Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by Rupesh Kumar Singh.
Chitosan is a biopolymer with various favorable properties (biotic/abiotic stress mitigation, qualitative improvement, bio-fertilizer, bio-stimulant and postharvest management) to meet multiple agricultural objectives. Grapevine is an important crop and has an enormous impact on the world’s economy due to its derived products, notably the different wine styles. In viticulture, chitosan application made significant developments towards higher contents of beneficial metabolites in grape berries as well as stress and postharvest management.
- sustainability
- elicitor
- secondary metabolites
- crop protection
1. Introduction
Chitin is an essential component of insect (crustacean) exoskeletons and fungal cell walls, and its deacetylation results into a linear polymer of two sub-units of d-glucosamine and N-acetyl-d-glucosamine that are linked through 1,4-glycosidic bonds, commonly known as chitosan [1]. It is biodegradable and biocompatible, elicits an antioxidant response, and is economically feasible and environmentally friendly [1,2,3,4,5][1][2][3][4][5]. The use of chitosan against plant pathogens was initiated by Allan and Hadwiger [6], and since then, chitosan has become an interesting biomaterial. Plant scientists have been testing this biopolymer to meet various objectives in agriculture for the last few decades [7,8,9,10][7][8][9][10]. Induced defense responses upon chitin and chitosan application led to its development as a bio-fertilizer, bio-fungicide, bio-bactericide, bio-virucide, natural rhizo-bacteria growth promoter, and bioremediation agent [6,11,12,13,14,15,16[6][11][12][13][14][15][16][17],17], although it has not yet been tested against phylloxera.
