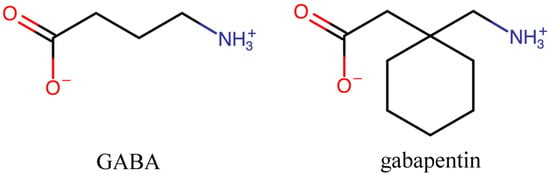
Gabapentin (GBP) is a common name for 1-(aminomethyl)cyclohexaneacetic acid (C9H17NO2, CAS Registry No. 60142-96-3), a GABA (γ-aminobutyric acid) derivative and a popular active pharmaceutical ingredient (API). It has a molecular weight of 171.34 and two pKa values of 3.68 and 10.70.
1. Introduction
Gabapentin (GBP) is a common name for 1-(aminomethyl)cyclohexaneacetic acid (C
9H
17NO
2, CAS Registry No. 60142-96-3), a GABA (γ-aminobutyric acid) derivative (
Figure 1) and a popular active pharmaceutical ingredient (API)
[1][2][1,2]. It has a molecular weight of 171.34 and two pKa values of 3.68 and 10.70
[3][4][3,4]. Therefore, at physiological pH, GBP exists in the form of a zwitterion. It was originally developed in 1977 in an effort to create a structural analog of gamma-aminobutyric acid (GABA) with higher lipophilicity than the original neurotransmitter, thus enhancing its ability to enter the central nervous system
[5].
Figure 1.
Chemical structure of GABA and its derivative, gabapentin (GBP).
Gabapentin is an antiepileptic drug that is considered a first-line treatment for the management of neuropathic pain. GBP is also approved for the treatment of focal seizures. However, it is ineffective in treating generalized epilepsy
[4][6][7][4,6,7]. Aside from neuropathic pain, off-label use in primary care is very common. These include the treatment of a wide range of conditions such as bipolar disorder, complex regional pain syndrome, attention deficit disorder, restless legs syndrome, and periodic limb movement, alongside sleep disorders, headaches, alcohol withdrawal syndrome, chronic back pain, fibromyalgia, visceral pain, and acute postoperative pain
[5].
Despite its quite simple formula, it took almost 25 years from the first synthesis to the crystal structure determination of GBP in 2001. However, during the subsequent 23 years, multiple forms of this API have been successfully obtained, and their crystal structures have been solved. GBP, due to its relatively short half-life, is usually administered three times daily. Therefore, exploration of the solid landscape of this drug has been, at least partially, motivated by the desire to improve its pharmacokinetic properties.
2. Pharmaceutical Properties of GBP
2.1. Pharmacological Properties
2.1.1. Mechanism of Action
Despite multiple extensive studies, the exact mechanisms of action of GPB remain unknown
[4][8][9][4,12,13]. It has been proven in vivo that GBP does not bind to GABA receptors
[8][12] despite its structural similarity to this neurotransmitter. However, it displays a high affinity for the α2δ-1 subunit of voltage-gated calcium channels (VGCCs)
[8][10][12,14]. Therefore, it is commonly considered that GBP’s analgesic effects are due to the suppression of calcium currents by binding to the α2δ-1 subunit, resulting in reduced postsynaptic excitability
[10][14]. This assumption, however, is inaccurate because GBP has not been demonstrated to reliably inhibit Ca
2+ currents
[8][12]. Despite this, GBP is helpful in the therapy of neuropathic pain, which is achieved by inhibiting the release of different neurotransmitters at neural synapses
[4][8][4,12].
