You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 1 by Axel Nierhaus.
Various techniques for extracorporeal blood purification can decrease levels of elevated proinflammatory cytokines in septic shock, potentially mitigating the severity of the systemic inflammatory response. Some methods are effective in removing endotoxins, especially in sepsis caused by Gram-negative bacteria, which may aid in stabilizing the patient’s condition. Blood purification can enhance hemodynamic stability and reduce the need for vasopressors, crucial for managing septic shock. Techniques like continuous renal replacement therapy (CRRT) offer simultaneous management of acute kidney injury—a frequent complication in septic shock—alongside the removal of toxins and cytokines.
- sepsis
- septic hyperinflammation
- blood purification
- immune response
- cytokines
- endotoxi
1. Introduction
1.1. Septic Hyperinflammation
Sepsis is a life-threatening clinical condition with extensive physiological and biochemical abnormalities. Each year, approximately 49 million people worldwide are affected by sepsis, and it is estimated that 11 million deaths can be attributed to this syndrome. This accounts for up to 19.7% of all global deaths [1]. Although there appears to be a global decline in the average mortality rate, the current mortality rate for sepsis can still reach up to 25%. In the case of septic shock, which is a subset of sepsis characterized by profound circulatory, cellular, and metabolic disturbances, the hospital mortality rate approaches almost 60% [2].
Over recent decades, the definition of “sepsis” has continuously evolved, adapting to the expanding scope of knowledge. The current definition, established by the Third International Consensus (Sepsis-3), characterizes sepsis as “organ dysfunction caused by a dysregulated host response to infection” [3]. This definition notably emphasizes, for the first time, the critical role of both the innate and adaptive immune responses in the development of the clinical syndrome. Sepsis, unlike an uncomplicated and localized infection, involves a complex disruption of the finely tuned balance between pro- and anti-inflammatory processes. Although understanding of the development, pathophysiology, and immunological mechanisms of sepsis has advanced significantly over the last three decades, the syndrome’s complexity—with its myriad interactions and effects on various organs—means that the opportunities for successful and specific therapeutic interventions remain limited.
Even approaches within the realm of personalized or “precision medicine”, where treatments are tailored to predefined conditions or the specific needs of individual patients, have yet to achieve widespread success.
Sepsis necessitates timely and effective treatment strategies that vary across its continuum, from early sepsis to sepsis syndrome/severe sepsis and septic shock. The treatment modalities are multidisciplinary and escalate in intensity with the progression of the disease.
1.2. Immune Response Mechanisms in Sepsis: From Recognition to Regulation
Both adaptive and innate immunity rely on a variety of intracellular, membrane-bound, and soluble receptors. These include pattern recognition receptors (PRRs) which detect not only pathogen-associated molecular markers (PAMPs, e.g., endo- and exotoxins, DNA, lipids) from foreign invaders but also endogenous, host-derived danger signals (damage-associated molecular patterns, DAMPs). The recognition of PAMPs or DAMPs triggers a cascade aimed at both containing and destroying invasive pathogens, as well as repairing damaged tissue. The resulting upregulation of pro- and anti-inflammatory signaling pathways leads to a systemic release of cytokines, mediators, and pathogen-related molecules. This, in turn, activates coagulation and complement cascades, contributing to the immune response [7][4]. Toll-like receptors (TLRs), a subclass of PRRs, are located on the outer membranes as well as in intracellular vesicles of antigen-presenting cells (APCs) and monocytes [8][5]. Their interaction with PAMPs and DAMPs (e.g., extracellular LPS or intracellular nucleic acids) initiates signal transduction, which triggers a translocation of the nuclear factor-kappa-light-chain-enhancer of activated B cells (NF-κB) into the cell nucleus. This, in turn, leads to the expression of “early activation genes”. These genes include proinflammatory interleukins (IL), such as IL-1, IL-12, IL-18, along with tumor necrosis factor (TNF) and interferons (IFN). These proinflammatory substances then promote the activation of complement and coagulation pathways and stimulate the release of further cytokines (e.g., IL-6, IL-8, IFN-γ). This excessive and widespread increase in pro- and anti-inflammatory cytokines, resulting from the upregulation of both proinflammatory and anti-inflammatory signaling pathways, is a classic hallmark of sepsis. It leads to progressive tissue damage in the host and can ultimately escalate into multiorgan dysfunction. In the later stages of sepsis, the downregulation of activating cell surface molecules, increased apoptosis of immune cells, and T-cell exhaustion often result in emerging immunosuppression, a phenomenon known as “immune paralysis”. This condition renders affected patients susceptible to nosocomial infections, viral reactivation, and opportunistic pathogens [10,13][6][7].1.3. Neutrophils in Sepsis: Roles in Defense, Hyperinflammation, and Organ Damage
