Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Georges Maestroni and Version 2 by Camila Xu.
The current view of the origin of melatonin (MLT), chemically defined as N-acetyl-5-methoxytriptamine, suggests that MLT appeared on earth about 2.5 billion years ago. Indeed, it is proposed that at that time, anaerobic bacteria developed the ability to synthesize MLT as an adaptative response to increasing concentrations of oxygen in the atmosphere.
- melatonin
- circadian rhythm
- immunomodulation
- anti-inflammatory effect
1. Introduction
The current view of the origin of melatonin (MLT), chemically defined as N-acetyl-5-methoxytriptamine, suggests that MLT appeared on earth about 2.5 billion years ago. Indeed, it is proposed that at that time, anaerobic bacteria developed the ability to synthesize MLT as an adaptative response to increasing concentrations of oxygen in the atmosphere. Thus, the very first function of MLT is considered to have been counteracting oxygen toxicity. These bacteria were then eventually phagocytized by eukaryotes where, according to the endosymbiotic theory, they established a symbiotic association and evolved in mitochondria or chloroplasts, retaining the ability to synthesize MLT [1]. This could explain why MLT may be synthesized in many, if not in all, extra-pineal organs. In mammals, MLT has been identified in skin, gastrointestinal tract, liver, kidney, the immune system, bone marrow, the testes, skeletal muscles and all body fluids [2]. In general, the concentration of extra-pineal MLT is reported to be several orders of magnitude higher than that of the circulating pool derived from the pineal gland. Nevertheless, it has recently been found that the gastrointestinal tract is not a major source of extra-pineal MLT, as was formerly believed [3][5]. It is, therefore, possible that some of the early studies in this field overestimated the MLT content because of methodological flaws.
Beyond being able to act as an antioxidant [4][6], during evolution, MLT acquired many other functions and became a pleiotropic molecule. In vertebrates, MLT has acquired the basic function of synchronizing the organism’s physiology with the 24-h environmental cycle (circadian rhythm) caused by the daily rotation of our planet. This vital effect is carried out by the circadian oscillation of MLT synthesis in the pineal gland accompanied by its immediate release into the blood circulation [5][7]. In contrast, extra-pineal MLT does not show any rhythmicity, and it is not secreted into the blood in significant amounts [2]. The environmental cue driving the MLT circadian rhythm is the light/dark cycle of the day. In particular, light is sensed in retinal ganglion cells through a photopigment called melanopsin that is activated by photons of 460–480 nm in wavelength (blue light). The ensuing nervous signal travels in the retino-hypothalamic tract and entrains the suprachiasmatic nucleus (SCN) of the hypothalamus, i.e., the central biological clock of the organism. In turn, the SCN activates a nervous pathway involving the paraventricular nucleus (PVN) of the hypothalamus, the intermediolateral cell column and the superior cervical ganglia (SCG) regulating MLT synthesis in the pineal gland [5][7]. Remarkably, blue light inhibits MLT synthesis; hence, MLT is synthesized and released during the darkness part of the daily photoperiod in all vertebrates [1][5][1,7], and this synchronization occurs in the organism in this part of the photoperiod by activating specific MLT receptors in both the SCN and peripheral biological clocks [1][5][1,7]. Basic biological rhythms such as the oscillations of the autonomic nervous system activity and the hypothalamic pituitary adrenal (HPA) axis are also driven by the SCN upon the synchronizing action of MLT [6][8].
