Mesenchymal stem/stromal cells (MSCs) are multipotent cells located in different areas of the human body. The oral cavity is considered a potential source of MSCs because they have been identified in several dental tissues (D-MSCs). Clinical trials in which cells from these sources were used have shown that they are effective and safe as treatments for tissue regeneration. Importantly, immunoregulatory capacity has been observed in all of these populations. Since this property is of clinical interest for cell therapy protocols, it is relevant to analyze the differences in immunoregulatory capacity, as well as the mechanisms used by each type of MSC. Interestingly, D-MSCs are the most suitable source for regenerating mineralized tissues in the oral region. Furthermore, the clinical potential of D-MSCs is supported due to their adequate capacity for proliferation, migration, and differentiation. There is also evidence for their potential application in protocols against autoimmune diseases and other inflammatory conditions due to their immunosuppressive capacity.
- mesenchymal stem/stromal cells
- dental tissue
- immune cells
1. Introduction
2. Mesenchymal Stromal Cells from Dental and Periodontal Tissues
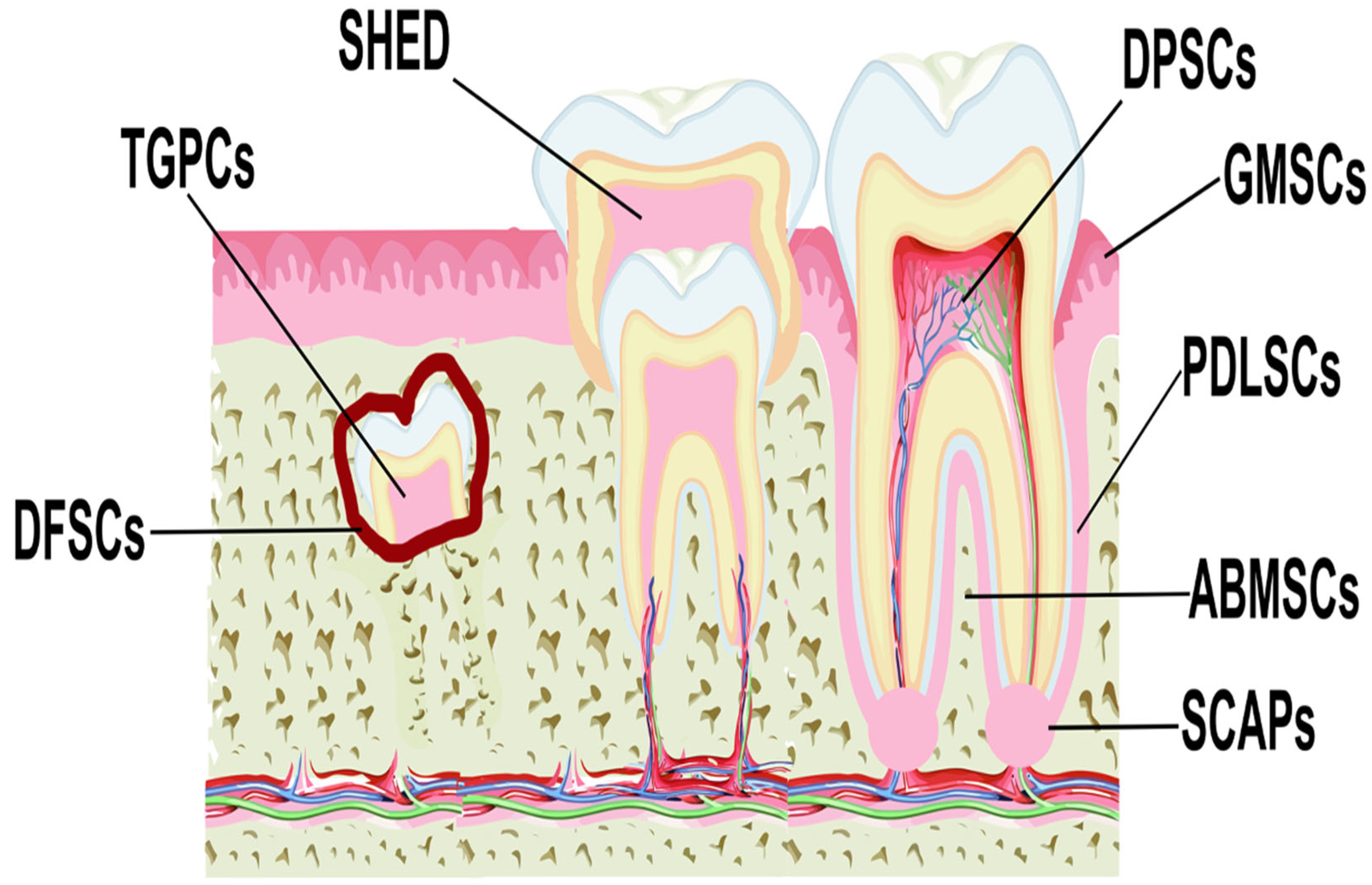
MSCs have been isolated from various anatomical regions of the oral cavity (
Figure 1), including the dental pulp (DPSCs) [
37,
38], periodontal ligament (PDLSCs) [
39,
40], gingival tissue (GMSCs) [
41,
42], apical papilla (SCAPs) [
43,
44], dental follicle (DFSCs) [
45,
46], human exfoliated deciduous teeth (SHED) [
47,
48], alveolar bone (ABMSCs), and tooth germ (TGPCs) [
34], and these are collectively defined as dental MSCs (D-MSCs) [
49].].