The immunological characterization of sepsis is complicated by its highly variable influence on the immunological phenotype, which can manifest itself either as hypo- or hyperreactive, or as a mixed form. Neutrophil granulocytes, a key component of the innate immune system, play a crucial role in the primary defense against pathogens. They contribute to hyperinflammation in sepsis through the release of proteases and reactive oxygen species. In response to severe bacterial infections, both mature and immature forms of neutrophils are released from the bone marrow in a process known as emergency granulopoiesis. However, when activated by interaction with PAMPs or DAMPs, immature neutrophils show reduced phagocytosis and limited oxidative burst capacity [14,15,16][8][9][10]. Neutrophil granulocytes are capable of releasing neutrophil extracellular traps (NETs) [17][11]. NETs are diffuse extracellular structures composed of decondensed chromatin with granular and nuclear proteins (histones) that can bind to endothelial or epithelial cells, potentially causing cell damage. This may promote the formation of intravascular thrombi and contribute to multiple organ damage [18,19,20][12][13][14]. NETs are also known for their ability to immobilize a wide range of pathogens. In addition to various cytokines like IL-8, IL-1β, and TNF, the release of NETs can also be triggered by platelet agonists such as adenosine diphosphate (ADP), arachidonic acid, collagen, thrombin, and some antibodies [17,18,23,24][11][12][15][16].1.4. Endothelial Dysfunction and Thromboinflammation in Hyperinflammatory Diseases
The endothelium, along with its protective layer of glycoprotein polysaccharides known as the glycocalyx, plays a significant role in the progression of diseases associated with hyperinflammation. Both are key targets in various mechanisms that perpetuate the inflammatory response [26,27][17][18]. In such conditions, endothelial cells may lose their antithrombotic properties. For example, the expression of surface-bound thrombomodulin can be reduced, leading to an increase in tissue factor (TF) expression. This, in combination with leukocytic microparticles and monocytes that also carry TF, triggers the activation of the coagulation cascade [28][19]. Feedback mechanisms consequently lead to progressive vascular hyperpermeability, increased recruitment of inflammatory cells, pronounced expression of adhesion molecules, and the release of additional cytokines. The binding of released TF to activated platelets and neutrophils, among others, further intensifies the prothrombotic situation. Simultaneously, the activity of antithrombotic factors, including antithrombin, the protein C system, and the tissue factor pathway inhibitor (TFPI), is reduced [30][20].1.5. Complement System Activation and Immunothrombosis in Sepsis and Systemic Inflammation
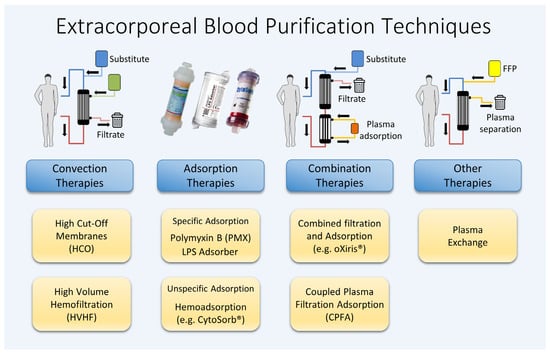
The complement system is a crucial component of innate immunity. In the initial phase of systemic hyperinflammation, elevated levels of activated complement factors such as the proinflammatory peptide fragments C3a, C4a, and C5a can be detected [24][16]. These anaphylatoxins, particularly C5a, intensify various responses, ranging from triggering apoptosis to the functional deactivation of neutrophils and the amplification of the hyperinflammatory response. C5a is known for its role in neutrophil chemotaxis; neutrophils, upon binding to the C5a receptor (C5aR), acquire the ability to migrate to and invade inflamed tissues. There, through the binding of PAMPs and DAMPs, they become activated and release granular enzymes, reactive oxygen species, and NETs [31][21]. Evolutionarily, the complement and coagulation systems share a common origin. The release of the proinflammatory complement factors C3a and C5a simultaneously not only leads to the recruitment but also the activation of platelets, endothelial cells, and leukocytes. Coagulation can be activated by coagulation factor XI, or, alternatively, through the cleavage of kininogen with release of bradykinin and antimicrobial peptides. Subsequent research suggests that, under certain conditions, thrombosis may play a significant physiological role in immune defense. Clinically, coagulopathy is a frequent complication of sepsis, and can be detected in up to one-third of critically ill patients. The International Society of Thrombosis and Haemostasis (ISTH) describes disseminated intravascular coagulopathy (DIC) as a syndrome “characterized by the intravascular activation of coagulation with loss of localization arising from different causes. It can originate from and cause damage to the microvasculature, which, if sufficiently severe, can produce organ dysfunction” [36][22]. The occurrence of DIC in sepsis is attributed to consumptive coagulopathy, driven by system-wide coagulation activation and accompanied by suppressed fibrinolysis. Alongside organ dysfunction due to systemic inflammation, decreased platelets, and increased PT-INR, the term “sepsis-induced coagulopathy (SIC)” has been introduced to describe this condition [37][23]. To summarize, many of the complex and diverse processes associated with septic hyperinflammation occur in the plasma. The various substances and messengers involved are systemically elevated and are present in a dissolved form, making them potential targets for treatment through blood purification. Despite numerous approaches being explored over the past decades, no single procedure nor combination of techniques has yet been identified that significantly improves the survival rates of patients with sepsis. Figure 1 illustrates a range of different techniques for extracorporeal blood purification, which are examined in this article for their application in treating sepsis and septic shock.
