Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by Miriam Dellino.
Human genital papilloma virus infection is the most prevalent sexually transmitted infection in the world. It is estimated that more than 75% of sexually active women contract this infection in their lifetime. In 80% of young women, there is the clearance of the virus within 18–24 months.
- human papilloma virus
- oral cavity infection
- genital infection
- HPV-related cancer
- squamous cell carcinoma
1. Oral–Cervical–Perinatal Human PVapilloma Virus Transmission
The extragenital sites, the oral and the anal area, each exhibit distinct probabilities of transmission, influenced by factors such as sexual relationships, duration of sexual relations, and the specific number of different acts within a partnership [25][1]. Additionally, fingers have been identified as a possible source of transmission or self-inoculation of human papilloma virus (HPV) to the oral cavity [25][1]. In men, oral HPV infection is significantly correlated with urinary HPV infection, with HPV16 being the most common type [20][2]. Similarly, the majority of female patients have concordant oral and urinary HPV types [20][2]. With regard to oral–genital transmission, although HPV is known to have the potential to affect the intimate lives of women and their partners, it has rarely been explored in the literature [16][3]. In fact, oral transmission of HPV and, consequently, the risk of oral cancer, increases in women with cervical cancer and their spouses [16][3]; this finding suggests a cross-transmission between the mouth and genitals. However, data in this regard are discordant, with only a few studies reporting concordant rates of oral and cervical HPV infection above 65% [26][4] (Figure 1). Among these, a recent study in Italian women compared the prevalence and concordance of HPV infections between the oropharynx and genital sites, reporting that the prevalence of oral HPV infections was high among women with concomitant genital HPV infections (22%) compared to genital HPV-negative women (0%) [26][4]. HPV16 was the most common high-risk genotype at both sites, and there was low concordance between HPV genotypes at the two anatomical sites (kappa = 0.125) [16][3]. On the other hand, other studies report that the overall prevalence of oral HPV infections is varied, with values ranging from 0.2% to 20.7%, and rates of concomitant infections with oral and cervical HPV are reported in less than 10% of cases [16][3]. The variability of these data can be attributed to different inclusion criteria, clinical contexts, sampling methods, and detection tests. In none of the women with oral HPV did wresearchers find relevant clinical lesions [16][3]. Therefore, an oral examination alone cannot exclude the possibility of an oral HPV infection, which is primarily subclinical. In addition, a multivariate analysis showed that oral infection in women with HPV-positive genitalia was significantly related to age and sexual habits, particularly oral sex [27][5]. In fact, it is highlighted that there is a higher incidence of HPV in the oral cavity in women aged between 36 and 50 years compared to patients under the age of 25 years [28][6]. Regarding the frequency of sexual intercourse, the probability of being positive for oral HPV increased by 77% if women had sexual intercourse more than 10 times a month (95% CI: 2.67, 2223.04), compared to those who had intercourse 0–1 times [16][3]. A study by Hemminki K et al. suggested a significant association between oral sex and oral HPV; among women who engaged in sexual activity “occasionally”, there was an 18.29% increase in the chances of testing positive for oral HPV compared to those who did not [29][7]. Furthermore, husbands of women with cervical cancer have two times the risk of tonsillar cancer. Transmission dynamics are influenced by sex, with female genitalia having a higher rate of transmission than male genitalia [30][8]. The keratinized penis epithelium is more resistant to HPV infection, and the amount of biological fluid that reaches the oropharynx can also be a factor in this gender difference [30][8]. Finally, it appears that women develop a stronger systemic immune response than men after a genital HPV infection [31][9]. This would more efficiently protect women than men in the case of subsequent exposure to HPV. In women aged 18 to 69 years, data from oral and cervicovaginal HPV DNA screening showed that cervicovaginal HPV infection was present in 45.2%, oral HPV infection in 4.1%, double infection in 3.0%, and concordant infection in 1.1%. Nearly half of U.S. men have HPV infection of the penis [31][9]. High burden of HPV infection of the penis has been associated with oral HPV infection. In cases of sexual intercourse with only one homosexual female partner, the risk of oral HR-HPV infection was 19.3% if the partner had a genital infection, increasing to 22.2% if oral sex was performed with two or more homosexual female partners. The incidence of oral HPV among young heterosexual men was lower than in same-sex relationships yet increased with a greater number of partners (17.9%), in those who engaged in oral sex (28.6%), and in partners with oral or genital infections (11.5%) [32][10]. In addition, prevalence increased with the frequency of oral sex among men whose partner had a genital infection with the same type of HPV, supporting evidence that oral HPV can be transmitted orally–orally or orally–genitally [32][10]. In fact, studies report how the low overall prevalence of specific genotype concordance across anatomical sites can be explained by variation in susceptibility to HPV infection, differences in exposure characteristics, and disparities in the natural history at each mucosal site [33][11]. Additionally, other carriers of infection and the frequency and early age of oral sex appears to contribute significantly to the probabilities of HPV-OSCC [33][11]. Notably, men and women with genital wart-like lesions represent a high-risk population, with 64.6% of patients with condyloma acuminata showing HPV types associated with an increased risk of dysplasia. The vast majority of these patients had HPV6 DNA detected at the wart site and also at other sites [20][2].