The potential use of chitosan in grapevine for crop protection/crop resilience induction is of the utmost importance as grapevine is a major crop across the globe and has a substantial impact on the economy. Winemaking, table grapes, juices, resins and other consumable products are being produced from grape berries, which provide numerous health benefits as grapevines contain diversified secondary metabolites [18]. The International Organisation of Vine and Wine (OIV) listed major grapevine-growing countries (Figure 1) and their primary grape consumption type [19].
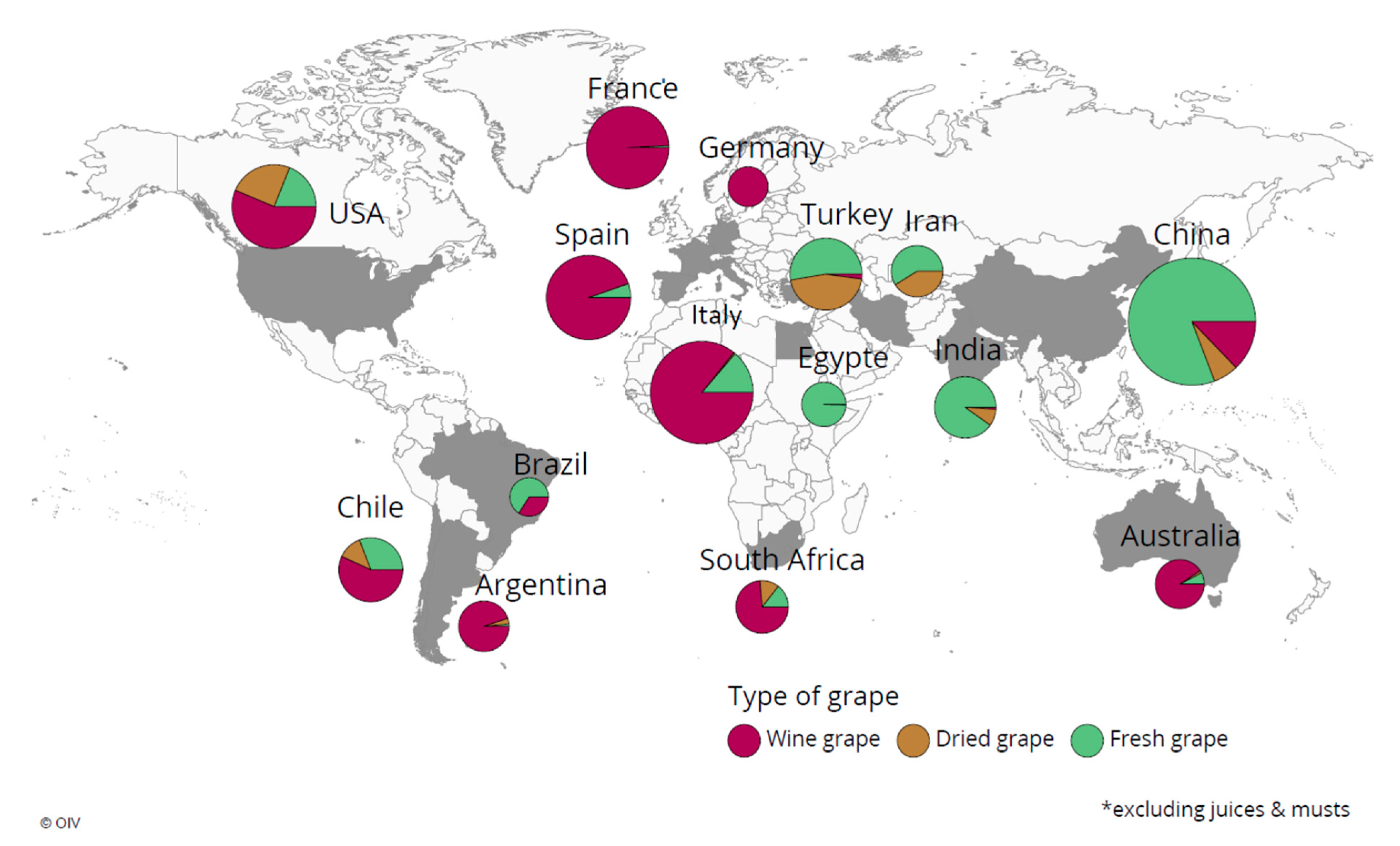
Figure 1.
Worldwide distribution of grapevine cultivation, adopted from OIV report 2019.
Grapevine is prone to infection from bacteria, viruses, mycoplasma, insects, worms, arthropods, oomycetes and fungi, where fungal and oomycetes pathogens lead to the maximum loss in terms of quality and quantity [20]. Grey mold disease by Botrytis cinerea Pers. (teleomorph: Botryotinia fuckeliana) (Helotiales: Sclerotiniaceae), a necrotrophic deuteromycete; powdery mildew by Erysiphe necator, a biotrophic ascomycete; downy mildew by Plasmopara viticola (Peronosporales: Peronosporaceae), a biotrophic oomycete; and trunk diseases by fungal association represent the major diseases worldwide [20]. In general, chemical pesticides and fungicide treatments are needed for fungal disease prevention in grapevine; however, no phytosanitary treatment is available yet for trunk diseases. A few active formulations with different modes of action were found to be effective in avoiding fungal resistance [21,22][21][22]. Fungicides and pesticides are potentially hazardous chemicals for winegrower’s health and can have serious consequences on environmental health resulting from contaminated water resources and diminished soil microbiota [23,24,25,26][23][24][25][26]. These toxic chemicals may affect the presence of natural yeasts in grapevine, leading to the deterioration of peculiar aromatic profiles by affecting the grapevine physiology and biochemistry, and subsequently, the wine quality [27,28,29,30][27][28][29][30]. Most importantly, traces of these chemicals identified in wines affect the taste [29].