2. MLT Receptors
Two high-affinity membrane receptors, MT1 and MT2, have been cloned from mammalian tissues, including human tissues. They share specific amino-acid sequences, indicating that they belong to a specific receptor subfamily [7][8][9,10]. Both are G-protein-coupled receptors (GPCR) activating similar signaling pathways, which result in the inhibition of adenyl cyclase and activation of phospholipase C influencing gene expression. However, GPCRs may also physically associate with intracellular proteins other than G proteins, and this may specifically allow the targeting of a cellular compartment with different outcomes. Furthermore, MT1 and MT2 may interact by forming heterodimers [9][11]. Another member of the MT1 and MT2 subfamilies is the melatonin-related receptor, also known as GPR50. This receptor is an orphan receptor because it does not bind MLT and its endogenous ligand is unknown. However, GPR50 can heterodimerize with both MT1 and MT2. Dimerization with MT1, but not with MT2, blocks MLT signaling [10][12]. A third low-affinity MLT binding site situated in the cytosol is the enzyme quinone reductase (QR2), whose activity may be related to the antioxidant and protective effects of MLT [9][11][11,13]. Finally, MLT can bind with the high-affinity calcium-binding proteins calmodulin and calretinin, thereby affecting the cell cycle [12][14], a phenomenon that has been related to the oncostatic action of MLT [13][15]. Due to its amphipathic nature, MLT can easily reach the cell nucleus and, according to many reports, bind members of the retinoid acid-related nuclear receptor (ROR) family [14][16]. ROR is strictly associated with the pleiotropic effect of MLT in various physiological and pathological processes, including circadian rhythms, immunity, inflammation, oxidative stress, and oncogenesis [14][16]. Studies have recently contested the conceptualization of RORs as MLT receptors [14][16]. However, the fact that both ROR and MLT are dependent on similar signaling pathways and have identical functions suggests that MLT can modulate ROR expression and function via indirect mechanisms involving MT1 and MT2 receptors or other mediators [14][16].3. MLT as an Antioxidant
Oxidative stress can be caused by excessive production of reactive oxygen species (ROS) or reduced activity of the antioxidant system. Oxidative stress is well known to increase inflammation and contribute to a variety of pathological conditions, including cancer, cardiovascular diseases, neurodegenerative diseases, lung diseases, renal diseases and aging. MLT is considered to be a major player in the antioxidant machinery because of its direct scavenging of ROS and its stimulation of antioxidant enzymes and suppression of pro-oxidant enzymes [15][17]. Its direct effect as a scavenger of free radicals has been clearly demonstrated in cell cultures, where MLT and its metabolites usually added at pharmacological concentrations may act by a variety of mechanisms, including electron transfer, hydrogen transfer and metal chelation [16][18]. However, it has been recently pointed out that in living organisms, the amount of substances that may react with MLT and its metabolites largely exceeds their concentration, even considering that the concentration of extra-pineal MLT is usually several orders of magnitude higher than that of plasma MLT [17][19]. This simple stoichiometric consideration casts some doubt on the conceptualization of MLT as an all-purpose in vivo scavenger of free oxygen or nitrogen radicals. However, a different interpretation suggests that MLT and its metabolites may act as stabilizers of the redox state of mitochondria when energy is produced via mitochondrial oxidative phosphorylation [16][18]. On the contrary, it is well recognized that the activation of MT1 and MT2 receptors enhances the expression of antioxidant enzymes such as superoxide dismutase, catalase, glutathione peroxidase and glutathione reductase [15][18][19][17,20,21]. In addition, the MLT binding to QR2 inhibits its enzymatic activity, reducing the generation of ROS [11][13].4. Melatonin and the Immune System