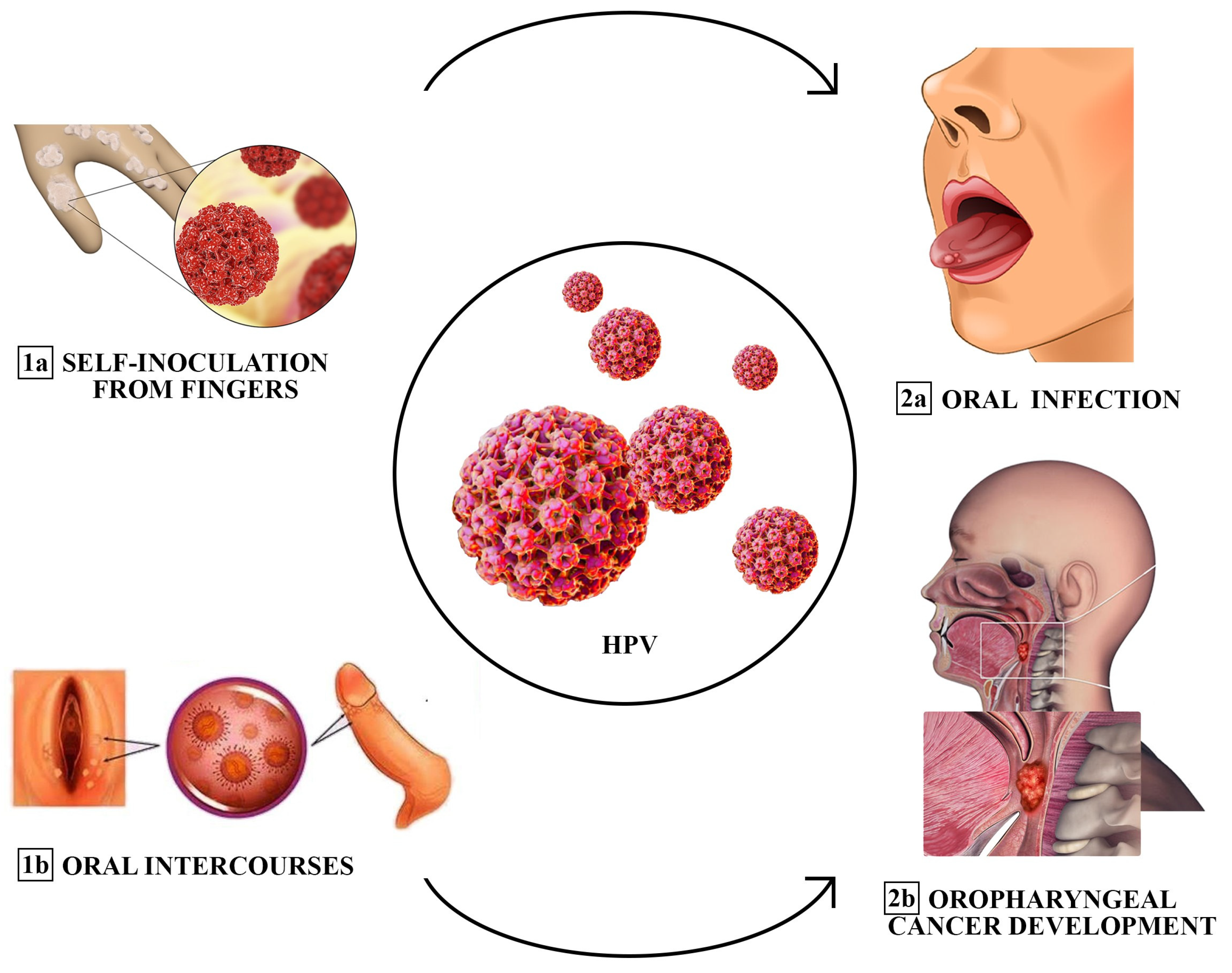
Figure 1. Different transmission modes of oral HPV infection (sequentially ordered: from 1a and 1b to 2a and 2b). Fingers have been described as a source of transmission or self-inoculation of HPV to the oral cavity. Oral sex could be another predominant cause of infection among adults. Risk factors associated with the development of the infection include promiscuity, prior diagnosis of sexually transmitted diseases (STDs), and a high frequency of oral intercourse.