2.1.2. Pharmacokinetics
GBP is absorbed in the small intestine. The only factor that affects GBP absorption is L-type amino acid transporter (LAT), which is easily saturable and causes dose-dependent pharmacokinetics
[11][15]. More specifically, LAT-1 actively carries GBP across the blood–brain barrier. The area under the plasma concentration–time curve (AUC) does not rise in proportion to an increase in GBP dosage. This API has no affinity for plasma proteins. Peak levels of cerebrospinal fluid require a median of 8 h to reach, which is a considerably longer time than peak plasma levels. GBP does not influence spinal neurotransmitter concentrations of glutamate, norepinephrine, substance P, or calcitonin gene-related peptide. The volume of distribution of GPB is 0.8 L/kg, and it is highly water soluble. Although GBP is not metabolized by the liver and does not impact the major isoenzymes of the cytochrome P450 system, case studies have reported drug-induced hepatotoxicity
[12][16]. Elimination is mostly performed by the kidney and is proportional to creatinine clearance. Adverse reactions may arise from accumulation, leading to renal failure
[5].
2.2. Medical Uses
2.2.1. Neuropathic Pain
Gabapentin is effective in the therapy of postherpetic neuralgia and diabetic neuropathy; however, there is limited evidence in other types of neuropathic pain
[8][12]. Numerous international and regional professional organizations have released clinical practice guidelines recommending gabapentinoids, including GBP, as first-line therapy. For neuropathic pain other than trigeminal neuralgia, the National Institute of Clinical Excellence (NICE) guidelines prescribe gabapentin, pregabalin, amitriptyline, or duloxetine as the first line of treatment
[8][12].
2.2.2. Seizures
GBP is a second-generation antiseizure drug, which has been shown to be effective as an addition to other anticonvulsants in the treatment of partial seizures and generalized tonic–clonic seizures in children over the age of 12
[4]. In three extensive multicenter, double-blind, randomized dosage, controlled studies, 649 patients were involved, and the results showed that gabapentin, when used alone, was both safe and effective in treating partial seizures
[4]. Gabapentin is ineffective in absence seizures
[13][17].
2.2.3. Drug Dependence
GBP is one of several anticonvulsants that have been studied for the treatment of drug abuse disorders. Their effectiveness in treating cocaine addiction has been shown to be ineffective
[14][18], and while the evidence for treating alcohol and cannabis addiction is promising, it is either not sufficient or of low quality
[15][16][19,20].
2.2.4. Restless Legs Syndrome
In a comparative analysis of suggested therapies for restless legs syndrome, GBP was found to be linked to comparable reductions in the International Restless Legs Syndrome, receiving a similar score as dopamine agonists
[17][21]. On the other hand, a higher improvement in the Periodic Limb Movement Index was linked to dopamine agonists
[17][21]. Regarding the Clinical Practice Guideline of the American Academy of Sleep Medicine, GBP has been accepted as a possible therapeutic choice for this syndrome
[18][22]. However, only GBP enacarbil is approved in the United States for the treatment of this illness
[5].
2.3. Dosages
Gabapentin is well tolerated at doses ranging from 800 to 1800 mg/day
[9][13]. However, according to the medication package insert of some drugs, patients may be treated with doses as high as 3600 mg/day
[4].
2.3.1. Dosages in Epilepsy
Gabapentin oral doses are administered three times daily due to its relatively short half-life
[4]. For adults and children over 12 years old with epilepsy, dosages up to 2400 mg per day are advised. Rapid titration can be performed with doses of 300 mg once daily on the first day, which are usually at bedtime to avoid side effects like sedation and drowsiness, 300 mg twice daily on the second day, and 300 mg three times daily on the third day. If efficacy is not obtained at this dose, the dosage may be increased further.
2.3.2. Dosages in Neuropathic Pain
The starting dose for the treatment of neuropathic pain is 300 mg three times per day, with escalation if necessary to a daily maximum of 3600 mg, although there have been reports of doses up to 4200 mg. The beneficial effects of gabapentin in neuropathic pain and in a variety of other chronic pain disorders are supported by evidence from both animal and human trials
[4].
2.4. Side Effects
Dizziness, sedation, somnolence, peripheral edema, and weight gain are the most frequent adverse effects; these side effects appear to be dose-dependent. GBP’s relative lack of interactions and severe side effects make it a desirable therapeutic alternative
[5].