Few reports associated the pineal gland with the immune system [20][21][22,23] before 1986, when it was shown, for the first time, that MLT could increase antibody production in mice and counteract the immunosuppressive effect of corticosterone and/or restrain stress via an opiatergic mechanism [22][23][24][24,25,26]. These findings were reproduced in different experimental models [25][26][27,28], and, in general, the immunoregulatory properties of MLT have been further extended in a variety of animal models and in humans [27][28][29][30][31][32][33][34][35][29,30,31,32,33,34,35,36,37]. Nevertheless, the overall picture of the immunological action of MLT is quite confused. Several reports indicate that MLT is a powerful in vivo immunoenhancing factor, suggesting its use as a therapeutic agent whenever it is needed to boost humoral and/or cellular immune responses, while others endow MLT with an anti-inflammatory effect [27][28][29][30][31][32][33][34][35][29,30,31,32,33,34,35,36,37]. This apparent contradiction might be due to the wide range of concentrations and dosages used, possibly linked to the pleiotropic nature of the molecule or, more probably, to the fact that the immunological consequences of the circadian action exerted by MLT via its specific receptors were seldom discriminated from the other non-circadian effects [36][38]. It is well known that the immune system is under a circadian control exerted by the SCN, which drives the activities of the sympathetic nervous system (SNS) and the hypothalamo-pituitary adrenal (HPA) axis. Both the SNS and HPA axis convey circadian information to peripheral organs by regulating clock gene expression [37][38][39,40] and basic immunological functions, such as the blood circulation of immunocompetent cells, their infiltration into peripheral organs and the circadian oscillation of their specific functions [39][41]. In general, circulating cells peak in the blood during the resting phase of the photoperiod, while their migration into peripheral tissues occurs during the active phase. These phenomena are essential to ensure tissue homeostasis and activate the appropriate immune response in case of infection. For example, it has been reported that lymphocyte migration into lymph nodes peaked in the phase with dendritic cells (DC) at the beginning of the active phase to optimize antigen presentation and the ensuing adaptive immune response [40][42]. In this context, MLT and its receptors play a fundamental role due to their ability to synchronize the circadian output of the SCN and/or drive circadian rhythms directly in other brain structures [41][43]. Thus, the immunoenhancing action of MLT is linked to its circadian properties, including the receptor-mediated modulation of cytokine production, cell migration and antigen presentation to immunocompetent cells [42][43][44,45]. Last but not least, MLT may suppress the nuclear translocation of glucocorticoid receptors [44][46] and, hence, modulate their effect on immunity [45][47]. In conclusion, the available evidence, including exogenous administration and studies in pinealectomized animals [43][45], suggests that the immunoenhancing action of MLT is exerted at physiological or supraphysiological concentrations via the activation of its specific receptors (Figure 1).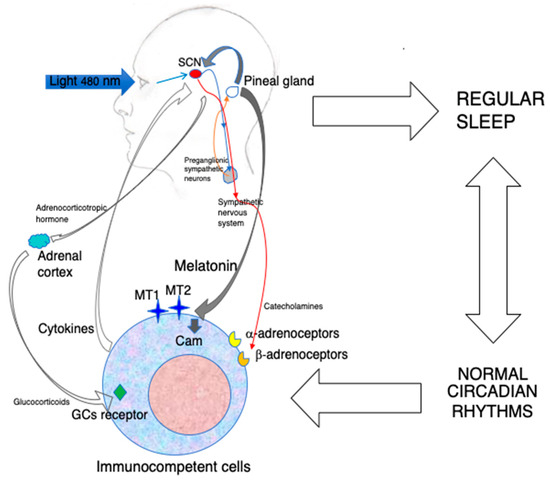
Figure 1.
The indirect and direct immunoenhancing action of MLT.
5. MLT and Viral Infections
The first evidence of an antiviral activity of MLT was shown against encephalomyocarditis virus (ECMV), a highly pathogenic virus that produces encephalitis and myocarditis in rodents. Exogenous MLT could prevent the paralysis and deaths of mice infected with EMCV [55][57]. Other encephalitogenic viruses proved to be affected by MLT. Normal mice were infected with the Semliki Forest virus (SFV), and stressed mice were injected with the attenuated non-invasive West Nile virus (WNV). SFV can produce viral encephalitis in normal mice, while the attenuated form of WNV can do it only in immunosuppressed mice. In both models, the administration of MLT significantly postponed the onset of the disease and reduced mortality [56][58]. A similar effect was then reported in mice infected with Venezuelan equine encephalomyelitis virus (VEEV) [57][59]. The protective effect of MLT in this model was shown to depend on increased IL-1β production, as it was abolished by IL-1β neutralization [58][60]. An inverse correlation between MLT and IL-12 plasma levels and disease progression has been described in HIV-1-infected individuals, suggesting a direct relationship between MLT and Th1 cell function [59][61]. MLT has also proven to be effective against respiratory syncytial virus (RSV). The in vitro infection of macrophages with RSV-activated TLR3 and NFkB and the subsequent inflammatory response was also identified. In this model, MLT was able to inhibit the response by suppressing NFkB activation [60][62]. This effect was reproduced in mice infected with RSV, where MLT could inhibit lung oxidative stress [61][63]. The anti-inflammatory and regenerative effects of MLT were also evident in rabbit with fulminant hepatitis of viral origin [62][64]. In the same model, another study showed that MLT could inhibit mitophagy and the innate immune response while