2. The Role of Oral and Vaginal Microbiota in Viral Oncogenesis
There is abundant evidence demonstrating how the alteration of vaginal or oral microbiota in patients with high-risk HPV can favor the onset of both cervical and oral cancer, respectively [71][12]. Particularly, there is evidence that HPV16 can integrate with host-cell DNA and activate oncogenes, and oral dysbiosis and synergistic effects in oral microbial communities may promote cancer development [72][13]. This is because different microorganisms in the oral cavity interact with each other instead of existing on their own and adhere together to form a microbial community through aggregation and coaggregation. A recent review article lists cancer as one of the comorbidities of periodontal disease; according to a case-control study, patients with periodontitis had 3.7 times the risk of developing oral cancer compared to controls, indicating a potential link between periodontitis and oral tumors [73][14]. In particular, microbial dysbiosis can act as a bridge between periodontitis and oral tumors. Several studies have shown substantial differences in bacterial diversity and the relative abundance of certain bacteria in the oral flora of cancer patients compared to controls with high-throughput sequencing techniques [74][15]. Several studies have reported the possibility of using oral swabs to analyze the microbiota on the surface of OSCC lesions and have found an increase in microbial diversity (alpha diversity) [75][16]. In addition, there was an increase in Fusobacterium and a decrease in Streptococcus at the tumor site since some authors examined tissue biopsy samples from OSCC patients and found that Prevotella, Corynebacterium, Pseudomonas, Deinococcus, and Noviherbaspirillum were enriched in tumor tissues, while Actinomyces, Sutterella, Stenotrophomonas, Anoxybacillus, and Serratia were significantly less abundant. Another study focused on microbiota changes in the saliva of OSCC patients and found that Prevotella melaninogenica, Fusobacterium sp., Prevotella pallens, Dialister, Streptococcus anginosus, Prevotella nigrescens, Campylobacter ureolyticus, Prevotella nanceiensis, and Peptostreptococcus anaerobius showed greater abundance than normal. Another study reports that at the genus level, the abundance of Fusobacterium periodonticum, Parvimonas micra, Streptococcus constellatus, Haemophilus influenzae, and Filifactor alocis was positively correlated with OSCC progression, while the abundance of Streptococcus mitis, Haemophilus parainfluenzae, and Porphyromonas pasteri was negatively correlated with the progression of OSCC [76][17]. Consequently, the results obtained from various tissue sources and sampling techniques varied. In terms of bacterial diversity, various sampling strategies produced contradictory results. In saliva and tissue biopsies of OSCC patients, bacterial diversity decreased, but bacterial diversity increased in oropharyngeal swab samples from tumor surfaces [77][18]. In addition, the oral microbiome alters as the cancer progresses. In fact, some studies found that the abundance of fusobacteria increased steadily with the advancement of cancer from stage 1 (4.35%) to stage 4 (7.92%), compared to 2.98% in healthy controls. Furthermore, age affects the microbiome of OSCC patients.