| Source | Efficiency of Isolation | Surface Markers | Embryonic Markers |
Neural Markers | Differentiation Potential |
|---|---|---|---|---|---|
| DPSCs | ++++ | CD13, CD29, CD44, CD59, CD73, CD90, CD105, CD146 | STRO-1, OCT-4, Nanog, SSEA-1, SEEA-4, SOX-2 | β3-tubulin, NFM, Nestin, CNPase, S100, CD271 | Adipogenic, osteogenic, odontoblast, angiogenic, and neuronal cells |
| PDLSCs | ++++ | CD10, CD29, CD44, CD73, CD105 | SSEA-1, SSEA-3, SSEA-4, TRA-1–60, TRA-1–81, OCT-4, Nanog, SOX-2, REX1, ALP |
Nestin, OCT-4, SSEA-4, CD271, SOX-10 | Adipogenic, chondrogenic, osteogenic, and neuronal cells |
| GMSCs | ++++ | CD73, CD90, CD105 | SSEA-4, OCT-4, Nanog | Nestin, SOX10, β3-tubulin, NFM, CNPase | Adipogenic, chondrogenic, osteogenic, angiogenic, and neuronal cells |
| SCAPs | +++ | CD24, CD44, CD90, CD146, STRO-1 | OCT-4, Nanog, NOTCH-1, SOX-2 | OCT-4, SOX2, Nestin | Adipogenic, chondrogenic osteogenic, odontogenic, and neuronal cells |
| DFSCs | ++ | CD13, CD29, CD44, CD56, CD59, CD90, CD105, CD106, CD166, STRO-1 | OCT-4, Nanog, NOTCH-1, SOX-2 | OCT-4, SOX2, Nestin | Osteogenic, odontogenic, and cementogenic |
| SHED | +++ | CD29, CD73, CD90, CD146, CD166 | OCT-4, Nanog, SSEA-3, SSEA-4, NOTCH-1, SOX-2 | β3-tubulin, NFM, Nestin, CNPase, GAD, NeuN, GFAP, CD271, Vimentin, OCT-4, PAX-6, NSE, MAP-2, PSA- NCAM | Adipogenic, chondrogenic, osteogenic, odontogenic, angiogenic, and neuronal cells |
| ABMSCs | ++++ | CD73, CD90, CD105, STRO-1 | Oct4, KLF4, Sox2, cMyc | NF-M, NeuN, GFAP | Adipogenic, chondrogenic, and osteogenic |
| TGPCs | + | CD29, CD73, CD90, CD105, CD166 | Nanog, OCT-4, SOX-2, Klf4, c-Myc | Nestin | Adipogenic, chondrogenic, osteogenic, and neuronal cells |
3. Immunomodulatory Properties of MSCs
In vitro, preclinical and clinical studies have shown that MSCs are capable of regulating inflammatory processes, an essential event for reducing tissue damage and promoting tissue repair [79][67]. To do this, they migrate to injured sites through the support of adhesion molecules, chemokines, and their receptors, as well as chemoattractant molecules, such as hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), transforming growth factor β (TGF-β) 1, granulocyte colony stimulating factor, and tumor necrosis factor α (TNF-α) [80,81,82,83][68][69][70][71]. Once they reach the site of inflammation, MSCs regulate the proliferation, differentiation, maturation, and production of soluble factors and the cytotoxicity of immune cells [84][72]. The regulatory mechanisms of MSCs include the secretion of soluble factors, the production of metabolites, cell–cell contact, and the release of extracellular vesicles (EVs). Some of the anti-inflammatory molecules that MSCs secrete include interleukin (IL)-10 [85[73][74][75][76],86,87,88], IL-6 [89[77][78],90], TGF-β [91[79][80][81],92,93], prostaglandin E2 (PGE2) [94,95[82][83][84],96], gene-6 stimulated by tumor necrosis factor (TSG-6) [97[85][86],98], HLA-G5 [99[87][88],100], galectins [101][89], and chemokine ligand 2 with a CC motif (CCL2) [101][89]. On the other hand, MSCs express intra- and extracellular enzymes that help the formation of anti-inflammatory metabolites, such as inducible nitric oxide synthase (iNOS), which allows for the generation of nitric oxide (NO) and reduces the migration of immune cells by decreasing chemoattractant molecules and the production of inflammatory cytokines [102[90][91][92],103,104], or indoleamine 2,3-dioxygenase (IDO), which depletes tryptophan in immune cells and generates anti-inflammatory molecules, including kinurenine and picolinic acid [105,106,107][93][94][95]. Finally, MSCs express the ectonucleotidases CD39 and CD73, which generate adenosine (ADO), modulating the production of inflammatory cytokines, cytotoxicity, apoptosis, and the proliferation of immune cells [108,109,110,111,112][96][97][98][99][100]. Among their membrane molecules, MSCs express programmed death ligands (PDLs) 1 and 2 [113,114][101][102], HLA-G1 [100][88], cytotoxic T lymphocyte antigen 4 (CTLA-4) [115][103], and intercellular adhesion molecule-1 (ICAM-1) [116][104]. Finally, the EVs secreted by MSCs transport and express immunomodulatory molecules and cytokines, such as microRNAs, TGF-β, galectin 1, and PDL-1 [117][105]. The immunomodulatory capacity of MSCs is of great relevance to the application of these cells in the clinical field. It is necessary to know in depth the biological mechanisms that allow them to sense their microenvironment to activate this property; Preclinical and clinical trials have given good results, and MSCs have been evaluated to improve adverse physiological conditions related to the immune system, which has created great expectations in cell therapy procedures.References
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The Development of Fibroblast Colonies in Monolayer Cultures of Guinea-Pig Bone Marrow and Spleen Cells. Cell Prolif. 1970, 3, 393–403.
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317.
- Gao, G.; Fan, C.; Li, W.; Liang, R.; Wei, C.; Chen, X.; Yang, Y.; Zhong, Y.; Shao, Y.; Kong, Y.; et al. Mesenchymal Stem Cells: Ideal Seeds for Treating Diseases. Hum. Cell 2021, 34, 1585–1600.
- Tyndall, A. Successes and Failures of Stem Cell Transplantation in Autoimmune Diseases. Hematol. Am. Soc. Hematol. Educ. Program. 2011, 2011, 280–284.
