Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Baojun Xu and Version 2 by Camila Xu.
Aging is a complex biological process that is influenced by both intrinsic and extrinsic factors. Recently, it has been discovered that reactive oxygen species can accelerate the aging process, leading to an increased incidence of age-related diseases that are characteristic of aging.
- Mushrooms
- anti-aging
- age-related disease
- cellular mechanisms
- bioactive compounds
1. Introduction
The global population is currently experiencing a significant expansion of aging populations compared to previous years. This trend is reflected in the increase in average life expectancy at birth, which has risen by 6.2 years from 65.3 years in 1990, to 71.5 years in 2013. Additionally, individuals who reach the age of 60 can now expect to live for another 22 years on average [1]. By the year 2040, it is projected that the average life expectancy will increase by 4.4 years for both men and women. Men can expect to live an average of 74.3 years, while women can expect to live an average of 79.7 years. However, these numbers may vary depending on individual health conditions [2]. As the population ages, there has been a noticeable increase in the prevalence of chronic degenerative diseases such as neurodegenerative and cardiovascular diseases, diabetes, and cancer. These diseases contribute to up to 70% of global mortality each year, including premature deaths occurring between the ages of 30 and 70 [1]. It is important to note that, while aging is often accompanied by deteriorative changes and an increased risk of functional declines or diseases, aging itself is not considered a disease. The focus of anti-aging strategies is not to reverse or halt the aging process, but rather to promote healthy aging and reduce the incidence of age-related diseases. The World Health Organization recommends adopting healthy dietary habits, engaging in regular physical activity, and controlling tobacco use as effective measures to alleviate or prevent the incidence of chronic diseases. By following these guidelines, the risk of developing age-related diseases can be reduced [3].
There is growing evidence to suggest that healthy aging can be promoted by consuming nutraceuticals and following various dietary patterns, such as caloric restriction, intermittent fasting, a Mediterranean diet, an Okinawan diet, and a Nordic diet. These dietary patterns have been evaluated for their negative correlation with aging and age-related conditions and diseases [4][5][4,5], which has led to a search for anti-aging components from food sources and an investigation of the underlying mechanisms of anti-aging pathways. Bioactive compounds derived from plant sources, including fruits and vegetables, roots, seeds, and edible flowers, have been suggested to exert anti-aging effects. These compounds include certain polysaccharides, phenolic compounds, and peptides [6][7][6,7]. In recent years, mushrooms—filamentous fungi with fruiting bodies—have also been shown to possess enormous pharmacological attributes that are valuable for healthy aging. These attributes include anti-oxidant, immunomodulatory, neuroprotective, anti-inflammatory, and anti-cancer properties [8][9][10][11][8,9,10,11].
Mushrooms are nutritious foods that are rich in carbohydrates and proteins, with a lower content of lipids [12]. In addition to their nutritional value, mushrooms contain various bioactive compounds, such as β-glucans, lectins, and linolenic acids, which can be isolated through different extraction methods. These compounds confer a variety of pharmacological activities and may enhance the immune system and strengthen the biological function of the body [13]. Regular intake of mushrooms or their extracts may help alleviate age-related diseases.