Figure 1.
Various extracorporeal blood purification methods available.
Figure 2 provides a schematic overview of the potential clearance properties of different blood purification methods based on the molecular weight of various mediators and toxins. Due to the large number of different commercially available membranes and technical settings, there is a wide variance in the actual effectiveness of the respective techniques.
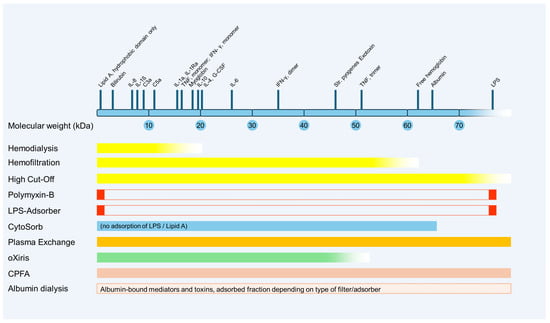

Figure 2. Schematic representation of potential clearance properties of blood purification methods based on the molecular weight of various mediators and toxins. CPFA, coupled plasma filtration adsorption; G-CSF, granulocyte-colony stimulating factor; IFN, interferon; IL, interleukin; kDa, kilodalton; LPS, lipopolysaccharide; TNF, tumor necrosis factor.
2. Renal Replacement Therapies (RRTs)
2.1. High-Cut-Off Membranes
Unlike standard high-flux membranes, high-cut-off (HCO) membranes feature increased pore size (20 nm instead of 10 nm), which theoretically allows for a more effective elimination of inflammatory mediators. The use of HCO membranes is similar to standard renal replacement therapy with a prescribed dose ranging between 25 and 40 mL/kg/h, as recommended by Kidney Disease Improving Global Outcomes (KDIGO). HCO membranes are employed in sepsis as well as in other conditions, such as acute kidney injury in the context of rhabdomyolysis or cast nephropathy in multiple myeloma. Initial studies on patients with sepsis-induced acute kidney injury predominantly indicated a more effective clearance of proinflammatory cytokines using HCO filters compared to classical high-flux filters. In a clinical trial involving 24 patients with sepsis-induced acute renal failure, Morgera et al. found that while the HCO membrane was effective in removing inflammatory mediators such as IL-1, IL-6, and TNF through convection, it also resulted in significant albumin loss compared to diffusion-based modalities [46][24]. Another study confirmed the higher sieving coefficient and mass removal rate of ultrafiltration for certain cytokines but failed to demonstrate a reduction in cytokine plasma levels in critically ill patients with acute kidney injury (AKI) within the first 72 h of therapy [47][25]. Following a small (n = 16) retrospective observational study that suggested a positive effect on mortality (37.5% mortality with HCO filter vs. 87.5% with continuous veno-venous hemodiafiltration, p = 0.03), these results were to be verified by the randomized High Cut-Off Sepsis Study (HICOSS) [48][26]. However, this trial was discontinued after a planned interim analysis showed no benefit in 28-day mortality (31% for the HCO group vs. 33% for the conventional group) or reductions in catecholamine use, days on mechanical ventilation, or duration of intensive care unit (ICU) stay. When diffusive modalities were used, albumin levels did not differ significantly. In summary, at present, there is no evidence supporting a positive effect of HCO filters in sepsis beyond established indications such as rhabdomyolysis.
2.2. High-Volume Hemofiltration
Continuous hemodialysis or hemodiafiltration with high filtration volume is likely the oldest method for extracorporeal removal of small molecules. Hemofiltration operates through convection, where dissolved substances are transported along with a solvent across a semipermeable membrane (ultrafiltration), driven by a positive transmembrane pressure gradient. The clearance in this process depends on the ultrafiltration rate, the sieving properties of the membrane for the solute, and the molecular size of the solute. According to the consensus definition, high-volume hemofiltration (HVHF) uses a convective target dose of more than 35 mL/kg/h, while a target dose of more than 45 mL/kg/h is classified as very-high-volume hemofiltration (VHVHF) [49][27]. As these methods do not require additional elements to be added to the standard circuit, they can be readily implemented as long as there is experience in the use of continuous renal replacement therapies. These techniques have been employed for immunomodulation in sepsis by aiming to eliminate inflammatory mediators through convection. Although most inflammatory molecules are medium-molecular substances and, in theory, can be removed by this technique, their endogenous release rate in sepsis is significantly higher compared to uremic toxins. Various studies have investigated the effects of different therapeutic regimens on outcome in sepsis and septic shock, using different target doses (HVHF and VHVHF) as well as comparing intermittent versus continuous usage [50,51,52,53][28][29][30][31]. Although a meta-analysis indicated lower mortality and improved hemodynamics, characterized by a lower heart rate and higher mean arterial pressure, it did not demonstrate a significant impact on disease severity or oxygenation index. Furthermore, most of the RCTs included in the meta-analysis were not of high quality, leading to questionable reliability of findings for various parameters (e.g., IL-6, mean arterial pressure) [54][32]. Therefore, the data available to date are insufficient for a conclusive assessment. Future studies should focus on exploring alternative extracorporeal therapies, rather than concentrating solely on HVHF as an adjunctive therapy for sepsis.