2. Different Objectives for Chitosan Application in Grapevine
2.1. Disease Management
The susceptibility of grapevine to various pathogens is well known; moreover, the grey mold disease caused by Botrytis cinerea is very common, which frequently affects the different plant parts at different developmental stages [44][31]. Recently, a commercial acidic solution of chitosan was tested against B. cinerea in grapevines, which demonstrated the induced defense responses by upregulating the jasmonic-acid- and ethylene-mediated responses, downregulation of salicylic acid signaling and modulation of trans-resveratrol [45][32]. The application of chitosan in grapevine as the effective and environmentally friendly approach against B. cinerea is suggested. The elicitation potential of chitosan was assessed in cell cultures of grapevine (V. vinifera cv. Limberger) tissues and various parameters were evaluated for, e.g., oxidative damage, extracellular alkalization, and defense responsive gene expression (such as pathogen responsive genes (PR-1, PR-9) and the phenylalanine ammonia-lyase gene (PAL)), suggesting an induced defense responses of chitosan on applied cells [46][33]. Culture medium supplemented with chitosan-based gel (1.75% v/v) was reported to induce the shoot and root biomass and photosynthesis and decrease the development of B. cinerea in cultured plantlets. Moreover, exogenous foliar application of same chitosan gel reduced the disease infestation [47][34]. Another investigation suggested high protection against grey mold by B. cinerea under controlled conditions with chitosan treatment (75–150 mg/l) on grapevine leaves, and a subsequent induction of the activity of key enzymes such as lipoxygenase (LOX), PAL and chitinase markers were observed at a molecular level that eventually inhibited grey mold mycelium development [48][35]. Chitosan-based application (Biochicol 020 PC) was reported to inhibit the growth of Phomopsis (Sacc.) Sacc. viticola (Diaporthales: Valsaceae) on stored grapevine shoot cuttings in comparison to the fungicide (azoxystrobin and mancozeb) [49][36]. Another study suggested a reduced disease level by B. cinerea and P. viticola on grapevine leaves upon chitosan application [50][37]. Chitosan induced the PAL enzymatic activity and phytoalexins in grapevine leaves within 2 h and improved the secondary metabolite synthesis and salicylic acid, subsequently enhancing the resistance against fungal pathogen infestation [50][37]. Different concentrations of chitosan were tested in grapes to evaluate its antifungal potential for Colletotrichum sp., and a higher amount of chitosan was observed to reduce the fungal growth significantly [51][38]. Chitosan-treated suspension cell culture showed increased expression of PR-10 family transcripts [52][39]. Treating fruits on the field with chitosan could directly inhibit the growth of B. cinerea and could be an alternative to traditional fungicides in post-harvest disease control of grapes [53][40]. Vineyards of Chardonnay and Sauvignon blanc grapevines were treated with chitosan (at concentrations greater than 0·125 g/L), and the results suggested a reduced infestation of B. cinerea in grape bunches [54][41].2.2. Induced Secondary Metabolism and Stress Management
The induction of secondary metabolite synthesis has shown the potential for qualitative and quantitative improvements in viticulture practices [62][42]. Chitosan was tested on grapevine leaves as an exogenous application, and the findings suggested a higher accumulation of trans and cis-resveratrol, viniferin and piceid [62][42]. Chitosan pre-harvest treatment improved the quality attributes in table grapes [63][43]. Chitosan was tested in in-vitro grown grapevine cuttings and resulted in the reduction of drought and temperature stress. Moreover, a higher root growth and plant development was recorded in chitosan-treated cuttings [64][44]. Physiological improvements were observed in pre-harvest chitosan-treated grapes [63][43]. Different components of grapes were studied for their antioxidant potential upon chitosan application [65][45]. Chitosan induced the stilbenes accumulation in suspension cell cultures of V. vinifera cv. Barbera grape [52][39]. Suspension cell cultures and regenerated calli (V. vinifera cv Italia) were tested for chitosan elicitation towards stilbene synthesis. However, the increased amount was not significantly different in comparison to control [66][46]. Higher antioxidant activity was observed upon the application of a new chitosan formulation product [55][47]. Higher terpenoid (ursolate, oleanoate and betulinate) levels were recorded in grape bunches upon chitosan treatment [61][48]. Volatile compounds were recorded in a higher concentration upon chitosan application in Tempranillo grapes [74][49]. The total phenolic content and total tannins were recorded as over-accumulated in the two Portuguese grapevine varieties [75][50]. Overall, the results demonstrated that chitosan has a stimulatory effect on the accumulation of phenolic compounds, including anthocyanins, which is mediated by the modifications in the transcription of key genes involved in their biosynthesis and transport in grape berries [75,76][50][51]. Furthermore, the antioxidant potential was improved in different tissues of V. vinifera cv. Touriga Franca and V. vinifera cv. Tinto Cao [75][50]. There were increased antioxidant and antimicrobial activities in different grape components treated with chitosan solution [77][52], and the seed extracts showed the highest antioxidant and antimicrobial activities. The studied individual components obtained from chitosan-treated grapevines could represent added value due to an increased antioxidant and antibacterial potential. The phenolic compounds found in the different components may be used in food and pharmaceutical industries as natural food preservers and antibiotic adjuvants [77][52].3. Postharvest Management