2. Aging
2.1. Aging and Age-Related Diseases
Aging is a complex process that involves the time-dependent accumulation of diverse deleterious changes in cells, tissues, organs, or systems that increase vulnerability to chronic illness and death [14][15][14,15]. Nine candidate hallmarks of aging have been identified and classified, including primary hallmarks (genomic instability, telomere attrition, epigenetic alterations, and loss of proteostasis), antagonistic hallmarks (deregulated nutrient sensing, mitochondrial dysfunction, and cellular senescence), and integrative hallmarks (stem cell exhaustion and altered intercellular communication), all of which are correlated with each other [16]. The antagonistic hallmarks exert positive effects at low levels but negatively affect the organism at high levels [16]. For example, reactive oxygen species (ROS) are important signaling molecules that play a role in regulating cellular functions, but excessive levels can lead to oxidative damage and contribute to aging. The primary hallmarks are the contributors to molecular damage during aging, while the integrative hallmarks are signs of failure of cellular homeostasis and metabolism mechanisms to ameliorate the damage. These hallmarks are interconnected with each other and could serve as a guidance to decipher the mechanistic molecular basis for prolonging health span and development of strategies for longevity, such as stem-cell-based therapies, epigenetic drugs, anti-inflammatory drugs, and dietary restrictions [16]. The free radical theory of aging, proposed in 1956 by Denham Harman [17], is a widely accepted theory of aging. The theory postulates that the aging process is triggered by the initiation of free radical reactions, leading to increased generation of free radicals by damaged mitochondria with increasing age [18]. Major sources of free radical reactions in mammals include non-enzymatic reaction of oxygen, ionizing radiation, cytochrome P-450 system, respiratory chain, phagocytosis, and prostaglandin synthesis, which lead to the accumulation of oxidative damage and may shorten the lifespan. Several defenses that alleviate the damage of the reactions include DNA repair mechanisms, superoxide dismutase, glutathione peroxidase, and anti-oxidants (e.g., carotenes and vitamin E) [15][19][15,19]. ROS are byproducts of oxidative metabolism that can induce cellular defense mechanisms against oxidative invasion at low doses, potentially prolonging health span and lifespan. However, long-term excessive exposure to ROS can lead to the oxidation of nucleic acids, proteins, and lipids, causing damage to macromolecules and mitochondrial dysfunction. This can disrupt cell homeostasis and result in cellular death [20]. ROS production is driven by progressive mitochondrial dysfunction with increasing age, creating a positive feedback loop of ROS generation and oxidative damage accumulation [18]. Concurrently, oxidative stress arises due to excessive ROS levels and limited anti-oxidant defense capability, leading to cellular senescence and a shortened lifespan. The accumulation of oxidative damage to macromolecules and mitochondria contributes to detrimental consequences, such as pathophysiological changes, functional decline, and accelerated aging, which are associated with age-related conditions such as inflammation, cardiovascular diseases, neurodegenerative diseases, autoimmune diseases, and cancer [21]. It is important to note that aging itself is not a disease. Age-related diseases can be considered “symptoms” of aging, initiated by minor disturbances that are intensified via vicious positive feedback loops, destabilizing the physiology of an organism and potentially leading to destruction (i.e., mortality) if no negative feedback loops are in place [22]. For example, low-grade inflammation can intensify in chronic inflammation, leading to decreased muscle mass, decreased physical activity, and excess fat deposition. This can further contribute to obesity, diabetes, and cardiovascular problems. Eventually, cardiovascular diseases can arise and worsen the physiological status of an individual by triggering chronic inflammation. To minimize cumulative damage to different organs and maintain cell function for healthy aging, interventions that can interrupt or break the vicious cycles of age-related diseases can be implemented, including medications, lifestyle adjustments, and dietary management (Figure 1).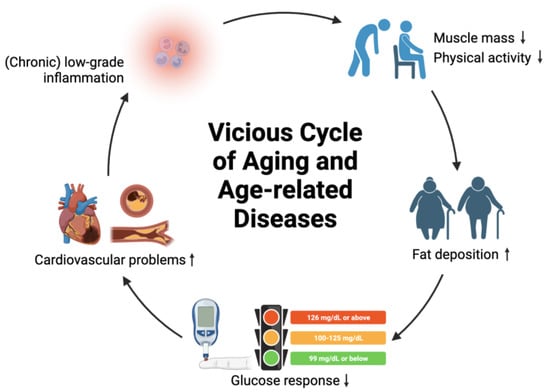
Figure 1.
Concept of vicious cycle of aging and age-related diseases. Symbol ↑ denotes increase; symbol ↓ denotes decrease.
