Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Deanna M Minich and Version 2 by Deanna M Minich.
Maca (Lepidium meyenii, Lepidium peruvianum) is part of the Brassicaceae family and grows at high altitudes in the Peruvian Andes mountain range (3500–5000 m). Historically, it has been used as a nutrient-dense food and for its medicinal properties, primarily in enhancing energy and fertility.
- maca
- menopause
- phenotype
- prostate
- reproductive health
1. Introduction
Maca refers to two distinct species known as Lepidium meyenii and Lepidium peruvianum [1], which are classified as the wild and cultivated forms of the plant, respectively. Maca is an annual cruciferous root vegetable and one of 249 known Lepidium species of plants [2]. It belongs to the same botanical Brassicaceae family as the turnip, cabbage, mustard, and broccoli, yet it is phytochemically distinct from this vegetable group [3]. Its predominant and native growing location is 3500–5000 m above sea level in Peru’s high, harsh-weathered Andean plateaus [4][5][6][7][8][4,5,6,7,8].
Due to its rising consumer demand in China, it has also begun to be cultivated in select areas with high altitudes, such as the Yunnan province of China (2800–3500 m) [9][10][9,10] and even Tibet (above 3000 m) [11][12][11,12]. However, the maca cultivated in these non-native locations exhibit different characteristics since the growing environment can significantly affect the plant phenotype and composition [8]. As reported by the American Botanical Council, there is a potential toxic feature of Chinese maca due to the use of pesticides and herbicides to accommodate for the difference in altitude relative to the Andean highlands, alongside chemical contamination in the agricultural settings in parts of China, especially the Yunnan province [13]. In 2018, the Botanicals Adulterants Prevention Program reported on maca powder being diluted with corn, wheat, and yam powders [14]. While there was an initial boom in the production and prices of Chinese maca, the demand and prices have declined drastically over the years [15]. Peruvian maca continues to uphold its superiority, as is seen by its relatively higher market prices [15]. Compared with Chinese maca, it is considered distinct in appearance and more pungent in aroma and taste [15] (Scheme 1).
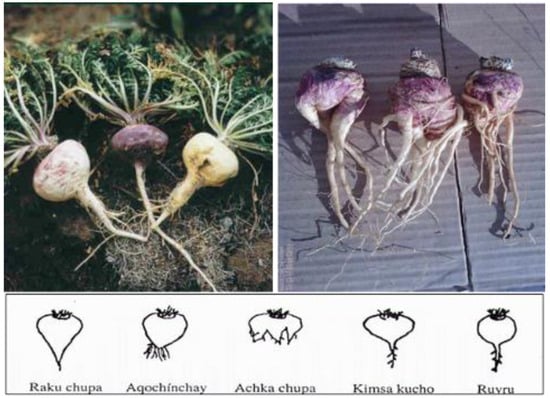
Scheme 1. Peruvian (left) and Chinese (right) maca. The Peruvian maca as shown on the left was cultivated in Junín (Ancash), Peru (4200 m above sea level), and the Chinese maca on the right is from Shangri-La, China (3180 m above sea level). The shapes presented below the photos indicate the variety in shapes of hypocotyls. As described by Meissner et al. [16], the Peruvian hypocotyl shape resembled the “Kimsa kucho” and “Ruyru” forms, while the Chinese maca hypocotyls were primarily classified as “Achka chupa” and “Aqochinchay,” resembling the appearance of ginseng, with a relatively small amount categorized as “Raku chupa”. Various explanations for the Chinese maca shapes have been provided, including injury from transplantation from greenhouses to outdoor, commercial plantation sites, invasion by nematodes or microbial and/or fungal soil infections from the relatively lower altitude, and less UV radiation to disinfect soil. The photo was provided with permission by Henry O. Meissner, Ph.D., as published in [16]. Reprinted from Meissner, H.O.; Xu, L.; Wan, W.; Yi, F. Glucosinolates profiles in Maca phenotypes cultivated in Peru and China (Lepidium peruvianum syn. L. meyenii). Phytochem. Lett. 2019, 31, 208–216., with permission from Elsevier (1874-3900/ @ 2019 Phytochemical Society of Europe. Published by Elsevier Ltd. All rights reserved.),The lower half of the photo (the figure) is reprinted originally from Meissner HO, Mscisz A, Kedzia B, Pisulewski P, Piatkowska E. Peruvian Maca: Two Scientific Names Lepidium Meyenii Walpers and Lepidium Peruvianum Chacon – Are They Phytochemical-ly-Synonymous? Int J