- Haddad, R.; Saldanha-Araujo, F. Mechanisms of T-Cell Immunosuppression by Mesenchymal Stromal Cells: What Do We Know So Far? Biomed. Res. Int. 2014, 2014, 216806.
- Siegel, G.; Kluba, T.; Hermanutz-Klein, U.; Bieback, K.; Northoff, H.; Schäfer, R. Phenotype, Donor Age and Gender Affect Function of Human Bone Marrow-Derived Mesenchymal Stromal Cells. BMC Med. 2013, 11, 146.
- Ganguly, P.; El-Jawhari, J.J.; Burska, A.N.; Ponchel, F.; Giannoudis, P.V.; Jones, E.A. The Analysis of In Vivo Aging in Human Bone Marrow Mesenchymal Stromal Cells Using Colony-Forming Unit-Fibroblast Assay and the CD45(low) CD271(+) Phenotype. Stem Cells Int. 2019, 2019, 5197983.
- Tokalov, S.V.; Gruner, S.; Schindler, S.; Wolf, G.; Baumann, M.; Abolmaali, N. Age-Related Changes in the Frequency of Mesenchymal Stem Cells in the Bone Marrow of Rats. Stem Cells Dev. 2007, 16, 439–446.
- Li, J.; Wong, W.H.; Chan, S.; Chim, J.C.; Cheung, K.M.; Lee, T.L.; Au, W.Y.; Ha, S.Y.; Lie, A.K.; Lau, Y.L.; et al. Factors Affecting Mesenchymal Stromal Cells Yield from Bone Marrow Aspiration. Chin. J. Cancer Res. 2011, 23, 43–48.
- Selle, M.; Koch, J.D.; Ongsiek, A.; Ulbrich, L.; Ye, W.; Jiang, Z.; Krettek, C.; Neunaber, C.; Noack, S. Influence of Age on Stem cells Depends on the Sex of the Bone Marrow Donor. J. Cell. Mol. Med. 2022, 26, 1594–1605.
- Katsara, O.; Mahaira, L.G.; Iliopoulou, E.G.; Moustaki, A.; Antsaklis, A.; Loutradis, D.; Stefanidis, K.; Baxevanis, C.N.; Papamichail, M.; Perez, S.A. Effects of Donor Age, Gender, and In Vitro Cellular Aging on the Phenotypic, Functional, and Molecular Characteristics of Mouse Bone Marrow-Derived Mesenchymal Stem Cells. Stem Cells Dev. 2011, 20, 1549–1561.
- Bonab, M.M.; Alimoghaddam, K.; Talebian, F.; Ghaffari, S.H.; Ghavamzadeh, A.; Nikbin, B. Aging of Mesenchymal Stem Cell In Vitro. BMC Cell Biol. 2006, 7, 14.
- Yang, Y.-H.K.; Ogando, C.R.; Wang See, C.; Chang, T.-Y.; Barabino, G.A. Changes in Phenotype and Differentiation Potential of Human Mesenchymal Stem Cells Aging In Vitro. Stem Cell Res. Ther. 2018, 9, 131.
- Lamas, J.R.; Fernandez-Gutierrez, B.; Mucientes, A.; Marco, F.; Lopiz, Y.; Jover, J.A.; Abasolo, L.; Rodriguez-Rodriguez, L. RNA Sequencing of Mesenchymal Stem Cells Reveals a Blocking of Differentiation and Immunomodulatory Activities Under Inflammatory Conditions in Rheumatoid Arthritis Patients. Arthritis Res. Ther. 2019, 21, 112.
- Sibov, T.T.; Severino, P.; Marti, L.C.; Pavon, L.F.; Oliveira, D.M.; Tobo, P.R.; Campos, A.H.; Paes, A.T.; Amaro, E.; Gamarra, L.F.; et al. Mesenchymal Stem Cells from Umbilical Cord Blood: Parameters for Isolation, Characterization and Adipogenic Differentiation. Cytotechnology 2012, 64, 511–521.
- Araújo, A.B.; Furlan, J.M.; Salton, G.D.; Schmalfuss, T.; Röhsig, L.M.; Silla, L.M.R.; Passos, E.P.; Paz, A.H. Isolation of Human Mesenchymal Stem Cells from Amnion, Chorion, Placental Decidua and Umbilical Cord: Comparison of Four Enzymatic Protocols. Biotechnol. Lett. 2018, 40, 989–998.
- Zuk, P.A.; Zhu, M.; Ashjian, P.; Ugarte, D.A.D.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol. Biol. Cell 2002, 13, 4279–4295.
- Mizuno, M.; Katano, H.; Mabuchi, Y.; Ogata, Y.; Ichinose, S.; Fujii, S.; Otabe, K.; Komori, K.; Ozeki, N.; Koga, H.; et al. Specific Markers and Properties of Synovial Mesenchymal Stem Cells in the Surface, Stromal, and Perivascular Regions. Stem Cell Res. Ther. 2018, 9, 123.
- Castro-Manrreza, M.E.; Bonifaz, L.; Castro-Escamilla, O.; Monroy-Garcia, A.; Cortes-Morales, A.; Hernandez-Estevez, E.; Hernandez-Cristino, J.; Mayani, H.; Montesinos, J.J. Mesenchymal Stromal Cells from the Epidermis and Dermis of Psoriasis Patients: Morphology, Immunophenotype, Differentiation Patterns, and Regulation of T Cell Proliferation. Stem Cells Int. 2019, 2019, 4541797.
- Sabatini, F.; Petecchia, L.; Tavian, M.; Jodon de Villeroche, V.; Rossi, G.A.; Brouty-Boye, D. Human Bronchial Fibroblasts Exhibit a Mesenchymal Stem Cell Phenotype and Multilineage Differentiating Potentialities. Lab. Investig. 2005, 85, 962–971.
- Najimi, M.; Khuu, D.N.; Lysy, P.A.; Jazouli, N.; Abarca, J.; Sempoux, C.; Sokal, E.M. Adult-Derived Human Liver Mesenchymal-Like Cells as a Potential Progenitor Reservoir of Hepatocytes? Cell Transplant. 2007, 16, 717–728.
