2. ConDiscluussions
Starting from the premise that anti-protozoal treatments are toxic, and their therapeutic regimens require prolonged treatment times and high concentrations of drugs,
in th
e researchersis systematic review, we demonstrate that 5-nitroimidazole derivatives offer a broader spectrum of activity against a variety of protozoal pathogens. Therefore, 5-nitroimidazole derivatives are proposed as pharmacophores against neglected tropical protozoan diseases. There are a plethora of chemical structures containing nitroimidazole rings that have been associated with multiple biological activities, such as anti-protozoan, anti-bacterial, anti-fungal, and anti-cancer effects
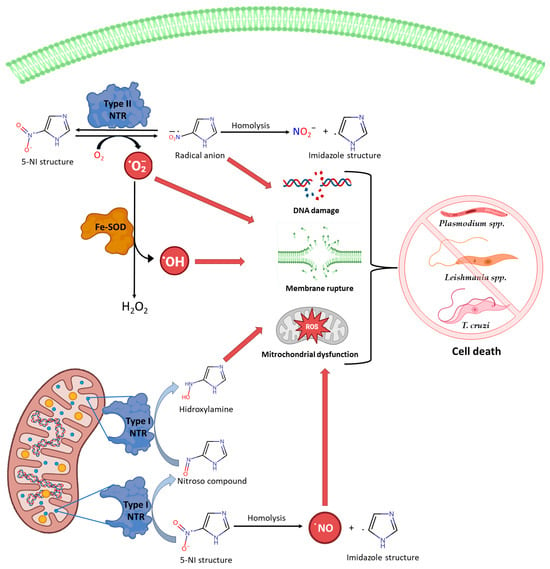
[4][61]. One of the main advantages is that nitroindazoles exhibit fewer toxic effects and better clinical tolerance than reference drugs. Furthermore, it is evident that the nitro group can induce improvements in the activity of compounds, enabling them to interfere with critical enzymes of these parasites, such as iron superoxide dismutase (Fe-SOD) and trypanothione reductase (TR), leading to the generation of reactive oxygen and nitrogen species in protozoans, resulting in their death
[5][58].
The anti-protozoal effect of all 5-NI derivatives is attributed to the nitro group reduction of 5-Nis, which results in intermediate compounds and free radicals that can interact with membranes, proteins, and nucleic acids through oxidation or covalent binding modifications. Due to this mechanism of action, the structures are considered “prodrugs”
[6][7][62,63].
It is worth mentioning that 5-NI can be metabolized by both humans and protozoa. However, recent achievements have been documented in the selectivity of 5-NI derivatives to combat protozoa endpoints, significantly reducing their side effects in humans
[8][64]. These new results have opened new perspectives for developing novel and safe anti-protozoan agents.
In addition to their anti-protozoan activity, 5-NI derivatives have also been associated with multi-inhibitory activity against different molecular targets involved in homeostasis, metabolism, and cancer
[9][10][11][12][13][14][65,66,67,68,69,70].
In general, the main metabolic pathway involves the oxidation of the aliphatic side chains of these compounds, resulting in the generation of oxidized metabolites (hydroxylated and acetylated metabolites). Some of these metabolites, along with the parent molecule, are conjugated and excreted via urine and feces along with free metabolites. The literature findings suggest that, although the initial metabolic step is the formation of a nitro anion radical, in the presence of oxygen, this radical is reoxidized, leading to the generation of a superoxide anion. This cycle is believed to be the source of reactive oxygen species (ROS), without the loss of the parent molecule. Subsequently, after the formation of the superoxide anion, in the presence of superoxide dismutase (SOD) and traces of metal ions such as Fe
2+ and Cu
2+, reactions would occur, leading to the formation of a hydroxyl radical
[15][71]. It is due to the action of ROS that nitroimidazole derivatives have been considered mutagenic, primarily in prokaryotic systems and to a lesser extent in eukaryotes, as mentioned earlier. However, the International Agency for Research on Cancer (IARC) does classify nitroimidazoles as carcinogens
[16][72]. For this reason, it is important to continue the study of their possible “off-target” effects.
In the present work, using a systematic review protocol,
thwe
researchers identified 213 5-NI derivatives with inhibitory activity against different protozoans:
Plasmodium (62/29.1%),
Leishmania (142/66.7%), and
T. cruzi (9/4.2%). The activity ranged from 0.0005 to 74 µM, 0.001 to 84 µM, and 1 to 602 µM, respectively. Notably, a greater number of 5-NI derivatives were tested against
Leishmania and demonstrated higher activity. The best scaffolds for each protozoan genus are 5-nitroimidazole-2-carbaldehyde derivatives for P
lasmodium falciparum, metronidazole derivatives for
Leishmania mexicana, and fexinidazole for
Trypanosoma cruzi. Interestingly, three 5-NI scaffolds have been associated with multi-protozoan activity, which are distributed in 23 compounds (nitroimidazopyridazine derivates, compounds
6–24; metronidazole, compound
54a; and fexinidazole derivatives, compounds
51–53).