2.2. Aging and Dietary Intervention
The lifestyle of an individual is closely linked to their health span and lifespan. One of the main ways to modify lifestyle for better health maintenance and to reduce the incidence of age-related diseases is through dietary management. Unhealthy dietary habits and lifestyle can accelerate the aging process by causing molecular and cellular damage. For example, a sedentary lifestyle, combined with a “Western diet”, that is high in energy but lacking in nutrition, has been associated with reduced lifespan and increased occurrence of age-related conditions such as obesity, type 2 diabetes, and cancer [23]. On the other hand, caloric restriction (CR) has been shown to slow down the rate of aging and extend health span. CR involves reducing total energy intake by 20% to 40% while ensuring optimal nutrition, compared to an ad libitum diet. This approach has been demonstrated to extend lifespan and health span in various experimental models, including yeast, fruit flies, mice, nonhuman primates, and even humans [24][25][26][24,25,26]. According to the theory of aging, CR enhances longevity by reducing oxidative damage and increasing resistance to oxidative stress through specific signaling pathways. The stress caused by CR, such as nutrient deprivation, activates defense mechanisms against oxidative damage, thereby slowing down the aging process [27]. CR also affects physiological pathways that may mediate anti-aging effects, such as the insulin-like growth factor-1 and insulin signaling pathways, the mammalian target of rapamycin (mTOR) pathway, and the sirtuins pathway [24][28][29][24,28,29]. Previous studies have demonstrated the potential of implementing CR as an anti-aging regimen, as adherence to this dietary management reduces biomarkers associated with the development of age-related diseases, including cardiovascular diseases, autoimmune disorders, neurodegenerative diseases, diabetes, and cancer [29][30][31][29,30,31]. Therefore, CR can be considered as the mechanistic foundation for healthy aging strategies involving dietary intervention, which can prolong lifespan and maintain physiological function for an extended health span. Despite the potential benefits of CR, it can be challenging for individuals to adhere to it in the long term due to various pitfalls and health concerns, such as hypotension, osteoporosis, slower wound healing, depression, and irritability [32]. As a result, scientists have explored alternative diet regimens and studied different dietary patterns that may offer similar benefits to CR but are more feasible for humans to sustain. One such approach is intermittent fasting, which shares the same concept as CR. Intermittent fasting activates cellular pathways that enhance the body’s intrinsic defense against oxidative stress, promotes the removal of damaged molecules, and facilitates tissue repair and growth. It also helps to suppress inflammation and improve stress resistance [33][34][33,34]. In addition to dietary modifications, researchers have developed anti-aging drugs that mimic the effects of CR. Examples include rapamycin and metformin, which have shown promising effects in various model organisms and clinical trials. Rapamycin delays aging by inhibiting mTOR, thereby maintaining the normal functioning of mitochondria and stem cells. Metformin, on the other hand, affects telomere length, reduces oxidative damage to DNA, and modulates the synthesis and degradation of age-related proteins [35][36][35,36]. However, it is important to note that there are concerns and side effects associated with the use of these drugs. For instance, rapamycin may lead to nephrotoxicity and thrombocytopenia, while metformin may cause vitamin B12 deficiency and lactic acid accumulation [37][38][37,38]. Therefore, there is a need to explore naturally occurring compounds that have significant anti-aging effects with minimal side effects. Nutraceuticals and dietary supplements are also viable alternatives for anti-aging and extending health span. Examples include curcumin, quercetin, ginseng, and medicinal mushrooms, which exhibit anti-inflammatory, immunomodulatory, and antioxidative effects [39][40][41][39,40,41]. A diet rich in fruits and vegetables, which provide a significant number of nutraceuticals and phytochemicals, is crucial for maintaining overall health. Interestingly, mushrooms, although not classified as animals or plants but as part of the fungal kingdom, are often considered as vegetables. They are low in calories, sodium, and fat, while being a valuable source of fiber, phenolic compounds, β-glucans, selenium, glutathione, B vitamins, and vitamin D. These components serve as protective agents against oxidative damage, which accelerates aging [12]. Medicinal mushrooms have also been used for centuries in traditional therapies, like Chinese medicine and Indian Ayurveda medicine, to alleviate symptoms of various diseases [42]. The bioactive compounds found in mushrooms may contribute to their anti-aging effects through various physiological pathways involved in aging and age-related diseases.2.3. Ageing, Mental Health and Gender