- Ouryazdanpanah, N.; Dabiri, S.; Derakhshani, A.; Vahidi, R.; Farsinejad, A. Peripheral Blood-Derived Mesenchymal Stem Cells: Growth Factor-Free Isolation, Molecular Characterization and Differentiation. Iran. J. Pathol. 2018, 13, 461–466.
- Lin, W.; Xu, L.; Lin, S.; Shi, L.; Wang, B.; Pan, Q.; Lee, W.Y.W.; Li, G. Characterisation of Multipotent Stem Cells from Human Peripheral Blood Using an Improved Protocol. J. Orthop. Transl. 2019, 19, 18–28.
- Chen, Y.R.; Yan, X.; Yuan, F.Z.; Ye, J.; Xu, B.B.; Zhou, Z.X.; Mao, Z.M.; Guan, J.; Song, Y.F.; Sun, Z.W.; et al. The Use of Peripheral Blood-Derived Stem Cells for Cartilage Repair and Regeneration In Vivo: A Review. Front. Pharmacol. 2020, 11, 404.
- Chong, P.P.; Selvaratnam, L.; Abbas, A.A.; Kamarul, T. Human Peripheral Blood Derived Mesenchymal Stem Cells Demonstrate Similar Characteristics and Chondrogenic Differentiation Potential to Bone Marrow Derived Mesenchymal Ctem Cells. J. Orthop. Res. 2012, 30, 634–642.
- Li, B.; Ouchi, T.; Cao, Y.; Zhao, Z.; Men, Y. Dental-Derived Mesenchymal Stem Cells: State of the Art. Front. Cell Dev. Biol. 2021, 9, 654559.
- Morris, A.L.; Tadi, P. Anatomy, Head and Neck, Teeth. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022.
- Hovorakova, M.; Lesot, H.; Peterka, M.; Peterkova, R. Early Development of the Human Dentition Revisited. J. Anat. 2018, 233, 135–145.
- Bartlett, J.D. Dental Enamel Development: Proteinases and Their Enamel Matrix Substrates. ISRN Dent. 2013, 2013, 684607.
- Samiei, M.; Alipour, M.; Khezri, K.; Saadat, Y.R.; Forouhandeh, H.; Abdolahinia, E.D.; Vahed, S.Z.; Sharifi, S.; Dizaj, S.M. Application of Collagen and Mesenchymal Stem Cells in Regenerative Dentistry. Curr. Stem Cell Res. Ther. 2022, 17, 606–620.
- Goldberg, M.; Kulkarni, A.B.; Young, M.; Boskey, A. Dentin: Structure, Composition and Mineralization. Front. Biosci. 2011, 3, 711–735.
- Ghannam, M.; Alameddine, H.; Bordoni, B. Anatomy, Head and Neck, Pulp (Tooth). In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2023.
- Olaru, M.; Sachelarie, L.; Calin, G. Hard Dental Tissues Regeneration-Approaches and Challenges. Materials 2021, 14, 2558.
- Cabana-Munoz, M.E.; Pelaz Fernandez, M.J.; Parmigiani-Cabana, J.M.; Parmigiani-Izquierdo, J.M.; Merino, J.J. Adult Mesenchymal Stem Cells from Oral Cavity and Surrounding Areas: Types and Biomedical Applications. Pharmaceutics 2023, 15, 2109.
- Torabi, S.; Soni, A. Histology, Periodontium. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022.
- Koller, A.; Sapra, A. Anatomy, Head and Neck, Oral Gingiva. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022.
- Agha-Hosseini, F.; Jahani, M.A.; Jahani, M.; Mirzaii-Dizgah, I.; Ali-Moghaddam, K. In Vitro Isolation of Stem Cells Derived from Human Dental Pulp. Clin. Transplant. 2010, 24, E23–E28.
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal Human Dental Pulp Stem Cells (DPSCs) In Vitro and In Vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630.
- Navabazam, A.R.; Sadeghian Nodoshan, F.; Sheikhha, M.H.; Miresmaeili, S.M.; Soleimani, M.; Fesahat, F. Characterization of Mesenchymal Stem Cells from Human Dental Pulp, Preapical Follicle and Periodontal Ligament. Iran. J. Reprod. Med. 2013, 11, 235–242.
- Seo, B.-M.; Miura, M.; Gronthos, S.; Mark Bartold, P.; Batouli, S.; Brahim, J.; Young, M.; Gehron Robey, P.; Wang, C.Y.; Shi, S. Investigation of Multipotent Postnatal Stem Cells from Human Periodontal Ligament. Lancet 2004, 364, 149–155.
- Mitrano, T.I.; Grob, M.S.; Carrion, F.; Nova-Lamperti, E.; Luz, P.A.; Fierro, F.S.; Quintero, A.; Chaparro, A.; Sanz, A. Culture and Characterization of Mesenchymal Stem Cells from Human Gingival Tissue. J. Periodontol. 2010, 81, 917–925.
- Zhang, Q.; Shi, S.; Liu, Y.; Uyanne, J.; Shi, Y.; Shi, S.; Le, A.D. Mesenchymal Stem Cells Derived from Human Gingiva Are Capable of Immunomodulatory Functions and Ameliorate Inflammation-Related Tissue Destruction in Experimental Colitis. J. Immunol. 2009, 183, 7787–7798.
- Sonoyama, W.; Liu, Y.; Yamaza, T.; Tuan, R.S.; Wang, S.; Shi, S.; Huang, G.T. Characterization of the Apical papilla and Its Residing Stem Cells from Human Immature Permanent Teeth: A Pilot Study. J. Endod. 2008, 34, 166–171.