Biomed Sci. 2015 Mar;11(1):1–15. PMCID: PMC4392557. This is an open-access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.5/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Depending on how the maca is processed and prepared, there can be significant alterations in parameters such as safety, stability, quality, bioactives, and clinical efficacy [8]. Exposure to environmental stressors, like altitude, ultraviolet radiation, sunlight, temperature extremes, intense wind, and varying moisture conditions, as well as soil, minerals, and microbiomes, all contribute to maca’s complex, therapeutic phytochemical profile [3][17][3,17], a concept referred to as xenohormesis [18]. Thus, these factors must be considered when evaluating maca in research studies and may even be implicated in disparate findings.
Maca, first described in 1553, serves as a dietary staple of native Peruvians, particularly in its dried hypocotyl (tuber) format at >20 g daily [3]. As a therapeutic agent, it has been used in many preparations primarily for energy, fertility, libido, and as a vitality tonic for aging [3][19][20][3,19,20]. Over the years, the research citations listed for the search term “maca” through the National Library of Medicine PubMed® database started with just two references in 1961. As of November 7, 2023, using the search terms “maca,” “maca” [in title], “maca” [in title/abstract], “Lepidium meyenii” [in title/abstract], and “Lepidium peruvianum” [in title/abstract], results in 843, 256, 528, 292, and 17 findings, respectively [21]. Beginning with the early 2000s until the present day (2023), preclinical and clinical research has provided data that would expand maca’s use into other areas of health such as menstrual cycle regulation [22][23][22,23], menopausal symptoms [24][25][26][27][24,25,26,27], osteoporosis [28], sperm quality [29][30][31][32][33][34][35][29,30,31,32,33,34,35], memory [36][37][38][39][36,37,38,39], mood [36][40][36,40], prostate health [41][42][43][44][45][46][41,42,43,44,45,46], and fitness optimization (e.g., reducing inflammation and increasing strength) [47][48][49][47,48,49]. Even though maca has broader applications, there has been historical research emphasis on its ability to modify the endocrine system, pioneered by the work of Gonzales et al. on males [29][31][33][42][50][51][29,31,33,42,50,51] and Meissner et al. on pre- and post-menopausal women [24][25][26][27][24,25,26,27]. Meissner et al. continue to conduct ongoing research at five universities in Poland to investigate the use of different maca phenotypes to treat specific medical conditions associated with various menopausal symptoms, men’s health, and even prevalent health areas of concern shared by both genders [52].
Over the past two decades, alongside its increasing presence in scientific publications, maca has become popular in the supplement industry as capsules, powders, gummies, tablets, liquids, and tinctures. The rise in consumer-friendly formats has risen in parallel with interest in a non-pharmaceutical, low-cost, non-toxic drug option for various conditions. Most notably, maca has been colloquially referred to as “Peruvian Viagra” as a substitute for medications typically used for erectile dysfunction, like sildenafil and tadalafil [8][53][54][8,53,54]. In addition to sexual health, the interest in maca as a viable alternative to medications has been more extensively explored for benign prostatic hyperplasia (BPH) and menopausal symptoms due to potential contraindications or side effects that can occur with drugs like finasteride [55][56][57][55,56,57] or even hormone replacement therapy (HRT) [58]. Some women have explored maca as another option or even in conjunction with in vitro fertilization (IVF). In a survey of 95 women seeking infertility treatment, maca was on the list of herbal supplements commonly used [59]. Of those participants taking maca, 71.4% took it for fertility, and 28.6% used it for general health and wellness [59].