There are additional compounds within 5-NI scaffolds that were identified in the early stages of this systematic review, but their content was either not directly related or did not meet the inclusion criteria. An example of this is secnidazole (1-(2-methyl-5-nitro-1-imidazole-1-yl) propan-2-o), which was tested in 2017 by Eke et al. against
Trypanosoma brucei, both in vitro and in vivo. They determined the minimum inhibitory concentration (MIC) that is required to inhibit motility in blood infected with 225,000 trypomastigotes, which was found to be 2.8–1.4 mg/mL after 10 min post-treatment. Regarding murine models, significantly lower parasitemia levels were observed (
p < 0.05) compared to the untreated infected group after 6 days post-infection (post-treatment)
[17][73]. While
Trypanosoma cruzi or
Leishmania spp. were not specifically evaluated, given that they are trypanosomatids, these results encourage further exploration of the application and development of new compounds based on 5-NI. For instance, compounds like tinidazole, ornidazole, ronidazole, and their derivatives could be potential candidates for future investigations
[18][74].
The data collected from this work allow for a systematic chemoinformatic study using DataWarrior software version 5.5.0
[13][69]. For each compound, their physicochemical properties were calculated. For example, the total molecular weight, cLogP (octanol/water coefficient, solubility estimation), and the number of sp3 atoms (stereochemical complexity estimation) were calculated and statistically analyzed
(Table S2). The results suggest that 3-nitroimidazopyridines derivatives (
6–24), metronidazole hybridized derivatives (
29–50), and quinoline–metronidazole derivatives (
63a–63m) have high molecular weights compared with other 5-NI derivatives. These kinds of compounds are in the upper recommended limit to be considered “drug-like” compounds, according to the empirical rules proposed by Lipinsky
[14][70].
Interestingly, these results also suggest that there are compounds with a hydrophobic profile, like the quinoline–metronidazole derivatives (
63a–63m), and others with a hydrophilic profile like fexinidazole derivatives. Finally, the authors remark on the stereochemical complexity of 5-NI derivatives, particularly for cases like metronidazole-hybridized and metronidazole-based compounds (
29–50,
55a–59i).
All data are available in the Supplementary Materials section and can be interactively analyzed using DataWarrior software version 5.5.0 [75].
Although the 5-NI derivatives offer an interesting starting point for developing novel and safe anti-protozoan agents, there are still other important issues to resolve. For example, different research groups are leading the development of novel drug formulations and new chemical vehicles to reduce the 5-NI doses to significantly reduce their associated side effects. In parallel, the main purpose of studying 5-NI derivatives is to develop new chemical derivates with better protozoan selectivity and better clinical administration regimens
[20][21][76,77]. Additionally, non-neglected tropical protozoan (e.g.,
Giardia lambia) strains have developed 5-NI derivative resistance through multiple mechanisms, which highlight the possibility that other types of protozoans (like
Trypanosoma brucei or
Trichomonas vaginalis) will develop similar resistance mechanisms in the near future
[22][23][78,79].
Finally, the authors highlight that this systematic review has limitations that must be noted. For example, the use of a systematic method does not guarantee that all 5-NI derivatives with anti-protozoan activity have been presented in the final list of this work
(see Supplementary Materials section). Also, this systematic review did not consider “non-open access journals” and “lower-impact journals” that could contain important data to discuss. However, this work shows representative successful cases that illustrate the potential of the 5-NI scaffold against protozoan endpoints.
Nevertheless, this systematic review shows us that the exploration of 5-NI derivatives and other new chemical entities in the treatment of NTPDs, including malaria, visceral leishmaniasis, and Chagas disease, underscores the importance of continued innovation and collaboration in drug discovery. These efforts are not only crucial in addressing the immediate challenges posed by these diseases but also in shaping future strategies for effective disease management in resource-limited settings
[24][80].
Nitro-imidazoles, in general, have provided relief for various human and livestock infections, resulting in significant economic benefits for humanity. However, in addition to the resistance, multidrug resistance, and pan-resistance that the excessive and uncontrolled use of antimicrobials has triggered, there is also environmental harm. Both soils and continental and marine waters are increasingly being invaded by waste containing metabolites and antibiotics that promote resistance in microorganisms, reactivating cycles that are difficult to halt. That is why collaborative efforts are underway to develop new, more easily degradable antibiotics, such as metal nanoparticles and biopolymer-based ones
[25][81]. Work is also being carried out on the design of devices that can identify contamination by nitroimidazoles or other antibiotics in water or soil, as well as on decontamination processes
[26][27][82,83]. However, as mentioned earlier, infections by protozoa-causing diseases that are classified as neglected primarily affect a larger number of victims in resource-poor countries that are unable to access these benefits.