Postharvest management in grapes is very important in order to maintain quality between harvesting and consumption. Grey mold is a major cause of the decay of perishable fruits such as grapes, in the field as well as during storage and transportation. Chitosan pre-harvest treatment was applied to evaluate the antifungal potential in table grapes, and the findings suggested a lower infestation of grey mold [78][53]. Postharvest coating with chitosan on grape bunches reduced the rate of fruit decay [63][43]. Chitosan formulation effectively reduced the gray mold disease in stored grapes, decreased CO2 and oxygen exchange, and prevented lesions in grape berries [79,80][54][55]. Coating with chitosan formulation resulted in a reduction in fruit decay and subsequent investments in quality management in grapes during storage, and such an approach may be a promising postharvest management method [81][56]. Chitosan coating was applied on grape berries (V. vinifera L. cv. Heiti) during storage at room temperature, and the observations suggested a lower respiratory metabolism and water loss, decreased titratable acid content, lower malonaldehyde accumulation, and improved SOD activity. Therefore, the treatment was found to have potential in the postharvest quality management of grape berries [82][57]. Gray mold is a major cause for the decay of perishable fruits such as grapes, in the field as well as during storage. Chitosan coating was found to be effective against the reduction in the gray mold lesions and for the induction of resistance in grape berries; hence, it can be considered as a potential protection agent for postharvest management in the grape berry industry [80][55]. Bunches of ‘Thompson Seedless’ grape berries were applied with three chitosan products (OII-YS, Chito Plant, and Armour-Zen) at berry set, pre-bunch closure, veraison, and at two or three weeks before final harvesting, and stored at 2 °C for 6 weeks. Chitosan application reduced the postharvest gray mold, improved the endochitinase activity, decreased the hydrogen peroxide content, and increased the quercetin, myrcetin, and resveratrol contents in the berries [83][58]. Chitosan nanoparticle formulation was used to make a coating on grape berries stored at 12 °C and 25 °C, and the potential for postharvest life, and total soluble solids, pH, titratable acidity, reducing sugar content, moisture content, and sensory characteristics of grapes were studied. The results demonstrated that the edible chitosan nanoparticle coating delayed the ripening of grapes and sensory characteristics, thus maintaining the quality parameters during storage [84][59].4. The Need for Sustainable Methods to Replace the Chemicals Used in Vineyards
Insecticides, miticides, herbicides, pesticides, and fungicides are toxic chemicals for human and environmental health and have been used extensively in grapevines worldwide [85,86][60][61]. Approximately 10 applications are essential in vineyards every year to prevent the disease infestations. These toxic chemicals have a drastic impact on the health of wine growers, as the direct exposure and contamination of surface water possess a potential threat to the environmental health. Moreover, traces of these chemicals have been found in different parts of grape plants, including berries [87][62]. Accumulative risks of these substances have provoked researchers to find unique sustainable solutions for multiple disease- and pest-related problems in vineyards [88][63]. Grape growers need an effective set of tools to reduce the disease in vineyards as well to eliminate chemical application-related health hazards. Some barrier methods have been proposed earlier, such as the use of plastic nets and covers to deter insects; however, such measures are not practically feasible on a large scale. Breeding traditional wines with resistant ones may provide an alternative, but this can also spoil the flavor of traditional wines. Genetic engineering-based approaches were also considered to promote the disease resistant traits; however, strong laws against GM crops and the societal acceptance of it represent major hindrances in this direction. Biofungicides and biopesticides were reported to be the best alternates to overcome this issue [89,90][64][65], but there were no significant answers for the control of downy mildew (P. viticola) in grapevines. Biocontrol agents have limitations due to their cost, quick degradability in fields, and temperature variations. Therefore, a significant potential biomaterial needs to be identified to overcome the multiple problems.References
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan-A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014.
- Vroman, I.; Tighzert, L. Biodegradable Polymers. Materials 2009, 2, 307–344.
- Muzzarelli, R.A.A. Natural Chelating Polymers, Alginic Acid, Chitin, and Chitosan; Pergamon Press: Oxford, UK; New York, NY, USA,, 1973.
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632.
- Shukla, S.K.; Mishra, A.K.; Arotiba, O.A.; Mamba, B.B. Chitosan-based nanomaterials: A state-of-the-art review. Int. J. Biol. Macromol. 2013, 59, 46–58.
- Allan, C.R.; Hadwiger, L.A. The fungicidal effect of chitosan on fungi of varying cell wall composition. Exp. Mycol. 1979, 3, 285–287.