Gender and mental health can significantly impact ageing experiences. Gender influences ageing in various ways, including health outcomes, social roles and expectations, and economic status. Women are more likely to experience depression, anxiety, and stress due to factors such as caregiving responsibilities, hormonal changes, and discrimination [43]. Women also tend to report higher levels of loneliness and social isolation in later life. In contrast, men may experience social isolation and mental health issues due to societal expectations of masculinity, which can lead to reluctance in seeking help for mental health problems [44]. Gender differences in health outcomes are well-documented, with women living longer but experiencing more chronic health conditions than men. Women are more likely to experience osteoporosis, urinary incontinence, and depression than men. Women also experience menopause, which can lead to physical and psychological symptoms [45]. Men, on the other hand, are more likely to experience heart disease, stroke, and certain types of cancer. Biological factors such as sex hormones, genetics, and lifestyle factors like diet, exercise, and smoking influence gender differences in health outcomes [46]. Gender roles and expectations can influence ageing experiences [47]. Women are often expected to take on caregiving roles for children, spouses, or ageing parents, which can lead to stress and impact their own health and well-being. Women may also face ageism and discrimination in the workplace, leading to financial insecurity in later life. Men, on the other hand, may experience pressure to maintain their independence and financial stability, leading to social isolation and mental health issues [43][44][48][43,44,48]. Gender differences in economic status can also impact ageing experiences. Women often earn less than men over their lifetimes, leading to lower retirement savings and financial insecurity in later life. Women are also more likely to work part-time or take career breaks to care for children or ageing parents, which can impact their pension entitlements. This can lead to poverty and social exclusion in later life [47][49][47,49]. Mental health issues, such as depression, anxiety, and cognitive impairment, can also impact ageing experiences. Depression is a common mental health issue among older adults and can lead to social isolation, physical illness, and suicide. Anxiety can affect quality of life and daily functioning. Cognitive impairment, including dementia, can result in memory loss, decision-making difficulties, and loss of independence and increased caregiving needs [50]. Studies have shown that gender and mental health can interact to influence ageing experiences [28][36][43][44][51][52][28,36,43,44,51,52]. Women with depression may be more prone to physical disability and cognitive decline in later life compared to men with depression. Similarly, men with higher levels of anxiety may be more likely to experience cognitive decline than women with anxiety [43]. Addressing gender and mental health in ageing policies and practices is crucial to ensure that older adults receive appropriate support and services. This includes promoting gender equity, addressing mental health stigma, and providing accessible and affordable mental health care for older adults [22].3. Components of Mushrooms and Their Anti-Aging Effects
Mushrooms have long been recognized for their nutritional value and potential health benefits. Edible mushrooms are not only rich in protein, fiber, vitamins, and minerals but also have low levels of fat, making them highly nutritious [53][54][53,54]. They contain all the essential amino acids and have a higher protein content compared to most vegetables, making them particularly beneficial for vegetarians. In addition to their nutritional value, edible mushrooms, as fungi, have the ability to produce a wide range of chemical compounds known as mycochemicals. These mycochemicals can act as bioactive substances with various advantages for human health [55]. Mushrooms have been found to contain significant levels of mycochemicals that serve as bioactive compounds, offering a range of health benefits against aging and age-related diseases [53][54][53,54].3.1. Bioactive Compounds in Mushrooms
Bioactive compounds extracted from mushrooms have been extensively studied for their ability to enhance cellular functions and provide health benefits. The following text summarizes four representative categories of bioactive compounds found in mushrooms: carbohydrates, proteins, lipids, and phenolic compounds.3.1.1. Carbohydrates
Carbohydrates derived from mushrooms have been extensively studied for their anti-tumor, anti-inflammatory, and immunomodulatory activities [56][57][56,57]. Numerous monosaccharides found in mushrooms, including arabinose, fructose, fucose, galactose, glucose, mannose, mannitol, rhamnose, trehalose, and xylose, have been identified as exhibiting these activities. They primarily achieve this through the activation of cytokines, such as interferons and interleukins, and involve cellular pathways that include dendritic cells, natural killer cells, neutrophils, and cytotoxic macrophages [57][58][57,58]. β-Glucans, the main type of carbohydrates found in mushrooms, have been shown to possess antioxidative, anti-cancer, immunomodulatory, and neuroprotective properties. They are considered potent agents for stimulating the immune system and protecting against carcinogens, pathogens, and toxins [59][60][61][62][63][64][59,60,61,62,63,64]. The biological activity and health benefits of β-glucans isolated from mushrooms, particularly in relation to immune health, are crucial for healthy aging. Supplementation with mushroom carbohydrates, which contain β-glucans, could be an effective strategy for anti-aging. Table 1 provides a list of various mushrooms that contain bioactive carbohydrates.Table 1.
Bioactive carbohydrates in selected mushrooms.
| Mushrooms | Common Names | Bioactive Compounds | Source and Yield | Bioactivities | References |
|---|---|---|---|---|---|
| Agaricus bisporus | Button mushroom | Heteropolysaccharide Abnp1001, Abnp1002, Abap1001, Abap1002 | Concentrated industrial wastewater of A. bisporus; 0.989 mg/g, 1.849 mg/g, 0.128 mg/g, and 0.68 mg/g (Abnp1001, Abnp1002, Abap1001, Abap1002) | Hepatoprotective | [65] |
| Heteropolysaccharide AcAPS, AcAPS-1, AcAPS-2, AcAPS-3, with rhamnose and glucose as major monosaccharide | Dried fruiting body; yield n.s. | Hepatoprotective, nephroprotective, antioxidative |
Table 2.