restoring the circadian dysregulation induced by the virus, recommending the use of MLT as a therapeutic option in human fulminant hepatic failure [63][65]. In an in vitro model of Hemorrhagic Shock Syndrome caused by the EBOLA virus, MLT was highlighted as a promising therapeutic agent because of its ability to neutralize endothelial cell disruption [64][66]. With the advent of the COVID-19 pandemic, an impressive number of studies have tested MLT for possible therapeutic and prophylactic effects. A PubMed search conducted with the terms MLT and COVID-19 retrieved 138 publications, including many clinical randomized studies. However, in spite of this outsized number of publications, it is difficult to draw any definite conclusion about the therapeutic efficacy of MLT in COVID-19 patients. In fact, there are reports showing the positive therapeutic effect of exogenous MLT [65][66][67][68][69][70][67,68,69,70,71,72], while others deny any effect [71][72][73][73,74,75]. Amid the beneficial effects exerted by MLT against COVID-19, it can be foud that the prevention of complications and reduction in mortality in severely ill patients [67][70][69,72], improvement in respiratory symptoms via the reduction in the lungs’ involvement [69][71] and reduced requirement for invasive mechanical ventilation, as well as overall improvement in clinical status [70][72]. On the other hand, a randomized retrospective study negates any effect of MLT on survival of COVID-19 patients [71][73], and another contemporaneous randomized clinical trial reached the same conclusion [72][74]. Perhaps this drastic discrepancy is due to the wide array of doses and treatment schedules used in these studies which continue to perpetuate misconceptions about the real therapeutic properties of MLT. For example, MLT has been administered once per day in the evening at a 10 mg dose for 14 days [67][69] or twice per day without mentioning the timetable at a dose-pro-dose of 3 mg [67][69] or 5 mg [68][70] in these studies. In the rationale of the studies, no authors considered a possible distinction between the circadian and non-circadian effects of MLT that could be related to its conceivable therapeutic effect against SARS-CoV-2. Most studies just mentioned, in a general fashion, the immunomodulatory and anti-inflammatory effects of MLT. Moreover, some new and peculiar mechanisms of action have been highlighted to explain the observed effects of MLT. Thus, the influence of MLT on the pathogenic enzyme p21-activated kinase 1, whose activation is involved in a variety of pathological conditions including viral infections [65][67], cluster differentiation 147 [66][68], viral phase separation and epitranscriptomics [68][70] and the coagulation system [67][69], has been reported. The emergency linked to the COVID-19 pandemic has somewhat boosted interest in the putative antiviral potential of MLT, generating studies about its effects on influenza infections. Even in this case, MLT has been administered at very high doses and, in some cases, with treatment schedules ignoring completely its circadian nature. A report claims that MLT ameliorates influenza A H1N1 infection in mice by virtue of its ability to inhibit pro-inflammatory cytokines while enhancing the anti-inflammatory cytokine IL-10. MLT was administered subcutaneously either 6 h before infection and/or 2, 4 and 6 days post-infection at a concentration of 200 mg/kg b.w. without specifying any timetable [74][76]. In another study of mice infected with influenza A H3N2, MLT was administered intraperitoneally at 30 mg/kg b.w. for 7 days in the evening. In this case, MLT was proved to reduce pulmonary damage, leukocyte infiltration and edema and switch the polarization of alveolar macrophages from the M1 to the M2 phenotype [75][77]. A third study provided the interesting observation that MLT-deficient mice show a significantly higher mortality rate in comparison to their wild-type counterpart after infection with influenza A H1N1 virus. In other experiments, BALB/c mice were pretreated for 3 days via the intranasal administration of MLT (3, 10 and 30 mg/kg b.w.) before virus inoculation. The MLT-treated animals were apparently significantly protected from the virus by the suppression of mast cell activation and inhibition of cytokine storm [76][78]. In particular, it seems rather problematic to combine the interesting observation of an augmented vulnerability to influenza infection of MLT-deficient mice with the effects of exogenous MLT administered at very high doses and by extremely different treatments. Table 1 shows the accessible preclinical studies investigating the possible therapeutic effects of MLT against viral diseases.Table 1.