Yu et al. analyzed the microbiota of 20 older patients (>60 years) and 20 younger patients (50 years) using 16S rRNA sequencing. They found that the younger group was significantly enriched in Ralstonia, Prevotilla, and Ochrobactrum, while Pedobacter was more abundant in the older group. This suggests that the role of bacteria in promoting OSCC may differ between older and younger populations [78][19]. Iron-ion transport, tryptophanase activity, peptidase activity, and superoxide dismutase increase considerably in the microbiota of OSCC patients compared to healthy controls and predict, to some extent, the carcinogenic mechanism [79][20]. Similarly, Stashenko et al. analyzed the metabolism of oral microbes in mice with OSCC and found that the microbiota was overexpressed in OSCC-associated metabolic pathways, including nitrogen transport, stress response, interspecies interactions, modulation of the Wnt pathway, and amino acid and lipid biosynthesis. Despite the clear link between oral microbiota dysbiosis and oral cancer, a precise mechanism of carcinogenesis has not yet been revealed. The 16s rRNA sequencing has been widely used for the analysis of oral microorganisms in cancer patients and controls [80][21]. However, due to differences in sampling sites, sampling methods and data processing, sequencing results have been inconsistent between studies, which, to some extent, reduced the reliability of sequencing results [80][21]. Therefore, a standardized technique is needed in the future to regulate sampling procedures. Chronic infection is considered a risk factor in the development of cancer [81][22]. Evidence suggests that periodontal pathogens, such as Porphyromonas gingivalis, Fusobacterium nucleatum, and Treponema denticola, are associated with OSCC [81][22]. They can stimulate tumorigenesis by promoting epithelial cell proliferation while inhibiting apoptosis and regulating the inflammatory microenvironment. Moreover, P. gingivalis is a known keystone pathogen related to chronic periodontitis. Clinical studies found that P. gingivalis was widely present in gingival squamous cell carcinoma tissue compared to normal gum tissue, suggesting a potential association between P. gingivalis and squamous cell gum cancer [82][23]. Indeed, in a specific oral microbiome, the interactions of microbial communities can also influence oral cancer. Polymicrobial synergy and dysbiosis are the etiology of periodontal disease. Moreover, the development of periodontal biofilm is similar to cancerous human cell communities. Furthermore, research into the mechanism of T. denticola-induced cancer is still at a very early stage. Recent research indicates that T. denticola directly promotes OSCC cell proliferation, as it has been found to promote OSCC migration by activating crosstalk between TLR/MyD88 and Integrin alfa V/FAK [83][24]. In short, T. denticola and Td-CTLP provide a microenvironment of tumor tissue conducive to invasion and metastasis. Candida, especially Candida albicans, is a typical oral component. Candida albicans is an opportunistic pathogen carried by about 80% of the general population and is harmful to immunocompromised individuals (HIV, cancer, or transplant patients) [84][25]. Regarding the association between Candida and tumors, it is a well-known fact that leukoplakia of the oral mucosa infected with Candida is more likely to progress to cancer than uninfected leukoplakia. Although Candida, particularly Candida albicans, has been highly related to oral cancer based on epidemiological studies, the molecular mechanism underlying Candida albicans-induced carcinogenesis remains controversial. Colonization of the host epithelium by Candida albicans depends on the imbalance between the virulence components of Candida albicans and the host defenses. Some mechanisms have been hypothesized for invasion and colonization of epithelial cells by Candida albicans [85][26]. The first mechanism is catabolic enzymes released by Candida albicans, such as aspartic proteases, which can degrade the subendothelial extracellular matrix as well as laminin 332 and E-cadherin, thus allowing the mycelium to penetrate the host cell or migrate through cells [86][27]. Another mechanism is that Candida albicans induces cellular endocytosis by secreting Als3, an invasion that binds to E-cadherin on host cells [87][28]. Moreover, a recent study determined that Candida albicans infection promoted oral cancer incidence in a 4-nitroquinoline 1-oxide (4NQO)-induced mouse tongue carcinogenesis model and promoted OC progression in a tongue tumor-bearing mouse model (C3H/HeN-SCC VII). Indeed, the tumor-associated macrophage (TAMs) infiltration was elevated during Candida albicans infection. Meanwhile, the attracted TAMs polarized into M2-like macrophages with high expression of programmed death ligand 1 (PD-L1) and galectin-9 (GAL-9) [87][28]. In addition, about 10% of Candida albicans isolates from the cervix and oral specimens produce gliotoxin and other mycotoxins that target and eliminate T lymphocytes, especially IFN-γ producers [88][29].