Bioactive proteins in mushrooms.
| Mushrooms | Common Names |
Bioactive Compounds/Substances * |
Bioactivities | References | ||||
|---|---|---|---|---|---|---|---|---|
| Agaricus bisporus | Button mushroom | Lectin | Immunomodulatory | [96] | ||||
| [ | 66 | |||||||
| Cerrena unicolor | ] | |||||||
| Mossy maze polypore | Laccase | Anti-tumor | [ | 97] | Polysaccharide extracts, main components n.s. | Whole mushroom; yield n.s. | Anti-tumor, immunostimulatory | [67] |
3.1.3. Lipids
Although mushrooms have a low fat content ranging from 0.1% to 16.3%, they are a good source of high-quality essential fatty acids such as oleic acid (1–60.3% of total fatty acids in 100 g), linoleic acid (0–81.1% of total fatty acids in 100 g), and linolenic acid (0–28.8% of total fatty acids in 100 g) [117]. Table 3 summarizes the lipid profiles of various mushrooms in terms of the content of saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), and polyunsaturated fatty acids (PUFA). Mushrooms are good sources of unsaturated fatty acids, as observed in a study by Günç Ergönül et al. [118] who investigated the fatty acid compositions of six wild edible mushroom species and found that unsaturated fatty acids predominated over saturated ones. In most nutritional characterization studies, mushroom fatty acids are commonly determined using gas-liquid chromatography coupled with a flame ionization detector. However, the sample extraction method used prior to measurement may impact the final outcome of lipid profiles. For instance, a study by Sinanoglou et al. [119] investigated the lipid profiles of Laetiporus sulphureus using different combinations of extraction methods and two individual solvents and found variations among the four combinations [119]. Ergosterol, the major sterol found in mushrooms, accounts for the major lipid component of fungal extracellular vesicles as well [120]. Ergosterol extracted from medicinal mushroom Ganoderma lucidum has been shown to exert anti-oxidant effects and reduce the risk of cardiovascular diseases while extending lifespan [55][121][122][55,121,122]. Compared to lipids from animal sources, edible mushrooms are advantageous due to their high levels of polyunsaturated fatty acids, which may regulate various physiological functions in age-related diseases, such as decreasing blood pressure and triglyceride levels, and reducing the risks of age-related cardiovascular diseases, arthritis, and neurodegenerative diseases [64][123][64,123]. Therefore, mushrooms may play a significant role in human nutrition and anti-aging regimens based on their fatty acid profiles.Table 3.
Lipid profiles in mushrooms.
| Mushrooms | Common Name | Total SFA (% of Total FA) |
Total MUFA (% of Total FA) |
Total PUFA (% of Total FA) |
Measurement Techniques | References | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Agaricus blazei | Almond mushroom | 24.4 | 2.0 | 73.6 | GC-FID | [83] | ||||||||
| Agaricus bisporus | White button mushroom | 20.3 | 1.4 | 78.3 | Capillary GLC-FID | [70] | ||||||||
| Coprinus comatus | Shaggy mane/chicken drumstick mushroom | |||||||||||||
| Brown button mushroom | 18.4 | Laccase | Anti-viral | [ | 1.898 | 79.8] | Heteropolysaccharide/Mannogalacoglucan mannose, galactose, glucose | Freeze-dried fresh fruiting body; 41.4% yield (w/w dry weight) | Anti-tumor | [68] | ||||
| Agrocybe cylindracea | Poplar mushroom | 28.1 | 2.83β-glucan | Dried fresh fruiting body; yield n.s. | Immunostimulatory | [69] | ||||||||
| 69.1 | Capillary GLC-FID | [ | Fructose, mannitol, trehalose | Fresh fruiting body; 5.79% (white mushroom) & 4.27% (brown mushroom) (w/w fresh weight) | n.s. | [70] | ||||||||
| Polysaccharide extracts, main components n.s. | Base of stipe; yield n.s. | Anti-tumor | [ | 72 | ] | |||||||||