3. Adsorption
3.1. Polymyxin B-Immobilized Fiber Columns (Specific Hemoadsorption)
In cases of Gram-negative sepsis, endotoxin (lipopolysaccharide (LPS) and its fragments) triggers the activation of different cell types, including endothelial cells, monocytes, polymorphonuclear neutrophils, and tissue-resident cells, as well as plasmatic systems like the complement and coagulation pathways. Endotoxin falls under the category of PAMP, and high serum activity of endotoxin is seemingly associated with increased disease severity and impacts survival rates in patients with sepsis or suspected sepsis [55,56][33][34]. That said, developing extracorporeal systems to remove this triggering stimulus from the bloodstream appears logical. One of the most promising approaches in this regard is hemoperfusion with polymyxin B-immobilized fiber columns (PMX). Polymyxin B, a cyclic lipophilic peptide antibiotic, is extensively studied for neutralizing LPS due to its high affinity for the lipid A moiety of endotoxin. This treatment approach was first applied to patients with abdominal sepsis. The device has been evaluated in two RCTs for sepsis or septic shock with an abdominal focus: EUPHAS and ABDO-MIX [57,58,59][35][36][37]. While the EUPHAS study showed a trend towards reduced mortality, this finding could not be confirmed by the ABDO-MIX study. One possible reason for this discrepancy might have been frequent “clotting” of the PMX cartridges, which resulted in only 70% of the cohort completing two treatments of two hours each.3.2. LPS Adsorber
The LPS Adsorber is a commercially available medical device designed for extracorporeal blood purification, specifically targeting the elimination of circulating endotoxin (lipopolysaccharide, LPS) from the bloodstream. This device features a cartridge filled with discs made of porous polyethylene (PE), characterized by surface pores averaging 100 μm in size. These surfaces, along with the pores, are coated with a specially designed peptide, synthesized entirely via solid phase peptide synthesis. This method ensures that the peptide is not genetically engineered and does not originate from human or animal sources. The peptide, covalently bound to the cartridge, is cationic and exhibits a high affinity for the negatively charged lipid A domain of LPS. Results from the pilot Phase IIa trial were published 2020 in Shock. This trial was aimed to allocate 32 septic shock patients with abdominal or urogenital focus in six Scandinavian ICUs who were randomized to either LPS Adsorber therapy or a Sham device. After 527 days, the investigation was terminated with only 15 patients included (eight in the LPS Adsorber group, seven in the control group). LPS levels in plasma were low without group differences; also, the chances in organ function and inflammatory markers were similar in both groups [41][38].3.3. CytoSorb
®
(Unspecific Hemadsorption)
The commercially available CytoSorb® device, which is approved for medical use, employs a nonselective hemadsorption process. It consists of a cartridge filled with beads made of a highly porous resin, coated with biocompatible polyvinylpyrrolidone. Despite these hollow spheres having a diameter of only around 300–600 μm, the active surface area of a cartridge is approximately 45,000 m2, significantly surpassing the surface area of conventional hemofilters, which is typically around 1.2–2.5 m2. When integrated into in a conventional extracorporeal system, such as continuous renal replacement therapy (CRRT) or extracorporeal membrane oxygenation (ECMO), the patient’s blood is passed over the adsorptive surface of the cartridge. This process facilitates the selective adsorption of various substances and molecules within the range of ~5–60 kDa, depending on their plasma concentration. An initial multicenter study conducted in 2013 indicated a reduction in the systemic IL-6 concentration following CytoSorb® application in septic patients. Yet, there was no evidence of a reduction in mortality, and the size of the study (n = 43 patients) was not sufficient to determine such outcomes [66][39]. In a case series involving 26 patients with septic shock and renal replacement therapy, a rapid stabilization of hemodynamic parameters, a reduced need for vasopressors, and a decrease in serum lactate were observed [67][40].4. Therapeutic Plasma Exchange (TPE)
The balance between circulating cells and the vascular endothelium is maintained through the interplay of various proteins and receptors. A key protein in this interaction is von Willebrand factor (vWF), which has a multimeric structure. The equilibrium of vWF is regulated by a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13), also known as von Willebrand factor-cleaving protease (vWFCP). A deficiency in ADAMTS13 activity can lead to markedly elevated levels of large vWF multimers, resulting in thrombocytopenic microangiopathy (TMA). Two notable and extreme forms of TMA are thrombotic thrombocytopenic purpura (TTP) and thrombocytopenia-associated multiple organ failure (TAMOF). In cases of thrombotic thrombocytopenic purpura, therapeutic plasma exchange (TPE) is part of the standard therapy. TPE, which is well known in nephrology