3. Vaccination Prophylaxis and Cancer Prevention
The implementation of primary prevention aims to reduce the burden of HPV infection and HPV-related disease [89][30]. There are five approved HPV vaccines, three of them in Europe, which prophylactically prevent HPV infection, despite each of them targeting different strains [90][31]. With more than 200 million doses administered to date, HPV vaccines are considered to be safe and effective in preventing HPV-related infections and cancers [89][30]. Therefore, in 2020, the FDA in the United States and the EMA in Europe extended the use of HPV vaccines to males [91][32]. Moreover, anti-HPV vaccines are also recommended in order to successfully counteract increasing incidence of OSCC [92][33], although time is needed in order to study the specific benefits of HPV vaccines for OSCC given the long interval between HPV infection and the development of oropharyngeal tumors. On the other hand, HPV vaccines do not increase clearance in patients with ongoing HPV infection [93][34]. Regarding secondary prevention, surprising data are arising. Indeed, current studies have focused on the role of vaccination after conservative surgery for high-grade cervical dysplasia. In particular, the study by Gherlardi et al. showed that quadrivalent HPV vaccination injected 30 days after conization reduced the risk of subsequent recurrence by 81.2% (95% CI, 34.3–95.7), irrespective of causal HPV type [94][35]. This does not imply a therapeutic effect of the vaccines but underlines their role as an adjuvant to surgical treatment. Nevertheless, a randomized placebo-controlled study, with a larger number of patients, would be required to confirm these findings. In the meantime, it is important to attract the younger population to improve HPV awareness and to educate them as to the importance of primary prevention to enhance the widespread adoption of HPV vaccination.
References
- Dzundova, M.N.; Sehnal, B.; Zikan, M.; Kocian, R.; Dubova, O.; Hubka, P.; Dostalek, L.; Kabele, P.; Brtnicky, T.; Slama, J. Risk Factors for the Anal and Oral Human Papillomavirus (HPV) Infections among Women with Severe Cervical Lesions: A Prospective Case-Control Study. Biomedicines 2023, 11, 3183.
- Wierzbicka, M.; San Giorgi, M.R.M.; Dikkers, F.G. Transmission and clearance of human papillomavirus infection in the oral cavity and its role in oropharyngeal carcinoma—A review. Rev. Med. Virol. 2023, 33, e2337.
- Bruno, M.T.; Boemi, S.; Caruso, G.; Sgalambro, F.; Ferlito, S.; Cavallaro, A.; Sudano, M.C.; Palumbo, M. Oral HPV Infection in Women with HPV-Positive Cervix Is Closely Related to Oral Sex. Diagnostics 2023, 13, 2096.
- Kalinganire, N.; Uwineza, A.; Kyokunda, L.; Banura, C. Oral-genital HPV infection transmission, concordance of HPV genotypes and genital lesions among spouses/ partners of patients diagnosed with HPV-related head and neck squamous cell carcinoma (HNSCC): A scoping review. Infect. Agent. Cancer 2023, 18, 63.
- Cossellu, G.; Fedele, L.; Badaoui, B.; Angiero, F.; Farronato, G.; Monti, E.; Liverani, C.A.; Gorni, C.; Botti, S. Prevalence and concordance of oral and genital HPV in women positive for cervical HPV infection and in their sexual stable partners: An Italian screening study. PLoS ONE 2018, 13, e0205574.
- Candotto, V.; Lauritano, D.; Nardone, M.; Baggi, L.; Arcuri, C.; Gatto, R.; Gaudio, R.M.; Spadari, F.; Carinci, F. HPV infection in the oral cavity: Epidemiology, clinical manifestations and relationship with oral cancer. Oral Implantol. 2017, 10, 209–220.
- Hemminki, K.; Dong, C.; Frisch, M. Tonsillar and other upper aerodigestive tract cancers among cervical cancer patients and their husbands. Eur. J. Cancer Prev. 2000, 9, 433–437.
- Balaji, R.; MacCosham, A.; Williams, K.; El-Zein, M.; Franco, E.L. Directionality of Genital Human Papillomavirus Infection Transmission Within Heterosexual Couples: A Systematic Review and Meta-analysis. J. Infect. Dis. 2020, 222, 1928–1937.
- Giuliani, E.; Rollo, F.; Dona, M.G.; Garbuglia, A.R. Human Papillomavirus Oral Infection: Review of Methodological Aspects and Epidemiology. Pathogens 2021, 10, 1411.