- Kaya, M.; Bitim, B.; Mujtaba, M.; Koyuncu, T. Surface morphology of chitin highly related with the isolated body part of butterfly (Argynnis pandora). Int. J. Biol. Macromol. 2015, 81, 443–449.
- Kaya, M.; Mujtaba, M.; Bulut, E.; Akyuz, B.; Zelencova, L.; Sofi, K. Fluctuation in physicochemical properties of chitins extracted from different body parts of honeybee. Carbohydr. Polym. 2015, 132, 9–16.
- Kaya, M.; Sofi, K.; Sargin, I.; Mujtaba, M. Changes in physicochemical properties of chitin at developmental stages (larvae, pupa and adult) of Vespa crabro (wasp). Carbohydr. Polym. 2016, 145, 64–70.
- Rinaudo, M. Main properties and current applications of some polysaccharides as biomaterials. Polym. Int. 2008, 57, 397–430.
- Barber, M.S.; Bertram, R.E.; Ride, J.P. Chitin oligosaccharides elicit lignification in wounded wheat leaves. Physiol. Mol. Plant Pathol. 1989, 34, 3–12.
- Chaouat, C. Design of New Formulation Systems for Depigmenting Active Ingredients, with a View to Their Use by the Cutaneous Route; Université Toulouse III-Paul Sabatier: Toulouse, France, 2013.
- Chirkov, S.N.; Surguchova, N.; Atabekov, J.G. Chitosan inhibits systemic infections caused by DNA†containing plant viruses. Arch. Phytopathol. Plant Prot. 1994, 29, 21–24.
- Agbodjato, N.A.; Noumavo, P.A.; Adjanohoun, A.; Agbessi, L.; Baba-Moussa, L. Synergistic Effects of Plant Growth Promoting Rhizobacteria and Chitosan on In Vitro Seeds Germination, Greenhouse Growth, and Nutrient Uptake of Maize (Zea mays L.). Biotechnol. Res. Int. 2016, 2016, 20.
- Akyuz, L.; Kaya, M.; Koc, B.; Mujtaba, M.; Ilk, S.; Labidi, J.; Salaberria, A.M.; Cakmak, Y.S.; Yildiz, A. Diatomite as a novel composite ingredient for chitosan film with enhanced physicochemical properties. Int. J. Biol. Macromol. 2017, 105 Pt 2, 1401–1411.
- Kaya, M.; Akyuz, L.; Sargin, I.; Mujtaba, M.; Salaberria, A.M.; Labidi, J.; Cakmak, Y.S.; Koc, B.; Baran, T.; Ceter, T. Incorporation of sporopollenin enhances acid-base durability, hydrophobicity, and mechanical, antifungal and antioxidant properties of chitosan films. J. Ind. Eng. Chem. 2017, 47, 236–245.
- Li, B.; Shi, Y.; Shan, C.; Zhou, Q.; Ibrahim, M.; Wang, Y.; Wu, G.; Li, H.; Xie, G.; Sun, G. Effect of chitosan solution on the inhibition of Acidovorax citrulli causing bacterial fruit blotch of watermelon. J. Sci. Food Agric. 2013, 93, 1010–1015.
- Iriti, M.; Faoro, F. Bioactivity of Grape Chemicals for Human Health. Nat. Prod. Commun. 2009, 4, 1934578X0900400502.
- OIV. World Vitiviniculture Situation; OIV: Dijon, France, 2017.
- Ferreira, R.B.; Monteiro, S.S.; Piçarra-Pereira, M.A.; Teixeira, A.R. Engineering grapevine for increased resistance to fungal pathogens without compromising wine stability. Trends Biotechnol. 2004, 22, 168–173.
- Corio-Costet, M.-F.; Dufour, M.-C.; Cigna, J.; Abadie, P.; Chen, W.-J. Diversity and fitness of Plasmopara viticola isolates resistant to QoI fungicides. Eur. J. Plant Pathol. 2011, 129, 315–329.
- Dufour, M.C.; Fontaine, S.; Montarry, J.; Corio-Costet, M.F. Assessment of fungicide resistance and pathogen diversity in Erysiphe necator using quantitative real-time PCR assays. Pest Manag. Sci. 2011, 67, 60–69.