and hematology, is a comparatively complex procedure. The potential for nondiscriminatory removal of cytokines and mediators of inflammation has led to the exploration of TPE as a therapeutic approach in sepsis and septic shock. TPE not only facilitates the effective elimination of damaging circulating molecules but also enables the replenishment of essential plasma components that are depleted by the disease process. These components include antipermeability factors such as ADAMTS13, angiopoietin-1, and protein C, all of which are abundantly present in fresh frozen plasma (FFP) [73][41]. A deficiency of ADAMTS13, the pathophysiological correlate of TTP, results in vWF released by the endothelium not being adequately cleaved into smaller fragments. This leads to stasis of the microcirculation, subsequently impairing metabolism in the affected organs [74][42]. ADAMTS13 levels are reduced in septic shock and this reduction is associated with increased mortality, suggesting that substitution through TPE might be a promising approach [75][43]. However, data on the use of plasmapheresis in sepsis and septic shock remain limited.5. Combination Methods
5.1. oXiris
®
The oXiris® hemofilter (Baxter, IL, USA) represents a novel approach in the simultaneous removal of inflammatory mediators, endotoxin, fluid, and uremic toxins. This is achieved through the inherent hydrogel structure of the AN69 membrane. The membrane is composed of a three-layer structure and is highly electrically charged. The first layer consists of AN69 copolymer hydrogel structure, in which negatively charged methallyl sulfonate molecules are incorporated, through which cytokines, among others, are adsorbed. In addition, solutes are removed by convection through membrane pores (cut-off 40 kDa). The middle zone consists of polyethyleneimine (PEI), a positively charged multilayer linear structure, which improves biocompatibility and can adsorb negatively charged endotoxin. The third layer, in direct contact with the blood, is coated with heparin, which minimizes local thrombogenicity [80,81,82][44][45][46]. The initial clinical results for the oXiris filter are encouraging, with significant catecholamine savings observed in retrospective studies [83][47].5.2. Coupled Plasma Filtration Adsorption (CPFA)
CPFA, developed in the 1990s as a treatment for sepsis, involves a two-step process. Initially, plasma is separated from cellular blood components using a highly permeable filter similar to standard plasmapheresis. Then, within the plasma component, adsorption therapy is performed using a styrenic polymer resin before the purified plasma is reinfused back into the patient. This method also allows for simultaneous CRRT for renal support and control of fluid balance. Due to the absence of direct contact between blood cells and sorbent material, CPFA is claimed to have high biocompatibility [84][48].6. Albumin Dialysis
Conventional dialysis utilizes diffusion, filtration, and osmosis to remove waste products, toxins, and excess fluids from the blood. Yet, this method has limitations in removing larger molecules, such as albumin-bound toxins or inflammatory mediators. Albumin, the most abundant protein in human blood plasma, plays a pivotal role in maintaining colloid osmotic pressure. This pressure primarily arises from the concentration gradient of albumin between the fluid in the blood vessels and the surrounding tissues. Albumin also binds and transports hydrophobic substances in the blood, including certain amino acids, hormones, and fat-soluble substances. Furthermore, albumin has also been recognized for its capacity to bind several inflammatory mediators, exhibiting an immunomodulatory effect in systemic inflammation and sepsis via toll-like receptor-mediated signaling [87,88][49][50]. In cases of renal insufficiency, bound molecules may accumulate as they are too large to pass through the pores of conventional dialysis membranes. Albumin dialysis is a highly effective treatment to remove such noncovalently albumin-bound substances utilizing specific semipermeable membranes. In this process, the blood flows along one side of the membrane, while a dialysis fluid containing albumin is present on the opposite side. This “fresh” albumin provides binding capacity for the toxins and other albumin-bound substances and binds them after diffusion through the membrane, thereby being effectively removed from the bloodstream. In the simplest technical variant of albumin dialysis, known as single-pass albumin dialysis (SPAD), the albumin-containing dialysate is discarded after a single contact with the membrane. Considering the high cost of albumin, the daily therapy costs for SPAD often become a limiting factor in its widespread clinical use [89][51].References
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211.
- Vincent, J.L.; Jones, G.; David, S.; Olariu, E.; Cadwell, K.K. Frequency and mortality of septic shock in Europe and North America: A systematic review and meta-analysis. Crit. Care 2019, 23, 196.
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810.
- Chousterman, B.G.; Swirski, F.K.; Weber, G.F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017, 39, 517–528.