In vivo preclinical studies of the antiviral activity of MLT.
| Pathogen | Species | MLT Dose | Treatment | Outcome | Ref. |
|---|---|---|---|---|---|
| EMCV | Mice | 1 μg/mouse | 10 days at 4 pm | Reversal of stress-induced death | [55][57] |
| SFV | Mice | 500 μg/kg | From 3 days before until 10 days after infection at 4 pm | Increased survival and decreased viremia | [56][58] |
| aWNV | Mice | 5 μg/mouse | From 2 days before until 8 days after infection at 4 pm | Reduced mortality | [56][58] |
| VEEV | MIce | 1 mg/kg | From 3 days before until 10 days after infection at 6 pm | Increased survival, decreased viremia and increased antibody response | [57][59] |
| RSV | Mice | 5 mg/kg | Twice daily for 3 days | Reduced oxidative damage of the lung | [61][63] |
| RHDV | Rabbits | 20 mg/kg | 0, 12 and 24 h after infection | Decreased mitophagy, inflammation and innate immunity | [62][63][64,65] |
| H1N1 | Mice | 3, 10, 30 mg/kg | Pretreatment for 3 days before infection | Decreased lung injury by the inhibition of mast cells and cytokine storm | [76][78] |
| H1N1 | Mice | 200 mg/kg | 6 h before and 2, 4 and 6 days post-infection | Inhibition of pro-inflammatory cytokines and stimulation of IL-10; synergy with an antiviral drug |
[74][76] |
| H3N2 | Mice | 30 mg/kg | For 7 days at 6 pm | Attenuated pulmonary damage, leukocyte infiltration and edema | [75][77] |
The features of the existing preclinical studies of the possible therapeutic effects of MLT in viral infections are reported. EMCV: encephalomyocarditis virus; SFV: Semliki Forest virus; aWNV: attenuated West Nile virus; VEEV: Venezuelan equine encephalitis virus; RSV: respiratory syncitial virus; RHDV: rabbit hemorrhagic disease virus; H1N1, influenza A H1N1; H3N2: influenza A H3N2.
It seems noteworthy to make the observation that early studies used supraphysiological doses of MLT administered according to a circadian schedule, while the recent ones employed high pharmacological doses and typically did not follow any circadian administration. Probably, this divergence reflects a different conceptual approach connected to the MLT property to be exploited in fighting the infection. In the initial studies, the authors investigated whether the immunoenhancing action could be used to fight the disease, while in the latest ones, the authors mostly focused on the antioxidant and anti-inflammatory effects.
