, M.R.; Lee, E.Y.; Kim, J.H.; Kim, Y.S.; Jeon, S.G.; Yang, J.M.; Lee, B.J.; Pyun, B.Y.; Gho, Y.S.; et al. Extracellular vesicles derived from Staphylococcus aureus induce atopic dermatitis-like skin inflammation. Allergy 2011, 66, 351–359.
- Seiti Yamada Yoshikawa, F.; Feitosa de Lima, J.; Notomi Sato, M.; Álefe Leuzzi Ramos, Y.; Aoki, V.; Leao Orfali, R. Exploring the Role of Staphylococcus aureus Toxins in Atopic Dermatitis. Toxins 2019, 11, 321.
- Bantel, H.; Sinha, B.; Domschke, W.; Peters, G.; Schulze-Osthoff, K.; Jänicke, R.U. alpha-Toxin is a mediator of Staphylococcus aureus-induced cell death and activates caspases via the intrinsic death pathway independently of death receptor signaling. J. Cell Biol. 2001, 155, 637–648.
- Essmann, F.; Bantel, H.; Totzke, G.; Engels, I.H.; Sinha, B.; Schulze-Osthoff, K.; Janicke, R.U. Staphylococcus aureus alpha-toxin-induced cell death: Predominant necrosis despite apoptotic caspase activation. Cell Death Differ. 2003, 10, 1260–1272.
- Jia, N.; Li, G.; Wang, X.; Cao, Q.; Chen, W.; Wang, C.; Chen, L.; Ma, X.; Zhang, X.; Tao, Y.; et al. Staphylococcal superantigen-like protein 10 induces necroptosis through TNFR1 activation of RIPK3-dependent signal pathways. Commun. Biol. 2022, 5, 813.
- Lee, E.Y.; Choi, D.Y.; Kim, D.K.; Kim, J.W.; Park, J.O.; Kim, S.; Kim, S.H.; Desiderio, D.M.; Kim, Y.K.; Kim, K.P.; et al. Gram-positive bacteria produce membrane vesicles: Proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics 2009, 9, 5425–5436.
- Hong, S.W.; Choi, E.B.; Min, T.K.; Kim, J.H.; Kim, M.H.; Jeon, S.G.; Lee, B.J.; Gho, Y.S.; Jee, Y.K.; Pyun, B.Y.; et al. An important role of α-hemolysin in extracellular vesicles on the development of atopic dermatitis induced by Staphylococcus aureus. PLoS ONE 2014, 9, e100499.
- Yang, J.; Shin, T.S.; Kim, J.S.; Jee, Y.K.; Kim, Y.K. A new horizon of precision medicine: Combination of the microbiome and extracellular vesicles. Exp. Mol. Med. 2022, 54, 466–482.
- Kulp, A.; Kuehn, M.J. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 2010, 64, 163–184.
- Sugaya, M. The Role of Th17-Related Cytokines in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 1314.
- Staudenmaier, L.; Focken, J.; Schlatterer, K.; Kretschmer, D.; Schittek, B. Bacterial membrane vesicles shape Staphylococcus aureus skin colonization and induction of innate immune responses. Exp. Dermatol. 2022, 31, 349–361.
- Kim, J.; Bin, B.H.; Choi, E.J.; Lee, H.G.; Lee, T.R.; Cho, E.G. Staphylococcus aureus-derived extracellular vesicles induce monocyte recruitment by activating human dermal microvascular endothelial cells in vitro. Clin. Exp. Allergy 2019, 49, 68–81.
- Jun, S.H.; Lee, J.H.; Kim, S.I.; Choi, C.W.; Park, T.I.; Jung, H.R.; Cho, J.W.; Kim, S.H.; Lee, J.C. Staphylococcus aureus-derived membrane vesicles exacerbate skin inflammation in atopic dermatitis. Clin. Exp. Allergy 2017, 47, 85–96.