- Kemball, C.C.; Alirezaei, M.; Whitton, J.L. Type B coxsackieviruses and their interactions with the innate and adaptive immune systems. Future Microbiol. 2010, 5, 1329–1347.
- Tamayo, E.; Fernandez, A.; Almansa, R.; Carrasco, E.; Heredia, M.; Lajo, C.; Goncalves, L.; Gomez-Herreras, J.I.; de Lejarazu, R.O.; Bermejo-Martin, J.F. Pro- and anti-inflammatory responses are regulated simultaneously from the first moments of septic shock. Eur. Cytokine Netw. 2011, 22, 82–87.
- Tang, B.M.; Huang, S.J.; McLean, A.S. Genome-wide transcription profiling of human sepsis: A systematic review. Crit. Care 2010, 14, R237.
- Drifte, G.; Dunn-Siegrist, I.; Tissieres, P.; Pugin, J. Innate immune functions of immature neutrophils in patients with sepsis and severe systemic inflammatory response syndrome. Crit. Care Med. 2013, 41, 820–832.
- Nierhaus, A.; Linssen, J.; Wichmann, D.; Braune, S.; Kluge, S. Use of a weighted, automated analysis of the differential blood count to differentiate sepsis from non-infectious systemic inflammation: The intensive care infection score (ICIS). Inflamm. Allergy Drug Targets 2012, 11, 109–115.
- Nierhaus, A.; Klatte, S.; Linssen, J.; Eismann, N.M.; Wichmann, D.; Hedke, J.; Braune, S.A.; Kluge, S. Revisiting the white blood cell count: Immature granulocytes count as a diagnostic marker to discriminate between SIRS and sepsis—A prospective, observational study. BMC Immunol. 2013, 14, 8.
- Zucoloto, A.Z.; Jenne, C.N. Platelet-Neutrophil Interplay: Insights Into Neutrophil Extracellular Trap (NET)-Driven Coagulation in Infection. Front. Cardiovasc. Med. 2019, 6, 85.
- Cox, L.E.; Walstein, K.; Vollger, L.; Reuner, F.; Bick, A.; Dotsch, A.; Engler, A.; Peters, J.; von Kockritz-Blickwede, M.; Schafer, S.T. Neutrophil extracellular trap formation and nuclease activity in septic patients. BMC Anesthesiol. 2020, 20, 15.
- Ortmann, W.; Kolaczkowska, E. Age is the work of art? Impact of neutrophil and organism age on neutrophil extracellular trap formation. Cell Tissue Res. 2018, 371, 473–488.
- Camicia, G.; Pozner, R.; de Larranaga, G. Neutrophil extracellular traps in sepsis. Shock 2014, 42, 286–294.
- Daix, T.; Guerin, E.; Tavernier, E.; Mercier, E.; Gissot, V.; Herault, O.; Mira, J.P.; Dumas, F.; Chapuis, N.; Guitton, C.; et al. Multicentric Standardized Flow Cytometry Routine Assessment of Patients With Sepsis to Predict Clinical Worsening. Chest 2018, 154, 617–627.
- Keshari, R.S.; Jyoti, A.; Dubey, M.; Kothari, N.; Kohli, M.; Bogra, J.; Barthwal, M.K.; Dikshit, M. Cytokines induced neutrophil extracellular traps formation: Implication for the inflammatory disease condition. PLoS ONE 2012, 7, e48111.
- Fernandez-Sarmiento, J.; Salazar-Pelaez, L.M.; Carcillo, J.A. The Endothelial Glycocalyx: A Fundamental Determinant of Vascular Permeability in Sepsis. Pediatr. Crit. Care Med. 2020, 21, e291–e300.
- Milusev, A.; Rieben, R.; Sorvillo, N. The Endothelial Glycocalyx: A Possible Therapeutic Target in Cardiovascular Disorders. Front. Cardiovasc. Med. 2022, 9, 897087.
- Swystun, L.L.; Liaw, P.C. The role of leukocytes in thrombosis. Blood 2016, 128, 753–762.
- Grover, S.P.; Mackman, N. Tissue Factor: An Essential Mediator of Hemostasis and Trigger of Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 709–725.
- Denk, S.; Taylor, R.P.; Wiegner, R.; Cook, E.M.; Lindorfer, M.A.; Pfeiffer, K.; Paschke, S.; Eiseler, T.; Weiss, M.; Barth, E.; et al. Complement C5a-Induced Changes in Neutrophil Morphology During Inflammation. Scand. J. Immunol. 2017, 86, 143–155.
- Taylor, F.B., Jr.; Toh, C.H.; Hoots, W.K.; Wada, H.; Levi, M.; Scientific Subcommittee on Disseminated Intravascular Coagulation of the International Society on Thrombosis and Haemostasis. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb. Haemost. 2001, 86, 1327–1330.