- Sequeira, D.B.; Oliveira, A.R.; Seabra, C.M.; Palma, P.J.; Ramos, C.; Figueiredo, M.H.; Santos, A.C.; Cardoso, A.L.; Peca, J.; Santos, J.M. Regeneration of Pulp-Dentin Complex Using Human Stem Cells of the Apical Papilla: In Vivo Interaction with Two Bioactive Materials. Clin. Oral Investig. 2021, 25, 5317–5329.
- Handa, K.; Saito, M.; Tsunoda, A.; Yamauchi, M.; Hattori, S.; Sato, S.; Toyoda, M.; Teranaka, T.; Narayanan, A.S. Progenitor Cells from Dental Follicle Are Able to Form Cementum Matrix In Vivo. Connect. Tissue Res. 2002, 43, 406–408.
- Qu, G.; Li, Y.; Chen, L.; Chen, Q.; Zou, D.; Yang, C.; Zhou, Q. Comparison of Osteogenic Differentiation Potential of Human Dental-Derived Stem Cells Isolated from Dental Pulp, Periodontal Ligament, Dental Follicle, and Alveolar Bone. Stem Cells Int. 2021, 2021, 6631905.
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem Cells from Human Exfoliated Deciduous Teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812.
- Nakajima, K.; Kunimatsu, R.; Ando, K.; Ando, T.; Hayashi, Y.; Kihara, T.; Hiraki, T.; Tsuka, Y.; Abe, T.; Kaku, M.; et al. Comparison of the Bone Regeneration Ability Between Stem Cells from Human Exfoliated Deciduous Teeth, Human Dental Pulp Stem Cells and Human Bone Marrow Mesenchymal Stem Cells. Biophys. Res. Commun. 2018, 497, 876–882.
- Sharpe, P.T. Dental Mesenchymal Stem Cells. Development 2016, 143, 2273.
- Banavar, S.R.; Rawal, S.Y.; Paterson, I.C.; Singh, G.; Davamani, F.; Khoo, S.P.; Tan, E.L. Establishing a Technique for Isolation and Characterization of Human Periodontal Ligament Derived Mesenchymal Stem Cells. Saudi Dent. J. 2021, 33, 693–701.
- Naz, S.; Khan, F.R.; Zohra, R.R.; Lakhundi, S.S.; Khan, M.S.; Mohammed, N.; Ahmad, T. Isolation and Culture of Dental Pulp Stem Cells from Permanent and Deciduous teeth. Pak. J. Med. Sci. 2019, 35, 997–1002.
- Raoof, M.; Yaghoobi, M.M.; Derakhshani, A.; Kamal-Abadi, A.M.; Ebrahimi, B.; Abbasnejad, M.; Shokouhinejad, N. A Modified Efficient Method for Dental Pulp Stem Cell Isolation. Dent. Res. J. 2014, 11, 244–250.
- Zhang, X.; Zeng, D.; Huang, F.; Wang, J. A Protocol for Isolation and Culture of Mesenchymal stem Cells from human Gingival Tissue. Am. J. Clin. Exp. Immunol. 2019, 8, 21–26.
- Remy, M.; Ferraro, F.; Le Salver, P.; Rey, S.; Genot, E.; Djavaheri-Mergny, M.; Thebaud, N.; Boiziau, C.; Boeuf, H. Isolation and Culture of Human Stem Cells from Apical Papilla under Low Oxygen Concentration Highlight Original Properties. Cells 2019, 8, 1485.
- Morsczeck, C. Dental Follicle Stem Cells. In Stem Cell Biology and Tissue Engineering in Dental Sciences; Elsevier: Amsterdam, The Netherlands, 2015; pp. 271–277.
- Hernandez-Monjaraz, B.; Santiago-Osorio, E.; Monroy-Garcia, A.; Ledesma-Martinez, E.; Mendoza-Nunez, V.M. Mesenchymal Stem Cells of Dental Origin for Inducing Tissue Regeneration in Periodontitis: A Mini-Review. Int. J. Mol. Sci. 2018, 19, 944.
- Ledesma-Martinez, E.; Mendoza-Nunez, V.M.; Santiago-Osorio, E. Mesenchymal Stem Cells Derived from Dental Pulp: A Review. Stem Cells Int. 2016, 2016, 4709572.
- Dave, J.R.; Tomar, G.B. Dental Tissue–Derived Mesenchymal Stem Cells: Applications in Tissue Engineering. Crit. Rev. Biomed. Eng. 2018, 46, 429–468.
- Ni, X.; Xia, Y.; Zhou, S.; Peng, H.; Wu, X.; Lu, H.; Wang, H.; Liu, R.; Blazar, B.R.; Gu, J.; et al. Reduction in Murine Acute GVHD Severity by Human Gingival Tissue-Derived Mesenchymal Stem Cells Via the CD39 Pathways. Cell Death Dis. 2019, 10, 13.
- De la Rosa-Ruiz, M.d.P.; Álvarez-Pérez, M.A.; Cortés-Morales, V.A.; Monroy-García, A.; Mayani, H.; Fragoso-González, G.; Caballero-Chacón, S.; Diaz, D.; Candanedo-González, F.; Montesinos, J.J. Mesenchymal Stem/Stromal Cells Derived from Dental Tissues: A Comparative In Vitro Evaluation of Their Immunoregulatory Properties Against T cells. Cells 2019, 8, 1491.
- Fracaro, L.; Senegaglia, A.C.; Herai, R.H.; Leitolis, A.; Boldrini-Leite, L.M.; Rebelatto, C.L.K.; Travers, P.J.; Brofman, P.R.S.; Correa, A. The Expression Profile of Dental Pulp-Derived Stromal Cells Supports Their Limited Capacity to Differentiate into Adipogenic Cells. Int. J. Mol. Sci. 2020, 21, 2753.
- Deng, C.; Sun, Y.; Liu, H.; Wang, W.; Wang, J.; Zhang, F. Selective Adipogenic Differentiation of Human Periodontal Ligament Stem Cells Stimulated with High Doses of Glucose. PLoS ONE 2018, 13, e0199603.