- Bony, S.; Gillet, C.; Bouchez, A.; Margoum, C.; Devaux, A. Genotoxic pressure of vineyard pesticides in fish: Field and mesocosm surveys. Aquat. Toxicol. 2008, 89, 197–203.
- Komarek, M.; Cadkova, E.; Chrastny, V.; Bordas, F.; Bollinger, J.-C. Contamination of vineyard soils with fungicides: A review of environmental and toxicological aspects. Environ. Int. 2010, 36, 138–151.
- Schreck, E.; Gontier, L.; Dumat, C.; Geret, F. Ecological and physiological effects of soil management practices on earthworm communities in French vineyards. Eur. J. Soil Biol. 2012, 52, 8–15.
- Sharma, L.; Bohra, N.; Singh, R.K.; Marques, G. Potential of Entomopathogenic Bacteria and Fungi. In Microbes for Sustainable Insect Pest Management: An Eco-Friendly Approach-Volume 1; Khan, M.A., Ahmad, W., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 115–149.
- Jermini, M.; Blaise, P.; Gessler, C. Quantitative effect of leaf damage caused by downy mildew (Plasmopara viticola) on growth and yield quality of grapevine ‘Merlot’ (Vitis vinifera). Vitis: J. Grapevine Res. 2010, 49, 77–85.
- Petit, A.N.; Wojnarowiez, G.; Panon, M.L.; Baillieul, F.; Clément, C.; Fontaine, F.; Vaillant-Gaveau, N. Botryticides affect grapevine leaf photosynthesis without inducing defense mechanisms. Planta 2009, 229, 497–506.
- Cesnik, H.B.; Gregorcic, A.; Cus, F. Pesticide residues in grapes from vineyards included in integrated pest management in Slovenia. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2008, 25, 438–443.
- Gonzalez Alvarez, M.; Noguerol-Pato, R.; Gonzalez-Barreiro, C.; Cancho-Grande, B.; Simal-Gandara, J. Changes of the sensorial attributes of white wines with the application of new anti-mildew fungicides under critical agricultural practices. Food Chem. 2012, 130, 139–146.
- Mohamed, N.L.J.; Farmer, M.J.; Fromentin, J.; Béno, N.; Houot, V.; Milat, M.L.; Blein, J.P. Defense Responses in Grapevine Leaves Against Botrytis cinerea Induced by Application of a Pythium oligandrum Strain or Its Elicitin, Oligandrin, to Roots. Phytopathology 2007, 97, 611–620.
- De Bona, G.S.; Vincenzi, S.; De Marchi, F.; Angelini, E.; Bertazzon, N. Chitosan induces delayed grapevine defense mechanisms and protects grapevine against Botrytis cinerea. J. Plant Dis. Prot. 2021, 128, 715–724.
- Repka, V. Elicitor-Stimulated Induction of Defense Mechanisms and Defense Gene Activation in Grapevine Cell Suspension Cultures. Biol. Plant. 2001, 44, 555–565.
- Ait Barka, E.; Eullaffroy, P.; Clement, C.; Vernet, G. Chitosan improves development, and protects Vitis vinifera L. against Botrytis cinerea. Plant Cell Rep. 2004, 22, 608–614.
- Trotel-Aziz, P.; Couderchet, M.; Vernet, G.; Aziz, A. Chitosan Stimulates Defense Reactions in Grapevine Leaves and Inhibits Development of Botrytis Cinerea. Eur. J. Plant Pathol. 2006, 114, 405–413.
- Król, E. Chitosan activity in an inhibition of in vitro growth of Phomopsis viticola and protection of grapevine canes against the pathogen. Phytopathol. Pol. 2006, 39, 155–162.
- Aziz, A.; Trotel-Aziz, P.; Conreux, A.; Dhuicq, L.; Jeandet, P.; Couderchet, M. Chitosan Induces Phytoalexin Synthesis, Chitinase and β-1,3-Glucanase Activities, and Resistance of Grapevine to Fungal Pathogens. In Macromolecules and Secondary Metabolites of Grapevine and Wine; Tec & doc: Gwabegar, Australia, 2007; pp. 83–88.
- Munoz, Z.; Moret, A.; Garces, S. Assessment of chitosan for inhibition of Colletotrichum sp. on tomatoes and grapes. Crop Prot. 2009, 28, 36–40.