- Iba, T.; Levi, M.; Levy, J.H. Sepsis-Induced Coagulopathy and Disseminated Intravascular Coagulation. Semin. Thromb. Hemost. 2020, 46, 89–95.
- Morgera, S.; Slowinski, T.; Melzer, C.; Sobottke, V.; Vargas-Hein, O.; Volk, T.; Zuckermann-Becker, H.; Wegner, B.; Muller, J.M.; Baumann, G.; et al. Renal replacement therapy with high-cutoff hemofilters: Impact of convection and diffusion on cytokine clearances and protein status. Am. J. Kidney Dis. 2004, 43, 444–453.
- Atari, R.; Peck, L.; Visvanathan, K.; Skinner, N.; Eastwood, G.; Bellomo, R.; Storr, M.; Goehl, H. High cut-off hemofiltration versus standard hemofiltration: Effect on plasma cytokines. Int. J. Artif. Organs 2016, 39, 479–486.
- Honore, P.M.; Jacobs, R.; Boer, W.; Joannes-Boyau, O.; De Regt, J.; De Waele, E.; Van Gorp, V.; Collin, V.; Spapen, H.D. New insights regarding rationale, therapeutic target and dose of hemofiltration and hybrid therapies in septic acute kidney injury. Blood Purif. 2012, 33, 44–51.
- Villa, G.; Neri, M.; Bellomo, R.; Cerda, J.; De Gaudio, A.R.; De Rosa, S.; Garzotto, F.; Honore, P.M.; Kellum, J.; Lorenzin, A.; et al. Nomenclature for renal replacement therapy and blood purification techniques in critically ill patients: Practical applications. Crit. Care 2016, 20, 283.
- Joannes-Boyau, O.; Honore, P.M.; Perez, P.; Bagshaw, S.M.; Grand, H.; Canivet, J.L.; Dewitte, A.; Flamens, C.; Pujol, W.; Grandoulier, A.S.; et al. High-volume versus standard-volume haemofiltration for septic shock patients with acute kidney injury (IVOIRE study): A multicentre randomized controlled trial. Intensive Care Med. 2013, 39, 1535–1546.
- Network, V.N.A.R.F.T.; Palevsky, P.M.; Zhang, J.H.; O’Connor, T.Z.; Chertow, G.M.; Crowley, S.T.; Choudhury, D.; Finkel, K.; Kellum, J.A.; Paganini, E.; et al. Intensity of renal support in critically ill patients with acute kidney injury. N. Engl. J. Med. 2008, 359, 7–20.
- Investigators, R.R.T.S.; Bellomo, R.; Cass, A.; Cole, L.; Finfer, S.; Gallagher, M.; Lo, S.; McArthur, C.; McGuinness, S.; Myburgh, J.; et al. Intensity of continuous renal-replacement therapy in critically ill patients. N. Engl. J. Med. 2009, 361, 1627–1638.
- Zhang, P.; Yang, Y.; Lv, R.; Zhang, Y.; Xie, W.; Chen, J. Effect of the intensity of continuous renal replacement therapy in patients with sepsis and acute kidney injury: A single-center randomized clinical trial. Nephrol. Dial. Transplant. 2012, 27, 967–973.
- Junhai, Z.; Beibei, C.; Jing, Y.; Li, L. Effect of High-Volume Hemofiltration in Critically Ill Patients: A Systematic Review and Meta-Analysis. Med. Sci. Monit. 2019, 25, 3964–3975.
- Yaroustovsky, M.; Plyushch, M.; Popov, D.; Samsonova, N.; Abramyan, M.; Popok, Z.; Krotenko, N. Prognostic value of endotoxin activity assay in patients with severe sepsis after cardiac surgery. J. Inflamm. 2013, 10, 8.
- Ikeda, T.; Kamohara, H.; Suda, S.; Nagura, T.; Tomino, M.; Sugi, M.; Wajima, Z. Comparative Evaluation of Endotoxin Activity Level and Various Biomarkers for Infection and Outcome of ICU-Admitted Patients. Biomedicines 2019, 7, 47.
- Vincent, J.L.; Laterre, P.F.; Cohen, J.; Burchardi, H.; Bruining, H.; Lerma, F.A.; Wittebole, X.; De Backer, D.; Brett, S.; Marzo, D.; et al. A pilot-controlled study of a polymyxin B-immobilized hemoperfusion cartridge in patients with severe sepsis secondary to intra-abdominal infection. Shock 2005, 23, 400–405.
- Cruz, D.N.; Antonelli, M.; Fumagalli, R.; Foltran, F.; Brienza, N.; Donati, A.; Malcangi, V.; Petrini, F.; Volta, G.; Bobbio Pallavicini, F.M.; et al. Early use of polymyxin B hemoperfusion in abdominal septic shock: The EUPHAS randomized controlled trial. JAMA 2009, 301, 2445–2452.