- Du, L.; Yang, P.; Ge, S. Isolation and Characterization of Human Gingiva-Derived Mesenchymal Stem Cells Using Limiting Dilution Method. J. Dent. Sci. 2016, 11, 304–314.
- Dong, R.; Yao, R.; Du, J.; Wang, S.; Fan, Z. Depletion of Histone Demethylase KDM2A Enhanced the Adipogenic and Chondrogenic Differentiation Potentials of Stem Cells from Apical Papilla. Exp. Cell Res. 2013, 319, 2874–2882.
- Koutsoumparis, A.E.; Patsiarika, A.; Tsingotjidou, A.; Pappas, I.; Tsiftsoglou, A.S. Neural Differentiation of Human Dental Mesenchymal Stem Cells Induced by ATRA and UDP-4: A Comparative Study. Biomolecules 2022, 12, 218.
- Gao, Y.; Tian, Z.; Liu, Q.; Wang, T.; Ban, L.K.; Lee, H.H.; Umezawa, A.; Almansour, A.I.; Arumugam, N.; Kumar, R.S.; et al. Neuronal Cell Differentiation of Human Dental Pulp Stem Cells on Synthetic Polymeric Surfaces Coated with ECM Proteins. Front. Cell Dev. Biol. 2022, 10, 893241.
- Rao, S.R.; Subbarayan, R.; Dinesh, M.G.; Arumugam, G.; Raja, S.T. Differentiation of Human Gingival Mesenchymal Stem Cells into Neuronal Lineages in 3D Bioconjugated Injectable Protein Hydrogel Construct for the Management of Neuronal Disorder. Exp. Mol. Med. 2016, 48, e209.
- Wu, T.; Xu, W.; Chen, H.; Li, S.; Dou, R.; Shen, H.; Liu, X.; Liu, X.; Hong, Y.; He, J. Comparison of the Differentiation of Dental Pulp Stem Cells and Periodontal Ligament Stem Cells into Neuron-Like Cells and Their Effects on Focal Cerebral Ischemia. Acta Biochim. Biophys. Sin. 2020, 52, 1016–1029.
- Li, N.; Dai, X.; Yang, F.; Sun, Y.; Wu, X.; Zhou, Q.; Chen, K.; Sun, J.; Bi, W.; Shi, L.; et al. Spontaneous Spheroids from Alveolar Bone-Derived Mesenchymal Stromal Cells Maintain Pluripotency of Stem Cells by Regulating Hypoxia-Inducible Factors. Biol. Res. 2023, 56, 17.
- Yan, X.; Yan, F.; Mohammed, H.A.G.; Liu, O. Maxillofacial-Derived Mesenchymal Stem Cells: Characteristics and Progress in Tissue Regeneration. Stem Cells Int. 2021, 2021, 5516521.
- Zhao, J.; Zhou, Y.H.; Zhao, Y.Q.; Gao, Z.R.; Ouyang, Z.Y.; Ye, Q.; Liu, Q.; Chen, Y.; Tan, L.; Zhang, S.H.; et al. Oral Cavity-Derived Stem Cells and Preclinical Models of Jaw-Bone Defects for Bone Tissue Engineering. Stem Cell Res. Ther. 2023, 14, 39.
- Mosaddad, S.A.; Rasoolzade, B.; Namanloo, R.A.; Azarpira, N.; Dortaj, H. Stem Cells and Common Biomaterials in Dentistry: A Review Study. J. Mater. Sci. Mater. Med. 2022, 33, 55.
- Zhai, Q.; Dong, Z.; Wang, W.; Li, B.; Jin, Y. Dental Stem Cell and Dental Tissue Regeneration. Front. Med. 2019, 13, 152–159.
- Liu, J.; Yu, F.; Sun, Y.; Jiang, B.; Zhang, W.; Yang, J.; Xu, G.T.; Liang, A.; Liu, S. Concise Reviews: Characteristics and Potential Applications of Human Dental Tissue-Derived Mesenchymal Stem Cells. Stem Cells 2015, 33, 627–638.
- Aydin, S.; Sahin, F. Stem Cells Derived from Dental Tissues. Adv. Exp. Med. Biol. 2019, 1144, 123–132.
- Santilli, F.; Fabrizi, J.; Santacroce, C.; Caissutti, D.; Spinello, Z.; Candelise, N.; Lancia, L.; Pulcini, F.; Delle Monache, S.; Mattei, V. Analogies and Differences between Dental Stem Cells: Focus on Secretome in Combination with Scaffolds in Neurological Disorders. Stem Cell Rev. Rep. 2024, 20, 159–174.
- Smojver, I.; Katalinic, I.; Bjelica, R.; Gabric, D.; Matisic, V.; Molnar, V.; Primorac, D. Mesenchymal Stem Cells Based Treatment in Dental Medicine: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 1662.
- Gan, L.; Liu, Y.; Cui, D.; Pan, Y.; Zheng, L.; Wan, M. Dental Tissue-Derived Human Mesenchymal Stem Cells and Their Potential in Therapeutic Application. Stem Cells Int. 2020, 2020, 8864572.
- Newman, R.E.; Yoo, D.; LeRoux, M.A.; Danilkovitch-Miagkova, A. Treatment of Inflammatory Diseases with Mesenchymal Stem Cells. Inflamm. Allergy Drug Targets 2009, 8, 110–123.
- Li, L.; Jiang, J. Regulatory Factors of Mesenchymal Stem Cell Migration into Injured Tissues and Their Signal Transduction Mechanisms. Front. Med. 2011, 5, 33–39.
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, A.G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784.
- Maeda, A. Recruitment of Mesenchymal Stem Cells to Damaged Sites by Plant-Derived Components. Front. Cell Dev. Biol. 2020, 8, 437.
- Sohni, A.; Verfaillie, C.M. Mesenchymal Stem Cells Migration Homing and Tracking. Stem Cells Int. 2013, 2013, 130763.