- Dahlstrom, K.R.; Burchell, A.N.; Ramanakumar, A.V.; Rodrigues, A.; Tellier, P.P.; Hanley, J.; Coutlee, F.; Franco, E.L. Sexual transmission of oral human papillomavirus infection among men. Cancer Epidemiol. Biomarkers Prev. 2014, 23, 2959–2964.
- Nunes, E.M.; Lopez, R.V.M.; Sudenga, S.L.; Gheit, T.; Tommasino, M.; Baggio, M.L.; Ferreira, S.; Galan, L.; Silva, R.C.; Lazcano-Ponce, E.; et al. Concordance of Beta-papillomavirus across anogenital and oral anatomic sites of men: The HIM Study. Virology 2017, 510, 55–59.
- McKee, K.S.; Carter, K.A.; Bassis, C.; Young, V.B.; Reed, B.; Harper, D.M.; Ruffin, M.T.t.; Bell, J.D. The vaginal microbiota, high-risk human papillomavirus infection, and cervical cytology: Results from a population-based study. Gynecol. Pelvic Med. 2020, 3, 18.
- Constantin, M.; Chifiriuc, M.C.; Mihaescu, G.; Vrancianu, C.O.; Dobre, E.G.; Cristian, R.E.; Bleotu, C.; Bertesteanu, S.V.; Grigore, R.; Serban, B.; et al. Implications of oral dysbiosis and HPV infection in head and neck cancer: From molecular and cellular mechanisms to early diagnosis and therapy. Front. Oncol. 2023, 13, 1273516.
- Komlos, G.; Csurgay, K.; Horvath, F.; Pelyhe, L.; Nemeth, Z. Periodontitis as a risk for oral cancer: A case-control study. BMC Oral Health 2021, 21, 640.
- Inchingolo, F.; Inchingolo, A.M.; Malcangi, G.; De Leonardis, N.; Sardano, R.; Pezzolla, C.; de Ruvo, E.; Di Venere, D.; Palermo, A.; Inchingolo, A.D.; et al. The Benefits of Probiotics on Oral Health: Systematic Review of the Literature. Pharmaceuticals 2023, 16, 1313.
- Peter, T.K.; Withanage, M.H.H.; Comnick, C.L.; Pendleton, C.; Dabdoub, S.; Ganesan, S.; Drake, D.; Banas, J.; Xie, X.J.; Zeng, E. Systematic review and meta-analysis of oral squamous cell carcinoma associated oral microbiome. Front. Microbiol. 2022, 13, 968304.
- Osman, M.A.; Neoh, H.M.; Ab Mutalib, N.S.; Chin, S.F.; Mazlan, L.; Raja Ali, R.A.; Zakaria, A.D.; Ngiu, C.S.; Ang, M.Y.; Jamal, R. Parvimonas micra, Peptostreptococcus stomatis, Fusobacterium nucleatum and Akkermansia muciniphila as a four-bacteria biomarker panel of colorectal cancer. Sci. Rep. 2021, 11, 2925.
- Gopinath, D.; Menon, R.K.; Wie, C.C.; Banerjee, M.; Panda, S.; Mandal, D.; Behera, P.K.; Roychoudhury, S.; Kheur, S.; Botelho, M.G.; et al. Differences in the bacteriome of swab, saliva, and tissue biopsies in oral cancer. Sci. Rep. 2021, 11, 1181.
- Yu, H.J.; Jing, C.; Xiao, N.; Zang, X.M.; Zhang, C.Y.; Zhang, X.; Qu, Y.N.; Li, Y.; Tan, Q.W. Structural difference analysis of adult’s intestinal flora basing on the 16S rDNA gene sequencing technology. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 12983–12992.
- Granato, D.C.; Neves, L.X.; Trino, L.D.; Carnielli, C.M.; Lopes, A.F.B.; Yokoo, S.; Pauletti, B.A.; Domingues, R.R.; Sa, J.O.; Persinoti, G.; et al. Meta-omics analysis indicates the saliva microbiome and its proteins associated with the prognosis of oral cancer patients. Biochim. Biophys. Acta Proteins Proteom. 2021, 1869, 140659.
- Sarkar, P.; Malik, S.; Laha, S.; Das, S.; Bunk, S.; Ray, J.G.; Chatterjee, R.; Saha, A. Dysbiosis of Oral Microbiota During Oral Squamous Cell Carcinoma Development. Front. Oncol. 2021, 11, 614448.
