Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by José Manuel Castro Torres and Version 2 by Catherine Yang.
The progress of artificial intelligence algorithms in digital image processing and automatic diagnosis studies of the eye disease glaucoma has been growing and presenting essential advances to guarantee better clinical care for the population.
- deep learning
- glaucoma
- image analysis
- artificial intelligence
1. Introduction
Glaucoma is a multifactorial neuropathy that can affect the fundus of the eye, causing gradual loss of vision and, in severe cases, blindness. Traditionally, the diagnosis of glaucoma is applied with the help of readily available ophthalmological teams and highly specialized equipment. The sensitivity of the diagnosis is generally high, as tests applied in ophthalmology offices have the clinical potential to identify virtually all cases of the disease. However, despite this sophisticated diagnostic scenario, the silent and slow evolution of the disease, the costs of exams and consultations, and the lack of access to public ophthalmological services in many cases prevent thousands of people from consulting an ophthalmologist during the early stages of this neuropathy. This contributes to the fact that around 70% of the patients are self-diagnosed, that is, alerted by their own visual impairment and not by an appropriate early diagnosis [1][2][1,2].
Glaucoma is considered a global problem; even in developed countries, it is estimated that at least 50% of patients with glaucoma do not know of their condition. This percentage is even worse in low-income countries [3]. It is considered a progressive, chronic, and incurable pathology; however, it can generally be efficiently controlled when treatment begins in the early stages of the disease.
There are several types of glaucoma: open-angle glaucoma, angle-closure glaucoma, congenital glaucoma and secondary glaucoma [4][5][4,5]. However, they all cause damage to the optic nerve, which in most cases occurs slowly, initially leading to the loss of midperipheral vision. In advanced stages, it affects central vision, leading to irreversible blindness.
Damage to the optic nerve can be analyzed using fundus examinations, also known as ophthalmoscopy or fundoscopy. The ophthalmoscopy examination is performed on the back part of the eye (fundus), which includes the retina, optic disc, choroid, and blood vessels. The funduscopic examination can be performed with a variety of equipment, such as direct ophthalmoscopy, indirect ophthalmoscopy, and slit lamp ophthalmoscopy. Found in almost all ophthalmology offices, these devices offer ophthalmologists a detailed view of the eyeball. As shown in Figure 1, the brightest part of the retina represents the optic disc (OD), which contains an excavation known as the optical cup (OC), depicted by the whitest part of the interior of the optic disc. Therefore, if the size of the optic cup increases, it is considered one of the main indicators of glaucoma [2][6][7][8][2,6,7,8].
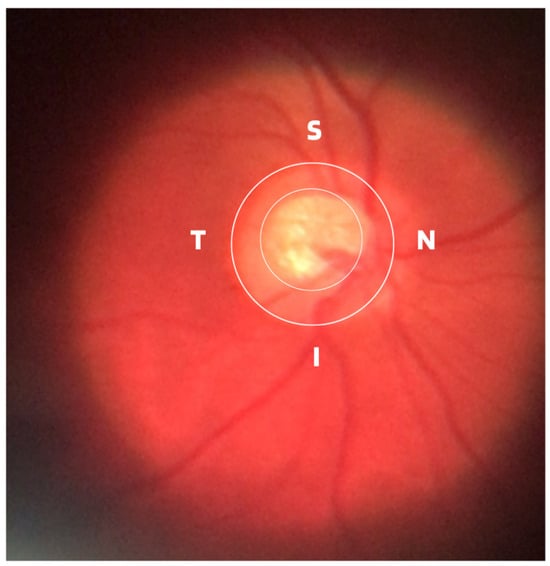
Figure 1.
ISNT (Inferior (I), Superior (S), Nasal (N) and Temporal (T)) Rule.
In terms of the basic and traditional methods of diagnosing glaucoma, in addition to the fundus examination to examine the optic disc and the retinal nerve fiber layer (RNFL), ophthalmologists generally use tonometry and visual field tests as adjuncts. Tonometry is an exam to assess the degree of dysfunction and measures intraocular pressure (IOP) in millimeters of mercury (mmHg). The common eye pressure range is 10 to 21 mmHg, which is based on the average eye pressure level of a normal person. Although tonometry examination is very important in the management and treatment of glaucoma, it cannot be considered a diagnosis due to the presence of cases of normal pressure glaucoma [9]. Perimetry through the perimetry or campimetry exam, as is also known, the degree of functional impairment resulting from the disease is examined through the results of the obtained visual field map. In clinical practice, visual field testing identifies so-called blind spots (scotomas) and their locations in human vision and is therefore widely used as the gold standard to assess whether a patient suffers from typical functional glaucomatous damage [10].