- Ferri, M.; Tassoni, A.; Franceschetti, M.; Righetti, L.; Naldrett, M.J.; Bagni, N. Chitosan treatment induces changes of protein expression profile and stilbene distribution in Vitis vinifera cell suspensions. Proteomics 2009, 9, 610–624.
- Munoz, Z.; Moret, A. Sensitivity of Botrytis cinerea to chitosan and acibenzolar-S-methyl. Pest. Manag. Sci. 2010, 66, 974–979.
- Reglinski, T.; Elmer, P.A.G.; Taylor, J.T.; Wood, P.N.; Hoyte, S.M. Inhibition of Botrytis cinerea growth and suppression of botrytis bunch rot in grapes using chitosan. Plant Pathol. 2010, 59, 882–890.
- Aziz, A.; Trotel-Aziz, P.; Dhuicq, L.; Jeandet, P.; Couderchet, M.; Vernet, G. Chitosan oligomers and copper sulfate induce grapevine defense reactions and resistance to gray mold and downy mildew. Phytopathology 2006, 96, 1188–1194.
- Meng, X.; Li, B.; Liu, J.; Tian, S. Physiological responses and quality attributes of table grape fruit to chitosan preharvest spray and postharvest coating during storage. Food Chem. 2008, 106, 501–508.
- Gornik, K.; Wgrzesik, M.; Duda, B.R. The effect of chitosan on rooting of grapevine cuttings and on subsequent plant growth under drought and temperature stress. J. Fruit Ornam. Plant Res. 2008, 16, 333–343.
- Vitalini, S.F.F.; Di Tommaso, G.; Di Tommaso, D.; Borgo, M.; Piaggesi, A.; Fico, G.; Tomé, F.; Iriti, M. Effects of chitosan and phosphite treatments on total polyphenol content and antioxidant power of different grape berry tissues and wines. In Proceedings of the Workshop Induced Resistance in the Control of Plant Diseases: Effectiveness and Mechanisms of Action of a Sustainable Approach, Ancona, Italy, 18 June 2009.
- Santamaria, A.R.; Mulinacci, N.; Valletta, A.; Innocenti, M.; Pasqua, G. Effects of elicitors on the production of resveratrol and viniferins in cell cultures of Vitis vinifera L. cv Italia . J. Agric. Food Chem. 2011, 59, 9094–9101.
- Iriti, M.; Vitalini, S.; DI Tommaso, G.; D’Amico, S.; Borgo, M.; Faoro, F. New chitosan formulation prevents grapevine powdery mildew infection and improves polyphenol content and free radical scavenging activity of grape and wine. Aust. J. Grape Wine Res. 2011, 17, 263–269.
- Lucini, L.; Baccolo, G.; Rouphael, Y.; Colla, G.; Bavaresco, L.; Trevisan, M. Chitosan treatment elicited defence mechanisms, pentacyclic triterpenoids and stilbene accumulation in grape (Vitis vinifera L.) bunches. Phytochemistry 2018, 156, 1–8.
- Gutiérrez-Gamboa, G.; Pérez-Álvarez, E.P.; Rubio-Bretón, P.; Garde-Cerdán, T. Changes on grape volatile composition through elicitation with methyl jasmonate, chitosan, and a yeast extract in Tempranillo (Vitis vinifera L.) grapevines. Sci. Hortic. 2019, 244, 257–262.
- Singh, R.K.; Soares, B.; Goufo, P.; Castro, I.; Cosme, F.; Pinto-Sintra, A.L.; Inês, A.; Oliveira, A.A.; Falco, V. Chitosan Upregulates the Genes of the ROS Pathway and Enhances the Antioxidant Potential of Grape (Vitis vinifera L. ‘Touriga Franca’ and ‘Tinto Cäo’) Tissues. Antioxidants 2019, 8, 525.
- Singh, R.K.; Martins, V.; Soares, B.; Castro, I.; Falco, V. Chitosan Application in Vineyards (Vitis vinifera L. cv. Tinto Cão) Induces Accumulation of Anthocyanins and Other Phenolics in Berries, Mediated by Modifications in the Transcription of Secondary Metabolism Genes. Int. J. Mol. Sci. 2020, 21, 306.