- Li, R.; Xiao, L.; Gong, T.; Liu, J.; Li, Y.; Zhou, X.; Li, Y.; Zheng, X. Role of oral microbiome in oral oncogenesis, tumor progression, and metastasis. Mol. Oral Microbiol. 2023, 38, 9–22.
- Wen, L.; Mu, W.; Lu, H.; Wang, X.; Fang, J.; Jia, Y.; Li, Q.; Wang, D.; Wen, S.; Guo, J.; et al. Porphyromonas gingivalis Promotes Oral Squamous Cell Carcinoma Progression in an Immune Microenvironment. J. Dent. Res. 2020, 99, 666–675.
- Peng, R.T.; Sun, Y.; Zhou, X.D.; Liu, S.Y.; Han, Q.; Cheng, L.; Peng, X. Treponema denticola Promotes OSCC Development via the TGF-beta Signaling Pathway. J. Dent. Res. 2022, 101, 704–713.
- Talapko, J.; Juzbasic, M.; Matijevic, T.; Pustijanac, E.; Bekic, S.; Kotris, I.; Skrlec, I. Candida albicans-The Virulence Factors and Clinical Manifestations of Infection. J. Fungi 2021, 7, 79.
- Engku Nasrullah Satiman, E.A.F.; Ahmad, H.; Ramzi, A.B.; Abdul Wahab, R.; Kaderi, M.A.; Wan Harun, W.H.A.; Dashper, S.; McCullough, M.; Arzmi, M.H. The role of Candida albicans candidalysin ECE1 gene in oral carcinogenesis. J. Oral Pathol. Med. 2020, 49, 835–841.
- Wang, X.; Zhang, W.; Wu, W.; Wu, S.; Young, A.; Yan, Z. Is Candida albicans a contributor to cancer? A critical review based on the current evidence. Microbiol. Res. 2023, 272, 127370.
- Wang, X.; Wu, S.; Wu, W.; Zhang, W.; Li, L.; Liu, Q.; Yan, Z. Candida albicans Promotes Oral Cancer via IL-17A/IL-17RA-Macrophage Axis. mBio 2023, 14, e0044723.
- Rouabhia, M.; Ross, G.; Page, N.; Chakir, J. Interleukin-18 and gamma interferon production by oral epithelial cells in response to exposure to Candida albicans or lipopolysaccharide stimulation. Infect. Immun. 2002, 70, 7073–7080.
- Bogani, G.; Ghelardi, A.; Sopracordevole, F.; Annoni, M.; Ciavattini, A.; Giannella, L.; De Vincenzo, R.; Cattani, P.; Barbero, M.; Vercellini, P.; et al. Human papillomavirus (HPV) vaccination: A call for action in Italy. Int. J. Gynecol. Cancer 2023, 33, 1132–1139.
- Colzani, E.; Johansen, K.; Johnson, H.; Pastore Celentano, L. Human papillomavirus vaccination in the European Union/European Economic Area and globally: A moral dilemma. Eurosurveillance 2021, 26, 2001659.
- Canepa, P.; Orsi, A.; Martini, M.; Icardi, G. HPV related diseases in males: A heavy vaccine-preventable burden. J. Prev. Med. Hyg. 2013, 54, 61–70.
- Hirth, J. Disparities in HPV vaccination rates and HPV prevalence in the United States: A review of the literature. Hum. Vaccin. Immunother. 2019, 15, 146–155.
- Simms, K.T.; Steinberg, J.; Caruana, M.; Smith, M.A.; Lew, J.B.; Soerjomataram, I.; Castle, P.E.; Bray, F.; Canfell, K. Impact of scaled up human papillomavirus vaccination and cervical screening and the potential for global elimination of cervical cancer in 181 countries, 2020–2099: A modelling study. Lancet Oncol. 2019, 20, 394–407.
- Ghelardi, A.; Parazzini, F.; Martella, F.; Pieralli, A.; Bay, P.; Tonetti, A.; Svelato, A.; Bertacca, G.; Lombardi, S.; Joura, E.A. SPERANZA project: HPV vaccination after treatment for CIN2. Gynecol. Oncol. 2018, 151, 229–234.
More