In the modern day, maca has been increasingly visible as part of nutritional regimens in the format of a powdered “superfood” [60] available in grocery stores, without much distinction or delineation as to the specific type (color or phenotype) of maca the product contains. An online search using the term “maca” on Amazon.com under the subcategory of “vitamins, minerals, and supplements” provides a listing of over 3000 results [61]. Furthermore, maca dietary supplements do not typically list standardized compounds to ensure reliable results related to known actives [62]. Therefore, there can plausibly be mixed, inconsistent physiological responses to maca powder due to this point being largely unknown. A few companies in the food and herb sector have begun to feature different colors of raw and gelatinized maca powders or tinctures (yellow, red, and black) for designated health functions, although this effort has been minimal [63][64][63,64]. Due to the limited and sometimes conflicting research on health applications based on phenotype, the recommendations of which color to use are, in some cases, debatable and may be, in other cases, potentially incorrect.
2. Species of Maca
The species of maca are distinct in appearance and phytochemical profiles, with Lepidium meyenii Walpers being the wildcrafted form of Peruvian maca first described by German botanist Gerhard Walpers in 1843 [8] and Lepidium peruvianium Chacón de Popovici, the cultivated, domesticated version of maca, identified by Dr. Gloria Chacón de Popovici, in which the colors were characterized [1][65][1,66]. There has been some taxonomic debate over the years about these two species, although a more detailed analysis suggests differences in physical appearance, phytochemicals, and DNA [8][66][67][8,67,68]. Along these lines, many scientific publications and even commercial natural products list Lepidium meyenii, which can include wild maca from a variety of countries such as Bolivia, Colombia, Brazil, and Argentina; however, it may be that what is used is Lepidium peruvianium [1]. While these two species’ names have been referred to as synonyms, distinctions exist [1]. Meissner et al. mentioned that much of the research has been conducted on the cultivated Peruvian maca (Lepidium peruvianum), though it is referred to as the wildcrafted maca (Lepidium meyenii) within the scientific literature [24]. There is an ongoing discussion about making a finalized decision on United States Pharmacopeia (USP) monographs for maca root, maca root powder, and maca root glucosinolates dry extract based on validated methods of analysis [68][69].3. The Nutrition of Maca
As a fresh root, maca contains more than 80% water [17]. Peruvians commonly drink it as a juice at home and when compared against non-consumers of maca, experience several health benefits, including higher levels of estradiol and testosterone, lower systolic blood pressure, and serum levels of interleukin-6 (IL-6), as well as an improvement in chronic mountain sickness and better performance on a lower-limb strength test [69][97]. Indeed, these benefits would seem to come from consistent consumption in the daily diet over years, if not decades, when considering that published clinical research over short periods (weeks or even months) from raw, gelatinized, or even extracts of maca have not demonstrated statistically significant efforts on hormones. There can be variability in maca’s nutrient levels depending on several factors: the location grown [7][70][7,72], its size and weight [66][67], the part of maca tested [60], and postharvest conditions involving drying, storage, and maceration [71][83]. Maca’s macronutrient composition as a dehydrated powder includes carbohydrates (55–73%, mostly starch), fiber (8.2–25.6%), protein (8.9–21%), and fat (0.6–2.2%) [7][10][17][7,10,17]. Amino acids identified in various parts of maca include aspartic acid, glutamic acid, serine, glycine, cysteine, alanine, arginine, tyrosine, hydroxyproline, proline, histidine, threonine, phenylalanine, D-phenylalanine, valine, methionine, isoleucine, leucine, lysine, and tryptophan [60]. The predominant starchy nature of the maca tuber resembles