Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Jason Zhu and Version 2 by Jason Zhu.
Membrane distillation (MD) is an attractive separation process that can work with heat sources with low temperature differences and is less sensitive to concentration polarization and membrane fouling than other pressure-driven membrane separation processes, thus allowing it to use low-grade thermal energy, which is helpful to decrease the consumption of energy, treat concentrated solutions, and improve water recovery rate.
- membrane distillation
- hybrid systems
- renewable energy
- forward osmosis
1. The Fundamentals and Configurations of MD
1.1. Fundamentals of MD
Membrane distillation is a separation technique that couples a thermally driven distillation process and a membrane separation process. This technique has been known for about 50 years [1][2][3][4][5][6]. Simultaneous heat and mass transfer through a micro-porous hydrophobic membrane occurs during the MD process [7]. Heat is transferred by the latent heat carried by water vapor and conduction through the membrane matrix and the vapor trapped inside the pores. With the volatile component being transported through the membrane pores, the mass is transferred [8]. The driving force of the MD process is the partial vapor pressure difference generated by the temperature difference between the hot liquid feed side and the cold permeate side of the membrane or the difference between the water activities of the two solutions [9][10].
1.2. MD Configurations
According to types of the driving forces, the configurations of MD can be divided into two categories. The first category includes direct contact membrane distillation (DCMD), air gap membrane distillation (AGMD), sweeping gas membrane distillation (SGMD), vacuum membrane distillation (VMD), and permeate gap membrane distillation (PGMD), the driving forces of which are created by temperature gradients. The other category includes osmotic membrane distillation (OMD), the driving force of which is a concentration gradient.
The most common configuration of MD is DCMD in which hot feed saline water and cold permeate water are in direct contact with the membrane [1]. More than 60% of MD studies related to DCMD systems have been carried out using the simplest configuration of DCMD, whose condensation step can be placed inside the MD module [11]. DCMD requires the least equipment, and its operation is simple [1]. Therefore, it is widely employed for desalination, especially for seawater desalination in remote rural areas where technical support is immature and budgets are small [12]. However, the main drawback of DCMD is the large amounts of heat loss from the hot side to the cold side [13].
The conductive heat loss of DCMD can be significantly reduced by introducing some stagnant air between the membrane and the condensation surface [13]. This configuration is called AGMD, and it has the highest energy efficiency among the single-stage MD modes [14]. In addition, internal heat recovery is more probable for AGMD compared to other modes [15]. Nonetheless, additional resistance to mass transfer is created by the stagnant air when the water vapor passes through the gap to the condensation surface, which results in lower permeate fluxes and is considered a disadvantage [16].
The lower permeate fluxes of AGMD can be avoided if a cold inert gas is used to sweep the vapor on the permeate membrane side to condense outside the membrane module, or if a vacuum pump is applied to the distillate side of the MD module to remove the permeated molecules from the distillate side [13][15]. These configurations are known as SGMD and VMD, respectively. Both of them enhance the mass transfer coefficient and reduce the heat loss due to conduction, and VMD presents the highest permeate flux and lowest heat loss due to conduction among DCMD, AGMD, SGMD, and VMD [7][13][15]. SGMD and VMD are typically used to remove a volatile organic or dissolved gas from an aqueous solution [17]. However, the main disadvantages of SGMD and VMD are that they increase the processing costs due to the additional condenser and vacuum pump, and that they make heat recovery difficult because the condensation takes place outside the membrane module [15].
To solve the decrease in flux caused by the air gap, special materials, such as polyurethane, polypropylene mesh, sand, or de-ionized water, can also be used to replace the stagnant air [18][19]. This configuration is known as material gap membrane distillation (MGMD), which can be understood as a modification of AGMD [20]. In reality, only permeate water can be used in the gap of the module to avoid distillate contamination [19]. Therefore, permeate gap membrane distillation (PGMD) is studied more than polyurethane, polypropylene mesh, and sand gap MD. Simultaneously, the PGMD configuration directly couples the heat recovery within the module better than SGMD and VMD [21].
OMD is a DCMD variant that combines DCMD and OD in one process [22]. The OMD process, which was patented at the end of the last century, is most often used to remove water from liquid foods, such as fruit and milk, and various nonfood aqueous solutions that are not thermally resistant [22]. The main advantages of OMD are that it can concentrate solutions at lower temperatures compared with other MD configurations and effectively increase the transmembrane fluxes by introducing an activity gradient [23][24].