6. MLT and Bacterial Infections
The first evidence suggesting that MLT could influence the outcome of bacterial infection was its protective effect in an animal model of septic shock. A single injection of MLT, a few hours after intraperitoneal inoculation of a lethal dose of LPS in mice, was able to protect the animals. The doses used were 1, 2, 3, 4, 5 and 10 mg/kg b.w., and the protective effect, which involved a reduction in NO synthesis, was significant in the 2–5 mg range but lost at 10 mg [77][79]. This finding was then confirmed and extended in a variety of animal models and in humans with sepsis [78][80]. In particular, MLT could ameliorate the clinical status and increase the survival of human newborns with sepsis [77][79]. Doses and treatment schedules ranged from oral administration of two single doses of 10 mg within 12 h of the diagnosis of sepsis to one injection of 20 mg/kg in septic newborns treated with antibiotics. In general, the effect of MLT is suggested to depend on the suppression of pro-oxidant and pro-inflammatory pathways [78][79][80,81]. However, a recent study shows that polymicrobial sepsis in mice enhanced the expression of MT2 receptors in neutrophils and MLT administration protected the mice by enhancing the bactericidal effects of neutrophils [80][82]. In this study, MLT was used at massive doses of 50 mg/kg in vivo and 100 μg/mL in vitro [80][82]. Another study reports that in mice exposed to short photoperiods and infected with Staphylococcus aureus or Escherichia coli, MLT administration at 10 mg/kg resulted in the improved clearance of bacteria from blood [81][83]. A further in vitro study using porcine macrophages claims that the impracticable concentration of 1 mM of MLT may improve the bacterial clearance of enterotoxigenic Escherichia coli and suggests that MLT is important for controlling this type of infection [82][84]. Similarly, in a model of Escherichia coli meningitis, mice were treated for 7 days with MLT at 10, 30 and 60 mg/kg, and the claim was that MLT may prevent meningitis by acting on the intestinal microbiota [83][85]. Also, bacterial mastitis and infection with Klebsiella pneumoniae are among the bacterial diseases in which MLT is suggested to exert a therapeutic action by virtue of its anti-inflammatory and antioxidant effects [84][85][86,87]. The doses and concentrations of MLT used in these studies are in line with those in the above-reported citations. Antimicrobial resistance is a growing emergency in public health. In particular, the transferable resistance–nodulation–division efflux pump TMexCD1-TOprJ1, conferring resistance to tigecycline, is becoming a serious health problem. A potentially very interesting and novel approach for combatting resistance to tigecycline used MLT, either in vitro or in vivo, in an infection model using tmexCD1-toprJ1-positive Klepsiella pneumoniae with encouraging results [86][88]. However, even in this study, MLT was used at extremely high concentrations (2–8 mg/mL) and doses (50 mg/kg) [86][88]. Table 2 lists the preclinical in vivo studies of the effects of MLT on bacterial infections.Table 2.
Preclinical and clinical studies on the anti-bacterial effects of MLT.
| Pathogen | Species | MLT dose | Treatment | Outcome | Ref. |
|---|---|---|---|---|---|
| Lethal dose of LPS | Mice | 1, 2, 3, 4, 5 and 10 mg/kg | 3 or 6 h after LPS injection | 2, 3, 4 and 5 mg/kg reduced mortality and NO synthesis | [77][79] |
| Sepsis | Human Newborns |
2 × 10 mg | Oral administration within 12 h after diagnosis | Increased survival and improved clinical status | [78][80] |
| Sepsis | Human newborns | 20 mg/kg | One injection plus antibiotics | Increased survival and improved clinical status | [79][81] |
| Polymicrobial sepsis | Mice | 50 mg/kg | Two doses, 30 min before and 30 min after cecal ligation puncture | Protection of mice via the induction of neutrophil extracellular trap | [80][82] |
| Staphylococcus aureus and Escherichia coli | Mice | 10 mg/kg | Once daily for 7 days | Improved clearance of bacteria from blood, reduced iNOS, plasma C-reactive protein and COX2 expression in the hypothalamus. | [81][83] |
| Escherichia coli | Mice | 30 mg/kg | Pretreatment for 7 consecutive days before infection | Prevention of and protection from bacterial meningitis by modulating the intestinal microbiota | [83][85] |
| Tigecyclin resistant Klebsiella pneumoniae | Mice | 50 mg/kg | One dose after infection | Restoring tigecycline activity | [86][88] |
The available preclinical and clinical studies of the therapeutic effects of MLT against bacterial infections are reported. NO: nitric oxide; iNOS: inducible nitric oxide synthetase; COX2: cyclooxygenase
As for viral diseases, also in these studies, the rationale for using gigantic amounts of MLT is not mentioned. It is somewhat surprising that in most studies, the well-known idea that circadian rhythms influence the outcome of and the susceptibility to infections [87][89] was completely ignored. In addition, both viral and bacterial infections may disrupt the circadian machinery [87][89], but whether such effects involve the immune–pineal axis or are exerted only on peripheral circadian clocks is still obscure. In my opinion, this is probably the crucial point that has to be carefully pondered in future studies aimed at improving the therapeutic approach for the use of MLT in infectious diseases.