Although the demographic and clinical characteristics associated with glaucoma are relatively well known, there is still no uniform definition of the diagnosis of this disease by ophthalmologists. In this way, many international efforts have been made to develop such a definition, but no real consensus standard has been reached. Therefore, those with an IOP greater than 21 mmHg, accompanied by characteristic damage to the optic disc or defects in the visual field compatible with glaucoma, are generally included as glaucomatous [11]. Due to this particularity, it is important to assess and document the appearance of an increase in the cup-to-disc ratio as a way of evaluating possible structural damage caused by the disease, as well as accompanying the patient to treatment or routine appointments. Therefore, from ophthalmoscopy images, ophthalmologists can evaluate at least four important informative characteristics of glaucoma, such as cup/disc ratio, inferior (I), superior (S), nasal (N), and temporal (T) rule (ISNT), cup asymmetry, and in addition other structural damage caused to the optic disc, namely the following:
-
Cup-to-Disc Ratio (CDR): An abnormal increase in disc cupping is important in the diagnosis of glaucoma; however, many people may have increased nerve cupping and not necessarily have glaucoma. This is especially true for myopic people, who tend to have a larger optical disc and consequently a larger optical cup. Therefore, during the diagnosis of glaucoma, it is important to assess not only the optical cup but also the cup-to-disc ratio (CDR). For better understanding, the CDR measurement is calculated from the relationship between the vertical diameter of the excavation (VCD) and the vertical diameter of the disc (VDD), as shown in Figure 2.To calculate the CDR ratio, the optical disc must be divided into 10 equal parts, as in Figure 3, and then the excavation scope must be taken into account in each division made. Therefore, it is considered a fractional percentage measurement, generally made horizontally, and can vary greatly between normal individuals. However, optical excavations greater than 0.65 indicate possible abnormalities, suggesting further investigation [2][12][2,12].
-
ISNT Rule: The border formed between the optic cup and the optic disc, called the neuroretinal ring or neural ring, is also considered an indication of glaucoma, for which there is a rule called ISNT, which alludes to the orientation (inferior, superior, nasal, and temporal) of the edges in the image of the fundus, as shown in Figure 1. When considering the ISNT rule, in nonglaucomatous eyes, it is suggested that the thickness of the neural ring should be greatest in the inferior quadrant, followed by the superior, nasal, and temporal quadrants. Misalignment in the guidelines of this rule leads to suspicion of glaucoma [13].
-
Cup-to-disc ratio (CDR) asymmetry: The CDR relationship between both eyes is symmetric in most people, and asymmetry is an important sign of suspected glaucomatous damage. This is due to the observation that 1% to 6% normal adults may have a discrepancy of 0.2 in the cup/disc ratio, while 1% of the general population may have an asymmetry of 0.3. Therefore, cup asymmetry is a finding on ophthalmological examination that requires additional tests to rule out the presence of glaucoma or other possible complications [14][15][14,15].
-
Other structural damage to the optic disc: The main descriptions of these types of damage related to glaucoma are as follows [2][16][17][2,16,17]:
-
Changes in RNFL: the presence of defects located in the retinal nerve fiber layer is called Hoyt’s sign and is characterized by a dark area that extends and widens from the optic disc, exhibiting an arched shape.
-
Peripapillary atrophy: According to the ophthalmological appearance, peripapillary atrophy can be divided into a peripheral alpha zone and a central beta zone. The alpha zone is characterized by patchy hypopigmentation and thinning of the layers of the chorioretinal tissue. It is laterally adjacent to the retina and medially in contact with the beta area, with the sclera and large choroidal vessels visible. In normal eyes, the alpha and beta areas are usually located in the temporal area, followed by the inferior and superior areas. In glaucomatous eyes, the beta area is more present in the temporal region and its extension is associated with thinning of the RNFL.
-
Excavation of the optic disc: In addition to disc excavation, the neuroretinal ring or neural rim must also be observed, as excavation is influenced by the size of the optic disc.
-
Disc hemorrhage: The presence of peripapillary hemorrhages is an important sign in both the diagnosis and the monitoring of glaucoma. Therefore, vessel deflection and nasal excavation must be examined.
-
Denudation of the lamina, cribriform: the presence of visible extinction of the cribriform lamina to the edge of the optic disc is called a notch, which represents the evolution of a defect located in the neural rim until there is a complete absence of tissue in the region, which exposes the cribriform lamina and allows visualization of its pores. Although it is very suggestive of glaucoma, this sign is not characteristic of the disease.
-
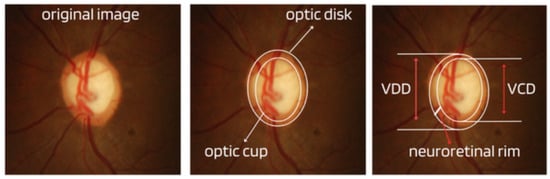
Figure 2.
Measures considered in the CDR calculation.
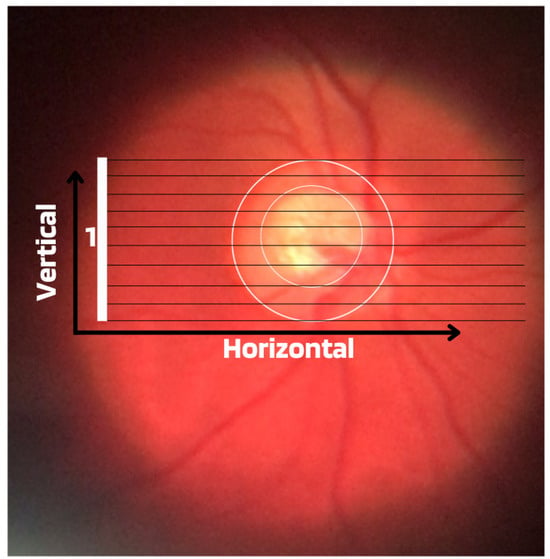
Figure 3.
Example of CDR calculation with figure showing excavation of 0.6.
Regarding the difficulties associated with the diagnosis of glaucoma, it is considered that in cases in the moderate or advanced stages of the disease, the diagnosis is usually more simplified. However, the best way is to detect early glaucoma, which is essential for adequate treatment, mainly because quality of life can be altered even with slight loss of visual field [18]. However, the early identification of this disease, although important, can be challenging for several reasons, including glaucomatous characteristics that can be ambiguous in the optic disc region, RNFL, or visual field results at the beginning of the disease.