the high carbohydrate content (70–85%) in other root vegetables such as potato, sweet potato, and cassava, as well as grains like wheat and maize [7]. This starch content in maca is relatively easy to gelatinize, altering its solubility and making it less shelf-stable [7]. Zhang et al. [7] tested yellow, purple, and black maca (the methods section stated the “roots” were tested) and reported no significant difference in amylose contents between them, with the range being 21.0–21.3%. However, each color of maca tested did exhibit differential viscosity, with yellow maca showing high viscosity relative to the purple and black formats, denoting that they may have defined applications in the food industry [7]. Some of these polysaccharides are being tested in vitro for their immunomodulating ability in cancer cells [72][73][98,99]. Maca is micronutrient dense, containing vitamins and minerals, such as vitamin A, vitamin B2, vitamin B6, boron, calcium, chromium, copper, iron, magnesium, manganese, niacin, sodium, potassium, and zinc [10][19][60][66][74][75][76][10,19,60,67,79,81,100]. The origin of maca may impact the micronutrient density. For example, one study [75][81] found that select phenotypes grown in a particular area of China had a wide range of sodium content (less than 30 to 2600 mg/kg dry weight), whereas Peruvian maca had a lower sodium concentration range (110–190 mg/kg dry weight). Conversely, another study [10] showed negligible differences in sodium content in maca from different locations. Researchers at Jinzhou Medical University have suggested that essential oils, lipids, and polysaccharides are the biologically active constituents in L. meyenii and may contribute to its antioxidant activity, with the essential oils being the most potent contributor to free-radical scavenging action [9]. There may be more complexity to the constituents of maca. Carvalho and Ribeiro [19] reported on 101 bioactive phytochemicals in L. meyenii extracts. These structurally diverse secondary metabolite compounds encompass a wide range of secondary phytometabolites such as glucosinolates, isothiocyanates, flavonols, phytosterols, polysaccharides, fatty acid derivatives (2-oxononadecanoic acid, anandamide, oleamide), and alkaloids such as macaenes, macamides, and thiohydantoins [19][77][19,101]. Meissner et al. list seven categories of phytochemicals within L. peruvianum with known physiological relevance: amides, carbolines, catechins, cyanogenic compounds, fatty acids, glucosinolates, and imidazole alkaloids [70][72].Nutritional Differences between Colors of Maca
Researchers quantified 79 different nutrients and metabolites in three colors of Chinese maca (yellow, black, and violet) [78][102]. In this study, yellow maca was found to be high in carbohydrates, black maca was rich in protein, and violet maca was highest in antioxidant capacity. While the overall macronutrient (protein, carbohydrate, fat, fiber) composition between the four main maca phenotypes (black, purple, red, and yellow) was within relatively similar ranges for the Peruvian maca analyzed, Meissner et al. identified one distinct difference in fatty acid composition [66][67]. Namely, the fatty acid, C18:1n-9 (elaidic acid), the trans isomer of oleic acid, is substantially higher in the yellow phenotype, although the implications of that higher concentration are unknown [66][67]. Another study indicated that red maca had higher protein and potassium content but less soluble reducing sugars, riboflavin, and iron than black maca [9]. Analytical attempts have been made to evaluate the phytochemical content of the different colorful varieties of L. meyenii [9][79][9,71] and L. peruvianum [39][66][70][80][39,67,72,103]. Seven compounds were reliably detected in yellow, black, white, and purple maca (L. meyenii) samples grown in China: p-hydroxybenzylglucosinolate, benzylglucosinolate, N-benzyl-9Z,12Z,15Z-octadecatrienamide, N-benzyl-9Z,12Z-octadecadienamide, N-(3-methoxybenzyl)-hexadecanamide, N-benzyl-hexadecanamide, and N-benzyl-9Z-octadecenamide [79][71]. Of these samples tested, yellow and black maca were highest in glucosinolates (1.55%), followed by white (0.93%) and