| Polysaccharide extracts, main components n.s. | Fresh whole-mushroom; yield n.s. | Neuroprotective | [73] | |||||||||||
| Fructose, mannitol, sucrose, trehalose | Fresh fruiting body; 8.29% (w/w fresh weight) | n.s. | [70] | |||||||||||
| Ganoderma | ||||||||||||||
| Flammulina velutipes | Enoki/Golden needle mushroom | FIP | Anti-inflammatory | [99] | ||||||||||
| 124 | ] | RIP | Anti-viral | [100] | ||||||||||
| Boletus reticulatus | Summer cep | 21.1 | 40.3 | 38.4 | GLC-FID | [118] | Ganoderma applanatum | Artist’s conk | Lectin | Anti-tumor | [ | |||
| Coprinus comatus | 101 | ] | Shaggy mane/Lawyer’s cap | 23.8 | 11.4 | 64.8 | Capillary GLC-FID | [124 | Calocybe indica |
Milky mushroom | Polysaccharide extracts, main components n.s. | Fresh fruiting body; 3.27% (w/w dry weight) | Anti-oxidant, neuroprotective | [ |
| ] | Ganoderma lucidum | Lingzhi | Laccase | Anti-viral | [102]71] | |||||||||
| Flammulina velutipes | Enoki/Golden needle mushroom | 18.5 | 7.2 | 74.3 | Capillary GLC-FID | [70] | Flammulina velutipes |
Enoki/Golden needle mushroom | ||||||
| Ganoderma tsugae | Hemlock reishi | FIP | ||||||||||||
| 20.7 | 18.6 | 60.7 | Immunomodulatory | GLC-FID[103] | ||||||||||
| [ | 118 | ] | Hypsizygus marmoreus | Jade mushroom | RIPs (hypsin, marmorin) | Anti-fungal, anti-tumor | [104][104[105],105] | |||||||
| Inonotus baumii | Sanghuang | Laccase | Anti-tumor | [106] | lucidum | Ling Zhi | Polysaccharide extracts, main components n.s. | Mycelia; 71.99% (w/w dry weight) | Anti-inflammation, ameliorating insulin resistance, suppressing lipid accumulation, regulation of gut microbiota | [74] | ||||
| Macrolepiota procera | Parasol mushroom | Lectin | Anti-tumor | [107] | Polysaccharide extracts, main components n.s. | Commercialized spray dried mycelia; 91.48% (w/w dry weight) | Improving intestinal barrier functions | [75] | ||||||
| Pleurotus cornucopiae | Golden oyster | Laccase | Anti-viral, anti-tumor | [108] | Arabinose, galactose, glucose, xylose | Whole mushroom; yield n.s. | Anti-tumor | [76] | ||||||
| Pleurotus eryngii | King oyster mushroom | Laccase | Anti-viral | [109] | Polysaccharide extracts, main components n.s. | Dried conidial powder; 2% (w/w dry weight, crude extracts) | Promote cognitive function and neural progenitor proliferation | |||||||
| Pleurotus ostreatus | Oyster mushroom | [ | Lectin77] | |||||||||||
| Immunomodulatory | [ | 110 | ] | Lentinula edodes | Shiitake mushroom | Glucose, galactose, mannose, arabinose | Fruiting body; 1.3% (w/w | |||||||
| Lactarius deliciocus | Saffron milkcap | 20.8 | 42.0 | 37.3 | Capillary GLC-FID | [124] | ||||||||
| Lactarius salmonicolor | Salmon milkcap | 19.0 | 19.6 | 61.6 | GLC-FID | [118] | ||||||||
| Lentinus edodes | Shiitake mushroom | 16.7 | 3.5 | 79.8 | GC-FID | [83] | ||||||||
| 15.1 | 2.9 | 82.0 | Capillary GLC-FID | [70] | ||||||||||
| Pleurotus eryngii | King oyster mushroom | 17.4 | 13.1 | 69.4 | Capillary GLC-FID | [70] | dry weight, purified polysaccharide cLEP1) | Therapeutic to cervical carcinoma | [78] | |||||
| Rhamnose | Residue/byproduct; yield n.s. | Anti-inflammatory, anti-oxidant | [79] | |||||||||||
| Pyranose, β-d-glucans (β-(1→3)-D-glucose as backbone & β-(1→6)-D-glucose as side chains) | Dried fruiting body; 0.76% (w/w dry weight) | Anti-tumor | [80] | |||||||||||
| Mannogalactoglucan-type polysaccharides WPLE-N-2, WPLE-A0.5-2 | Fruiting body; yield n.s. | Anti-cancer, immunomodulatory | [81] | |||||||||||
| Lentinan (β-(1,3)-glucan with β-(1,6) branches) | Dried fruiting body (commercial product); 2.6% (w/w dry weight) | Anti-tumor | [82] | |||||||||||
| Mannitol, trehalose, arabinose | Dried powder; 23.3% (mannitol), 13.2% (trehalose), 1.79% (arabinose) ( | |||||||||||||
| Pleurotus ostreatus | Oyster mushroom | 17.0 | 13.6 | 69.4 | Capillary GLC-FID | [70] | ||||||||
| 21.8 | 11.4 | 66.5 | GLC-FID | [118] | ||||||||||