- Hass, R.; Kasper, C.; Bohm, S.; Jacobs, R. Different Populations and Sources of Human Mesenchymal Atem Cells (MSC): A Comparison of Adult and Neonatal Tissue-Derived MSC. Cell Commun. Signal 2011, 9, 1–14.
- Wang, J.; Ren, H.; Yuan, X.; Ma, H.; Shi, X.; Ding, Y. Interleukin-10 Secreted by Mesenchymal Stem Cells Attenuates Acute Liver Failure through Inhibiting Pyroptosis. Hepatol. Res. 2018, 48, E194–E202.
- Nakajima, M.; Nito, C.; Sowa, K.; Suda, S.; Nishiyama, Y.; Nakamura-Takahashi, A.; Nitahara-Kasahara, Y.; Imagawa, K.; Hirato, T.; Ueda, M.; et al. Mesenchymal Stem Cells Overexpressing Interleukin-10 Promote Neuroprotection in Experimental Acute Ischemic Stroke. Mol. Ther. Methods Clin. Dev. 2017, 6, 102–111.
- Zhang, C.; Delawary, M.; Huang, P.; Korchak, J.A.; Suda, K.; Zubair, A.C. IL-10 mRNA Engineered MSCs Demonstrate Enhanced Anti-Inflammation in an Acute GvHD Model. Cells 2021, 10, 3101.
- Xiao, S.; Huang, G.; Wei, Z.; Nie, K.; Liu, Z.; Deng, C.; Wang, D. IL-10 Gene-Modified Human Amniotic Mesenchymal Stem Cells Augment Regenerative Wound Healing by Multiple Synergistic Effects. Stem Cells Int. 2019, 2019, 9158016.
- Dorronsoro, A.; Lang, V.; Ferrin, I.; Fernandez-Rueda, J.; Zabaleta, L.; Perez-Ruiz, E.; Sepulveda, P.; Trigueros, C. Intracellular Role of IL-6 in Mesenchymal Stromal Cell Immunosuppression and Proliferation. Sci. Rep. 2020, 10, 21853.
- Huang, P.; Zhang, C.; Delawary, M.; Korchak, J.A.; Suda, K.; Zubair, A.C. Development and Evaluation of IL-6 Overexpressing Mesenchymal Stem Cells (MSCs). J. Tissue Eng. Regen. Med. 2022, 16, 244–253.
- Niu, J.; Yue, W.; Le-Le, Z.; Bin, L.; Hu, X. Mesenchymal Stem Cells Inhibit T Cell Activation by Releasing TGF-β1 from TGF-β1/GARP Complex. Oncotarget 2017, 8, 99784–99800.
- Lynch, K.; Treacy, O.; Chen, X.; Murphy, N.; Lohan, P.; Islam, M.N.; Donohoe, E.; Griffin, M.D.; Watson, L.; McLoughlin, S.; et al. TGF-beta1-Licensed Murine MSCs Show Superior Therapeutic Efficacy in Modulating Corneal Allograft Immune Rejection In Vivo. Mol. Ther. 2020, 28, 2023–2043.
- Liu, F.; Qiu, H.; Xue, M.; Zhang, S.; Zhang, X.; Xu, J.; Chen, J.; Yang, Y.; Xie, J. MSC-Secreted TGF-beta Regulates Lipopolysaccharide-Stimulated Macrophage M2-Like Polarization Via the Akt/FoxO1 Pathway. Stem Cell Res. Ther. 2019, 10, 345.
- Yanez, R.; Oviedo, A.; Aldea, M.; Bueren, J.A.; Lamana, M.L. Prostaglandin E2 Plays a Key Role in the Immunosuppressive Properties of Adipose and Bone Marrow Tissue-Derived Mesenchymal Stromal Cells. Exp. Cell Res. 2010, 316, 3109–3123.
- Qi, J.; Tang, X.; Li, W.; Chen, W.; Yao, G.; Sun, L. Mesenchymal Stem Cells Inhibited the Differentiation of MDSCs Via COX2/PGE2 in Experimental Sialadenitis. Stem Cell Res. Ther. 2020, 11, 325.
- Sun, X.; Su, Y.; Liu, X.; Liu, F.; Zhang, G.; Chen, Q.; Wang, C.; Fu, H.; Zhu, X.; Liu, K.; et al. PGE2 Dependent Inhibition of Macrophage Pyroptosis by MSCs Contributes to Alleviating aGVHD. Blood 2020, 136, 15.
- Li, Q.; Song, W.J.; Ryu, M.O.; Nam, A.; An, J.H.; Ahn, J.O.; Bhang, D.H.; Jung, Y.C.; Youn, H.Y. TSG-6 Secreted by Human Adipose Tissue-Derived Mesenchymal Stem Cells Ameliorates Severe Acute Pancreatitis Via ER Stress Downregulation in Mice. Stem Cell Res. Ther. 2018, 9, 255.
- Zhao, Y.; Zhu, X.Y.; Song, T.; Zhang, L.; Eirin, A.; Conley, S.; Tang, H.; Saadiq, I.; Jordan, K.; Lerman, A.; et al. Mesenchymal Stem Cells protect Renal Tubular Cells Via TSG-6 Regulating Macrophage Sunction and Phenotype Switching. Am. J. Physiol. Ren. Physiol. 2021, 320, F454–F463.
- Selmani, Z.; Naji, A.; Zidi, I.; Favier, B.; Gaiffe, E.; Obert, L.; Borg, C.; Saas, P.; Tiberghien, P.; Rouas-Freiss, N.; et al. Human Leukocyte Antigen-G5 Secretion by Human Mesenchymal Stem Cells is Required to Suppress T Lymphocyte and Natural Killer Function and to Induce CD4+CD25highFOXP3+ Tegulatory T Cells. Stem Cells 2008, 26, 212–222.
- Ding, D.C.; Chou, H.L.; Chang, Y.H.; Hung, W.T.; Liu, H.W.; Chu, T.Y. Characterization of HLA-G and Related Immunosuppressive Effects in Human Umbilical Cord Stroma-Derived Stem Cells. Cell Transplant. 2016, 25, 217–228.