purple (0.76%) maca [79][71]. Macamides were lowest in black maca (0.15%) compared with the other colors (0.23–0.29%) [79][71]. The quantity of certain phytochemicals may vary with the growing stage, as demonstrated in a study assessing the transcriptomics of black maca [81][82]. The secondary metabolites are presumed to be responsible for the purported health benefits of maca alone or in combination. As noted above, distinct differences have been observed between phenotypes, phytochemicals, and cultivation location [79][71], suggesting the genes’ ability to express uniquely in those respective environments [78][102].4. Clinical Application: Endocrine System Support
The rationale for this focus is that this area is where its central claims for use (e.g., energy, fertility, menopausal symptoms, prostate health, reproductive function) reside from a mechanistic point of view and where there exists most of the research, relative to its effects on other parts of the body.4.1. Adrenal Health
In botanical medicine, maca is classified as an adaptogen [3][82][3,145]. Adaptogenic herbs are unique from other substances in their ability to modulate hormones and the immune system, assisting with maintaining optimal homeostasis. Adaptogens have a modulating effect on the body and can either tone down the activity of hyper-functioning systems or strengthen the activity of hypo-functioning systems [83][150]. While the primary proposed mechanism of action for adaptogens is the impact on the hypothalamic–pituitary–adrenal [84][151] axis, especially cortisol output, they can also display anti-inflammatory and antioxidant effects and influence gene regulation involved in modulating pathways of detoxification and stress regulation [83][150]. Indeed, maca’s function would fit the definition of an adaptogen; however, as discussed herein, could maca be in a category by itself? It may be separate from being solely classified as an adaptogen due to the various phenotypes having different physiological effects and modulating other systems beyond the adrenal glands. The research utilizing standardized, quality-controlled maca formulations indicates that the entire endocrine axis, consisting of the hypothalamus, pituitary gland, thyroid gland, adrenal gland, and gonads (HPTAG axis), is impacted.4.2. Ovarian Health
Scientific research suggests that when combining the purported benefits of select colors, maca’s mechanisms surpass that of a classically defined adaptogen [27]. Importantly, research conducted more than fifty years ago by Dr. Gloria Chacón de Popovici [85][152] touted that alkaloids in maca were stimulating the testes and ovaries of rats, extending its beneficial effects past the hypothalamus–pituitary–adrenal [84][151] axis into the hypothalamus–pituitary–adrenal–gonad (HPAG) axis [27]. However, to date, only one select formulation of specific concentrated maca phenotypes (referred to in research as Maca-GO®, commercially as Femmenessence®) has exhibited modulation of the hypothalamic–pituitary–ovarian (HPO) axis in early postmenopausal women via clinical changes in estradiol (E2), progesterone (P), follicle-stimulating hormone (FSH), and luteinizing hormone (LH) [24][25][26][27][24,25,26,27]. Conversely, in another clinical trial, postmenopausal Chinese women from Hong Kong taking 3.3 g daily of an unspecified color of maca known commercially as Maca Power (Healthychoices, Murwillumbah, NSW, Australia) for six weeks did not experience any changes in E2, FSH, or LH, compared with six weeks on a placebo [86][153]. It was not stated in this study whether the maca used was raw or gelatinized. Similar to these latter findings, the researchers found no differences in these hormones in fourteen postmenopausal women taking 3.5 g of Maca Power (Incan Food, Murwillumbah, NSW, Australia) daily for six weeks with a six-week crossover on placebo. There were no significant differences in serum concentrations of these hormones (E2, FSH, LH), even though symptoms on the Greene Climacteric Scale, such as anxiety, depression, and sexual dysfunction, were improved using Maca Power [87][154]. A study in healthy, perimenopausal Japanese women given a maca extract