| Polyporus squamosus | Dryad’s saddle | 25.2 | 34.3 | 40.6 | GLC-FID | [w/w dry weight) | n.s. | [83] | ||||||
| Pleurotus eryngii |
||||||||||||||
| Sparassis latifolia | Cauliflower mushroom | Lectin | Anti-fungal, anti-bacteria | [111] | 118 | ] | ||||||||
| Russula anthracina | - | 23.7 | 53.3 | 22.9 | GLC-FID | [118] | ||||||||
| Laetiporus sulphureus | Sulphur polypore | 21.6 | 17.6 | 60.8 | GC-FID, TLC-FID |
[119] | ||||||||
| Suillus collinitus | - | 17.5 | 34.4 | 47.4 | Capillary GLC-FID | [124] | King oyster | |||||||
| Tricholoma myomyces | Grey knight mushroom | 15.8 | 46.3 | 37.8 | Capillary GLC-FID | [124] | mushroom |
Mannose, glucose, galactose | Fresh whole-mushroom; 5.4% (w/w dry weight) | Anti-tumor | [84] | |||
| Heteropolysaccharides, novel fractions PEPE-1, PEPE-2, PEPE-3 (mannose, glucose, galactose, xylose) | Fresh mushroom residue; yield n.s. | Anti-tumor | [85] | |||||||||||
| Mannose, glucose, galactose | Fresh whole-mushroom; 28.3% (w/w dry weight) | Immunomodulatory | [86] | |||||||||||
| Pleurotus ostreatus |
Oyster mushroom | Crude polysaccharide extracts | Fresh whole-mushroom; 61% (w/w) | Alleviation of cognitive impairment | [87] | |||||||||
| Crude polysaccharide extracts | Fresh whole-mushroom; 63.98% (w/w) | Regulation of dislipidemia | [88] | |||||||||||
| Homogeneous polysaccharides, fractions POMP1, POMP2, POMP3 | Mycelia; yield n.s. | Anti-tumor | [89] |
n.s., not specified; Abnp, Agaricus bisporus polysaccharides between 5 kDa and 100 kDa; Abap, Agaricus bisporus polysaccharides under 5 kDa; AcAPS, purified fractions of acidic-extractable polysaccharides; WPLE, mannogalactoglucan-type polysaccharides from Lentinus edodes; POMP, Pleurotus ostreatus mycelium polysaccharide.
The ribosome inactivating protein family acts as rRNA N-glycosylase, inactivating 60S ribosomal subunits through an N-glycosidic cleavage that eliminates one or more adenosine residues from rRNA to inhibit protein synthesis [112]. Members of the ribosome inactivating protein family, such as trichosanthin, luffin, ricin, and abrin, have been of considerable interest due to their potent activity against viral infections and their potential use as immunotoxins for cancer treatment by conjugating with monoclonal antibodies [113][114][115][113,114,115
3.1.2. Proteins
* Include various categories and sub-categories of proteins. FIP, fungal immunomodulatory protein. RIP, ribosome inactivating protein.
SFA, saturated fatty acid. MUFA, monosaturated fatty acid. PUFA, polysaturated fatty acid. GC-FID, gas chromatography coupled with flame ionization detector. GLC-FID, gas-liquid chromatography coupled with flame ionization detection. TLC-FID, thin layer chromatography–flame ionization detection.
3.1.4. Phenolic Compounds
Phenolic compounds found in mushrooms are typically considered secondary metabolites. The most prominent phenolic compounds in mushrooms include heteroglycans, lectins, phenolic acids (such as ferulic, gallic, and cinnamic acids), flavonoids (including hesperetin, quercetin, kaempferol, and naringenin), steroids, alkaloids, tannins, chitinous substances, terpenoids, and tocopherols. These compounds exhibit various biological activities, including anti-oxidant, anti-tumor, anti-inflammatory, anti-hyperglycemic, anti-osteoporotic, anti-tyrosinase, and anti-microbial effects, primarily due to their strong antioxidative properties [125][126][127][128][125,126,127,128]. Some of the preferred mushroom species for extracting phenolic compounds include Agaricus brasiliensis (almond mushroom), Cantharellus cibarius (chanterelle), Lactarius indigo (indigo milk cap), Inonotus obliquus (chaga mushroom), and Melanoleuca cognate [126][129][130][131][126,129,130,131]. Table 4 provides a summary of representative phenolic compounds extracted from various mushroom species.Table 4.