- Payen, D.M.; Guilhot, J.; Launey, Y.; Lukaszewicz, A.C.; Kaaki, M.; Veber, B.; Pottecher, J.; Joannes-Boyau, O.; Martin-Lefevre, L.; Jabaudon, M.; et al. Early use of polymyxin B hemoperfusion in patients with septic shock due to peritonitis: A multicenter randomized control trial. Intensive Care Med. 2015, 41, 975–984.
- Lipcsey, M.; Tenhunen, J.; Pischke, S.E.; Kuitunen, A.; Flaatten, H.; De Geer, L.; Sjolin, J.; Frithiof, R.; Chew, M.S.; Bendel, S.; et al. Endotoxin Removal in Septic Shock with the Alteco LPS Adsorber Was Safe But Showed no Benefit Compared to Placebo in the Double-Blind Randomized Controlled Trial-the Asset Study. Shock 2020, 54, 224–231.
- Schädler, D.; Porzelius, C.; Jörres, A.; Marx, G.; Meier-Hellmann, A.; Putensen, C.; Quintel, M.; Spies, C.; Engel, C.; Weiler, N.; et al. A multicenter randomized controlled study of an extracorporeal cytokine hemoadsorption device in septic patients. Crit. Care 2013, 17, P62.
- Kogelmann, K.; Jarczak, D.; Scheller, M.; Druner, M. Hemoadsorption by CytoSorb in septic patients: A case series. Crit. Care 2017, 21, 74.
- David, S.; Stahl, K. To remove and replace-a role for plasma exchange in counterbalancing the host response in sepsis. Crit. Care 2019, 23, 14.
- Bockmeyer, C.L.; Claus, R.A.; Budde, U.; Kentouche, K.; Schneppenheim, R.; Losche, W.; Reinhart, K.; Brunkhorst, F.M. Inflammation-associated ADAMTS13 deficiency promotes formation of ultra-large von Willebrand factor. Haematologica 2008, 93, 137–140.
- Azfar, M.F.; Khan, M.F.; Habib, S.S.; Aseri, Z.A.; Zubaidi, A.M.; Aguila, D.O.; Suriya, M.O.; Ullah, H. Prognostic value of ADAMTS13 in patients with severe sepsis and septic shock. Clin. Investig. Med. 2017, 40, E49–E58.
- Broman, M.E.; Hansson, F.; Vincent, J.L.; Bodelsson, M. Endotoxin and cytokine reducing properties of the oXiris membrane in patients with septic shock: A randomized crossover double-blind study. PLoS ONE 2019, 14, e0220444.
- Malard, B.; Lambert, C.; Kellum, J.A. In vitro comparison of the adsorption of inflammatory mediators by blood purification devices. Intensive Care Med. Exp. 2018, 6, 12.
- Monard, C.; Rimmele, T.; Ronco, C. Extracorporeal Blood Purification Therapies for Sepsis. Blood Purif. 2019, 47 (Suppl. S3), 1–14.
- Schwindenhammer, V.; Girardot, T.; Chaulier, K.; Gregoire, A.; Monard, C.; Huriaux, L.; Illinger, J.; Leray, V.; Uberti, T.; Crozon-Clauzel, J.; et al. oXiris(R) Use in Septic Shock: Experience of Two French Centres. Blood Purif. 2019, 47 (Suppl. S3), 1–7.
- Tetta, C.; Cavaillon, J.M.; Schulze, M.; Ronco, C.; Ghezzi, P.M.; Camussi, G.; Serra, A.M.; Curti, F.; Lonnemann, G. Removal of cytokines and activated complement components in an experimental model of continuous plasma filtration coupled with sorbent adsorption. Nephrol. Dial. Transplant. 1998, 13, 1458–1464.
- Alcaraz-Quiles, J.; Casulleras, M.; Oettl, K.; Titos, E.; Flores-Costa, R.; Duran-Guell, M.; Lopez-Vicario, C.; Pavesi, M.; Stauber, R.E.; Arroyo, V.; et al. Oxidized Albumin Triggers a Cytokine Storm in Leukocytes Through P38 Mitogen-Activated Protein Kinase: Role in Systemic Inflammation in Decompensated Cirrhosis. Hepatology 2018, 68, 1937–1952.
- Casulleras, M.; Flores-Costa, R.; Duran-Guell, M.; Alcaraz-Quiles, J.; Sanz, S.; Titos, E.; Lopez-Vicario, C.; Fernandez, J.; Horrillo, R.; Costa, M.; et al. Albumin internalizes and inhibits endosomal TLR signaling in leukocytes from patients with decompensated cirrhosis. Sci. Transl. Med. 2020, 12, eaax5135.
- Schmuck, R.B.; Nawrot, G.H.; Fikatas, P.; Reutzel-Selke, A.; Pratschke, J.; Sauer, I.M. Single Pass Albumin Dialysis-A Dose-Finding Study to Define Optimal Albumin Concentration and Dialysate Flow. Artif. Organs 2017, 41, 153–161.
More