- Jiang, W.; Xu, J. Immune Modulation by Mesenchymal Stem Cells. Cell Prolif. 2020, 53, e12712.
- Li, W.; Ren, G.; Huang, Y.; Su, J.; Han, Y.; Li, J.; Chen, X.; Cao, K.; Chen, Q.; Shou, P.; et al. Mesenchymal Stem Cells: A Double-Edged Sword in Regulating Immune Responses. Cell Death Differ. 2012, 19, 1505–1513.
- Maria, A.T.J.; Rozier, P.; Fonteneau, G.; Sutra, T.; Maumus, M.; Toupet, K.; Cristol, J.P.; Jorgensen, C.; Guilpain, P.; Noel, D. iNOS Activity Is Required for the Therapeutic Effect of Mesenchymal Stem Cells in Experimental Systemic Sclerosis. Front. Immunol. 2018, 9, 3056.
- Tedgui, A.; Mallat, Z. Anti-Inflammatory Mechanisms in the Vascular Wall. Circ. Res. 2001, 88, 877–887.
- Routy, J.P.; Routy, B.; Graziani, G.M.; Mehraj, V. The Kynurenine Pathway Is a Double-Edged Sword in Immune-Privileged Sites and in Cancer: Implications for Immunotherapy. Int. J. Tryptophan Res. 2016, 9, 67–77.
- Meesuk, L.; Tantrawatpan, C.; Kheolamai, P.; Manochantr, S. The Immunosuppressive Capacity of Human Mesenchymal Stromal Cells Derived from Amnion and Bone Marrow. Biochem. Biophys. Rep. 2016, 8, 34–40.
- Torres Crigna, A.; Uhlig, S.; Elvers-Hornung, S.; Kluter, H.; Bieback, K. Human Adipose Tissue-Derived Stromal Cells Suppress Human, but Not Murine Lymphocyte Proliferation, via Indoleamine 2,3-Dioxygenase Activity. Cells 2020, 9, 2419.
- Kerkelä, E.; Laitinen, A.; Rabina, J.; Valkonen, S.; Takatalo, M.; Larjo, A.; Veijola, J.; Lampinen, M.; Siljander, P.; Lehenkari, P.; et al. Adenosinergic Immunosuppression by Human Mesenchymal Stromal Cells Requires Co-Operation with T Cells. Stem Cells 2016, 34, 781–790.
- Saldanha-Araujo, F.; Ferreira, F.I.; Palma, P.V.; Araujo, A.G.; Queiroz, R.H.; Covas, D.T.; Zago, M.A.; Panepucci, R.A. Mesenchymal Stromal Cells Up-Regulate CD39 and Increase Adenosine Production to Suppress Activated T-Lymphocytes. Stem Cell Res. 2011, 7, 66–74.
- Zhang, X.; Huang, F.; Li, W.; Dang, J.L.; Yuan, J.; Wang, J.; Zeng, D.L.; Sun, C.X.; Liu, Y.Y.; Ao, Q.; et al. Human Gingiva-Derived Mesenchymal Stem Cells Modulate Monocytes/Macrophages and Alleviate Atherosclerosis. Front. Immunol. 2018, 9, 878.
- Luo, Y.; Wu, W.; Gu, J.; Zhang, X.; Dang, J.; Wang, J.; Zheng, Y.; Huang, F.; Yuan, J.; Xue, Y.; et al. Human Gingival Tissue-Derived MSC Suppress Osteoclastogenesis and Bone Erosion Via CD39-Adenosine Signal Pathway in Autoimmune Arthritis. EBioMedicine 2019, 43, 620–631.
- Antonioli, L.; Fornai, M.; Blandizzi, C.; Haskó, G. Adenosine Regulation of the Immune System. In The Adenosine Receptors; Borea, P.A., Varani, K., Gessi, S., Merighi, S., Vincenzi, F., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 499–514.
- Davies, L.C.; Heldring, N.; Kadri, N.; Le Blanc, K. Mesenchymal Stromal Cell Secretion of Programmed Death-1 Ligands Regulates T Cell Mediated Immunosuppression. Stem Cells 2017, 35, 766–776.
- Di Tinco, R.; Bertani, G.; Pisciotta, A.; Bertoni, L.; Pignatti, E.; Maccaferri, M.; Bertacchini, J.; Sena, P.; Vallarola, A.; Tupler, R.; et al. Role of PD-L1 in Licensing Immunoregulatory Function of Dental Pulp Mesenchymal Stem Cells. Stem Cell Res. Ther. 2021, 12, 598.
- Gaber, T.; Schonbeck, K.; Hoff, H.; Tran, C.L.; Strehl, C.; Lang, A.; Ohrndorf, S.; Pfeiffenberger, M.; Rohner, E.; Matziolis, G.; et al. CTLA-4 Mediates Inhibitory Function of Mesenchymal Stem/Stromal Cells. Int. J. Mol. Sci. 2018, 19, 2312.
- Montesinos, J.J.; López-García, L.; Cortés-Morales, V.A.; Arriaga-Pizano, L.; Valle-Ríos, R.; Fajardo-Orduña, G.R.; Castro-Manrreza, M.E. Human Bone Marrow Mesenchymal Stem/Stromal Cells Exposed to an Inflammatory Environment Increase the Expression of ICAM-1 and Release Microvesicles Enriched in This Adhesive Molecule: Analysis of the Participation of TNF-α and IFN-γ. J. Immunol. Res. 2020, 2020, 8839625.
- Lopez-Garcia, L.; Castro-Manrreza, M.E. TNF-alpha and IFN-gamma Participate in Improving the Immunoregulatory Capacity of Mesenchymal Stem/Stromal Cells: Importance of Cell-Cell Contact and Extracellular Vesicles. Int. J. Mol. Sci. 2021, 22, 9531.