Extractable phenolic compounds in mushrooms.
| Phenolic Compound Categories |
Phenolic Compounds |
Mushroom Sources | References |
|---|---|---|---|
| Phenolic acids | Ferulic acid | Agaricus brasiliensis, Agrocybe aegerita, Calocybe indica, Cantharellus cibarius | [126][127][[134][126132][133],127,132,133,134] |
| Gallic acid | Agaricus brasiliensis, Agrocybe aegerita, Calocybe indica, Cantharellus cibarius, Ganoderma lucidum, Pleurotus citrinopileatus, Pleurotus pulmonarius, Russula aurora | [126][130][132][133][134]138][126,130,132,133[135][136][,134137,135],136,137[,138] | |
| Cinnamic acid | Amanita crocea, Ganoderma lucidum, Pleurotus ostreatus, Suilus belinii | [135][139][140][141][135,139,140,141] | |
| Caffeic acid | Calocybe indica, Cantharellus cibarius, Hyphodontia paradoxa, Inonotus obliquus, Pleurotus citrinopileatus, Pleurotus pulmonarius, | [127][130][133][134][142][143][127,130,133,134,142,143] | |
| p-Coumaric acid | Agaricus brasiliensis, Agaricus subrufescens, Amanita crocea, Hyphodontia paradoxa, Laccaria amethystea, Melanoleuca cognate, Pleurotus ostreatus | [56][126][129][139][140][142][144][56,126,129,139,140,142,144] | |
| p-Hydroxybenzoic acid | Agaricus brasilensis, Amanita crocea, Cantharellus cibarius, Lactarius indigo, Lentinus edodes, Melanoleuca cognate, Suillus belinii | [126][129][134][138][139][141][126,129,134,138,139,141] | |
| Fumaric acid | Agaricus brasiliensis | [126] | |
| Vanillic acid | Morchella esculenta (L.) Pers., Russula emetic | [136][137][136,137] | |
| Syringic acid | Hyphodontia paradoxa, Morchella esculenta (L.) Pers. | [129][130][136][142][129,130,136,142] | |
| Protocatechuic acid | Agrocybe aegerita, Calocybe indica, Cantharellus cibarius, Hyphodontia paradoxa, Inonotus obliquus, Melanoleuca, Morchella esculenta (L.) Pers., Suillus belinii, Russula emetic | [129][130][132][133][134][136][137]137[141][142][129,130,132,133,134,136,,141,142] | |
| Rosmarinic acid | Hyphodontia paradoxa, Russula aurora, Russula emetic | [137][142][145][137,142,145] | |
| Flavonoids | Quercetin | Ganoderma lucidum, Laccaria amethystea, Pleurotus citrinopileatus, | [135][143][135,143] |
| Kaempferol | Ganoderma lucidum, Lactarius indigo | [135][146][135,146] | |
| Hesperetin | Calocybe indica, Ganoderma lucidum | [133][135][133,135] | |
| Naringenin | Calocybe indica, Ganoderma lucidum | [133][135][133,135] | |
| Catechin | Laccaria amethystea, Russula emetic | [137][144][137,144] | |
| Myricetin | Cantharellus cibarius, Lactarius indigo | [134][146][134,146] | |
| Procyanidin | Lactarius indigo | [146] | |
| Rutin | Pleurotus citrinopileatus, Russula emetic | [137][143][137,143] | |
| Tannins | Tannic acid | Agaricus silvaticus, Hydnum rufescens, Meripilus giganteus, Pleurotus citrinopileatus, Pleurotus ostreatus, Pleurotus tuber-regium(fries) | [147][148][149][147,148,149] |
| Tocopherols | α-Tocopherol | Agaricus bisporus, Boletus badius, Lepista inversa, Pleurotus ostreatus, Russula delica | [150][151][150,151] |
| β-Tocopherol | Laccaria laccata | [150] | |
| γ-Tocopherol | Clitocybe alexandri | [150] | |
| δ-Tocopherol | Lepista inversa | [150] |