standardized to at least 1.2% benzyl glucosinolate, known as Maca-BG1.2TM (CPX PERU S.A.C., not currently sold commercially), for eight weeks found a 2.2-fold increase in E2 levels, although this was not statistically significant [88][155]. These findings, in conjunction with the Maca-GO® results, highlight how important phenotype and concentration are in eliciting statistically significant effects on hormones in peri- and post-menopausal women. Concerning menopausal symptoms, one of the studies using Maca Power mentioned above reported non-significant menopausal symptom reduction compared with a placebo (using Greene’s Climacteric Scale, the total score after six weeks of maca supplementation was 17.6 ± 10.0, p = 0.07, and the placebo group was 16.8 ± 9.1, p < 0.05) [86][153]. Alternatively, the clinical trials using Maca-GO® reported highly significant reductions in menopausal symptoms in early postmenopausal women, notably hot flushes and night sweats, and overall symptoms, including nervousness, depression, sleep, and heart palpitations, using Kupperman’s Menopausal Index and Greene’s Menopausal Score (p < 0.001) [24][25][26][24,25,26]. These results further validate how important phenotype and concentration are for ensuring optimal clinical outcomes. Eighty-five percent of peri- and postmenopausal women experience various menopausal symptoms, including hot flushes, night sweats, sleep disturbances, mood imbalances, loss of libido, weight gain, and vaginal dryness, that can last for decades [89][90][156,157]. Apart from debilitating symptoms impacting the quality of life, the significant loss of hormones experienced at these stages of life results in substantial increases in morbidity and life-threatening conditions, ranging from osteoporosis to cardiovascular disease [91][158]. These serious health implications have naturally given rise to the use of HRT and bioidentical hormone therapies (BHRT). While the introduction of exogenous hormones is a logical solution, the fluctuations of hormones experienced during perimenopause and the simultaneous increase or proportional increase, such as between E2 and testosterone (T), indicates that a potentially more optimal functional medicine approach would be to support the declining function of the endocrine (hypothalamus–pituitary–thyroid–adrenal–ovarian, HPTAO) axis, thereby creating hormonal “balance”. With this novel mechanism of action of supporting the endocrine axis and regulating the endogenous production of hormones despite the absence of the introduction of exogenous hormones, it would seem that this would be an ideal first step as a treatment for many women. From that point forward, a clinician could assess if there is a need for additional exogenous hormone support and, if so, which hormones and to what degree, aligning with the current North American Menopausal Society (NAMS), Endocrine Society, and National Health Service (NHS) recommendations of personalizing the dose (often the lowest) and the duration of HRT (often the shortest) to the individual patient to maximize benefits and minimize risks [92][93][94][159,160,161]. Furthermore, this strategy offers an alternative for women who are over ten years beyond their menopausal transition, where there are heightened concerns about starting hormone therapy at an advanced age. It may also be a helpful, more natural approach for women who are looking for long-term hormone support throughout their life without side effects or risk or women for whom hormone therapy may not be an option or desired for various reasons (77% of women surveyed in one study were reluctant to do hormone therapy despite having symptoms) [95][162]. Apart from identifying phenotype (color), another point to consider is dose and concentration. Both Maca-GO® and Maca-BG1.2TM were noted as a concentration and an extract, respectively, versus Maca Power, which was undefined. Daily doses used in the abovementioned studies ranged between 300 mg and >3000 mg. Still, they did not define active ingredient parameters, except for Maca-GO®, which had glucosinolates as one of its standardized biomarkers and more information on sourcing described in the study methods (country, elevation, hypocotyl size, and manufacturing procedures). This additional information would enable greater accuracy in assessing and differentiating maca ingredients. Moreover, the genetic and/or cultural aspects of study participants could relate to the menopausal transition in a way that would result in maca products having differential effects. In a published case report of a Caucasian female in her thirties taking a maca extract to improve energy and libido, there was evidence of increased plasma testosterone; however, no symptoms related to virilization [96][163]. Upon further investigation, the researchers noted that there was analytical inference in the immunoassay caused by maca supplementation [96][163]. Therefore, it is worth noting from a laboratory test point of view that there can be potential interference in the testosterone immunoassay in women taking a maca supplement. However, this is a single subject, and these results have not been reported elsewhere. Of note, maca is also not a phytoestrogen with high concentrations of isoflavones like soybean and red clover [27][85][97][27,135,152], but it has been proposed to have progestin-like activity due to increases in uterine weight seen in a study with ovariectomized mice [98][164]. While it may not be a classical phytoestrogen, by nature of its potential ability to enhance endogenous E2 and P levels depending on the possible phenotypes used and the form it is in, its use would be contraindicated in those with a personal history of hormone-sensitive cancer. However, with several chemopreventative compounds present in some forms and phenotypes of maca, there will no doubt be additional investigation on this topic in future research [99][95].4.3. Testicular and Prostate Health
Standardized formulations known as MacaPure M-01 and MacaPure M-02 derived from unspecified colors of maca (L. meyenii) root were shown to enhance sexual function in mice and rats, suggesting possible regulatory modulation of gonads through specific phytochemicals like macaenes, macamides, and certain unsaturated fatty acids and their amides [100][126]. It is of interest to note that increased libido in men due to taking maca may not be due to hormone changes. In a clinical study, men aged 21–56 years old were given two doses of gelatinized dehydrated maca root or placebo for twelve weeks. While sexual desire increased at eight and twelve weeks, the effect was not attributed to changes in serum T or E2 [50]. In another study in men of the same age range, there was no statistically significant difference in T, FSH, LH, prolactin, or E2 in men when given gelatinized maca root (no colors specified) or placebo in doses known to be used for aphrodisiac enhancement [101][165]. Men with mild asthenozoospermia and/or mild oligozoospermia given a non-specified color of maca (2 g/day) or placebo for 12 weeks presented statistically significant changes in sperm concentration [102][166]. However, the effects on sperm with this maca product are rather specific, considering the researchers found no significant differences in other parameters (sperm volume, mobility, and morphology) between the maca-supplemented and placebo groups [102][166]. Conversely, other studies using black maca in animal models have shown an improvement in parameters related to sperm, which may suggest that the color of maca may be relevant to men’s health [29][32][33][34][103][104][29,32,33,34,167,168]. A dried maca extract (of unspecified color) supplement given to non-Peruvian men for twelve weeks resulted in greater improvements in erectile dysfunction and well-being compared with those given a placebo [105][169]. Another important mechanism related to the endocrine system is its anti-inflammatory effect in the prostate gland through red maca’s impact on decreasing prostate weight (Figure 23) [51]. While the preponderance of data on prostate health advocates the use of red maca, there may be other colors of maca or combinations of maca that would also benefit specific physiological effects related to the function of this gland.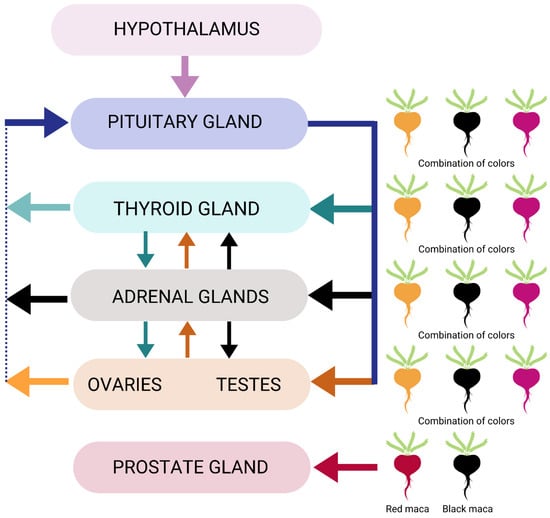
Figure 23.
Endocrine system modulation by maca phenotypes.