- Silva, V.; Singh, R.K.; Gomes, N.; Soares, B.G.A.; Silva, A.; Falco, V.; Capita, R.; Alonso-Calleja, C.; Pereira, J.E.; Amaral, J.S.; et al. Comparative Insight upon Chitosan Solution and Chitosan Nanoparticles Application on the Phenolic Content, Antioxidant and Antimicrobial Activities of Individual Grape Components of Sousao Variety. Antioxidants 2020, 9, 178.
- Romanazzi, G.; Gabler, F.M.; Smilanick, J.L. Preharvest Chitosan and Postharvest UV Irradiation Treatments Suppress Gray Mold of Table Grapes. Plant Dis. 2006, 90, 445–450.
- Romanazzi, G. Chitosan Treatment for the Control of Postharvest Decay of Table Grapes, Strawberries and Sweet Cherries. In Fresh Produce: New Trends in Postharvest Management of Fresh Produce; Sivakumar, D., Ed.; University of Pretoria: Pretoria, South Africa, 2009; Volume 4, pp. 111–115. ISBN 978-4-903313-40-5.
- Romanazzi, G.; Gabler, F.M.; Margosan, D.; Mackey, B.E.; Smilanick, J.L. Effect of chitosan dissolved in different acids on its ability to control postharvest gray mold of table grape. Phytopathology 2009, 99, 1028–1036.
- Meng, X.-H.; Qin, G.-Z.; Tian, S.-P. Influences of preharvest spraying Cryptococcus laurentii combined with postharvest chitosan coating on postharvest diseases and quality of table grapes in storage. LWT-Food Sci. Technol. 2010, 43, 596–601.
- Wang Simeng, R.Y.; Junyu, H.; Linchuan, F.; Houyu, L. Effects of Single and Complex Chitosan Coating on Grape Preservative; College of Agriculture, Guizhou University: Guiyang, China, 2010.
- Feliziani, E.; Smilanick, J.L.; Margosan, D.A.; Mansour, M.F.; Romanazzi, G.; Gu, S.; Gohil, H.L.; Ames, Z.R. Preharvest Fungicide, Potassium Sorbate, or Chitosan Use on Quality and Storage Decay of Table Grapes. Plant Dis. 2013, 97, 307–314.
- Melo, N.F.C.B.; MendonçaSoares, B.L.d.; Diniz, K.M.; Leal, C.F.; Canto, D.; Flores, M.A.; Tavares-Filho, J.H.d.C.; Galembeck, A.; Stamford, T.M.; Stamford-Arnaud, T.M.; et al. Effects of fungal chitosan nanoparticles as eco-friendly edible coatings on the quality of postharvest table grapes. Postharvest Biol. Technol. 2018, 139, 56–66.
- Sharma, L.; Oliveira, I.; Raimundo, F.; Torres, L.; Marques, G. Soil Chemical Properties Barely Perturb the Abundance of Entomopathogenic Fusarium oxysporum: A Case Study Using a Generalized Linear Mixed Model for Microbial Pathogen Occurrence Count Data. Pathogens 2018, 7, 89.
- Sharma, L.; Bohra, N.; Rajput, V.D.; Quiroz-Figueroa, F.R.; Singh, R.K.; Marques, G. Advances in Entomopathogen Isolation: A Case of Bacteria and Fungi. Microorganisms 2021, 9, 16.
- Sergazina, M.V.L.; Llompart, M.; Dagnac, T. Occurrence of Fungicides in Vineyard and the Surrounding Environment. Molecules 2021, 26, 6152.
- Sharma, L.; Gonsalves, F.; Oliveira, I.; Torres, L.; Marques, G. Insect-associated fungi from naturally mycosed vine mealybug Planococcus ficus (Signoret) (Hemiptera: Pseudococcidae). Biocontrol. Sci. Technol. 2018, 28, 122–141.
- (EC), E.P.R., Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 Concerning the Placing of Plant Protection Products on the Market and Repealing Council Directives 79/117/EEC and 91/414/EEC. 2009. Available online: https://www.legislation.gov.uk/eur/2009/1107/contents?view=plain (accessed on 28 August 2022).
- Sharma, L.; Oliveira, I.; Gonsalves, F.; Raimundo, F.; Singh, R.K.; Torres, L.; Marques, G. Effect of Soil Chemical Properties on the Occurrence and Distribution of Entomopathogenic Fungi in Portuguese Grapevine Fields. Pathogens 2021, 10, 137.
More