2. Renewable Energy and Waste Heat Coupled with MD
2.1. Solar Energy-Driven MD
2.1.1. Solar Radiation-Assisted MD
The most abundant and focused renewable energy source is solar radiation, which provides virtually unlimited energy [25][26]. Solar-driven MD desalination processes have been extensively studied by many researchers and practitioners [27]. One of the reasons for interest in solar energy driving MD is that MD has the ability to tolerate discontinuous, fluctuating, and unpredictable operating conditions and to run with a small temperature difference [28][29][30]. Solar radiation can be used to heat a feed solution directly or indirectly via solar collectors, such as flat plate collectors (FPCs), evacuated tube collectors (ETCs), PV panels, and compound parabolic collectors (CPCs), and can also be applied to generate electricity via photovoltaics (PVs) to run auxiliary equipment such as circulation pumps and valves for automatically operated systems [25][27][31].
Two different layouts can be adopted in solar-powered MD desalination systems: single-loop and two-loop systems [32][33]. In a single-loop system (often referred to as a compact system), the seawater is heated directly by solar collectors, and then enters into the membrane module (Figure 31a) [33][34]. The advantages of such systems are that they are suitable for small-capacity production and that the configuration is very simple, without a heat exchanger or heat storage facility [30][35]. However, the materials used in the solar collectors must be anti-corrosion and anti-scaling due to the seawater recirculating through the single loop [27][33]. Compared to a compact system, a two-loop system (Figure 31b) is more complex. This configuration includes two independent loops, a solar loop and a desalination loop, connected by a heat exchanger [27][36][37]. The solar loop is operated with tap water as a heat transfer fluid, while the desalination loop is operated with seawater [31]. Hence, it can achieve better control of corrosion and scaling problems [33]. A two-loop system can also use a heat storage facility to store surplus energy if enough radiation is available, which allows the extended operation of MD modules after sunset [27][33]. However, the maximum feed temperature of the seawater in a desalination loop is lower than the highest temperature in a solar loop due to the energy loss of the heat exchanger [33].
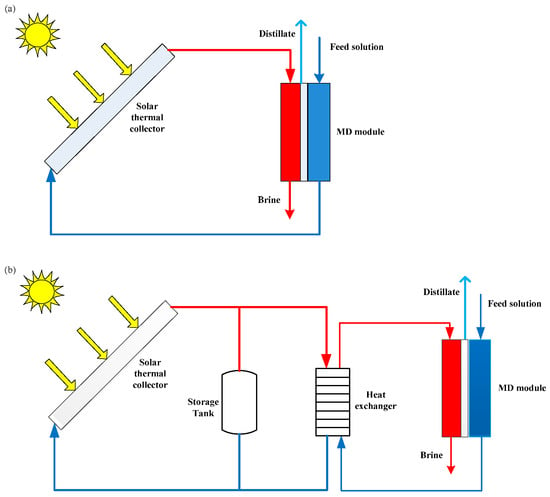
Figure 31. Scheme of solar-driven MD system: (a) single-loop system; (b) two-loop system.
Many experiments and simulations of solar-assisted MD systems for desalination have been carried out by several researchers. Chang et al. [38] have analyzed the performance of a solar membrane distillation desalination system using both experimental and simulation approaches. The authors used a dynamic mathematical model, which included a control algorithm and was verified using experimental results, to optimize the analysis of the system and revealed the operation strategy for maximum water production. The performance of the system was about 80% of the maximum water production for sunny day operation. Zaragoza et al. [39] evaluated various configurations of solar-driven MD and came to the conclusion that spiral-wound modules or multi-effect systems should be considered to improve efficiency. Raluy et al. [40] presented a five-year operational period of a solar-assisted single-loop MD system installed in Spain. They found that the water production ranged between 5 and 120 L/d, and the conductivity of the distillate water varied between 20 and 200 μS/cm. Gil et al. [41] designed four direct control schemes and a reference governor in a solar-powered MD facility. They reached the conclusion that settling times were reduced by more than half and could obtain the operation temperature at the inlet of the distillation module. Gil et al. [42] also proposed a two-layer hierarchical control system that has been tested in a simulation and an experiment to optimize the solar-driven MD facility according to distillate production and thermal energy. The authors concluded that the daily distillate production could improve to 14–20 L and the thermal energy demand could be reduced to 0.41–1.21 kWh/m3. Chen et al. [43] proposed a pseudo steady-state method to assess the total annual cost (TAC) of a discontinuous and fluctuating solar-assisted MD desalination facility. They reached the conclusion that the optimal TAC was about USD 280,000 at 500 W/m2. Abdallah et al. [44] designed a completely autonomous solar-driven MD unit, in which the feed solution was heated by solar collectors and the electric power of the auxiliary equipment was generated by PV panels. Simulations of the unit operating showed that distillate production varied from 35 L/h to 70 L/h.
Some efforts have also been made to evaluate the economy of solar-driven MD systems. Saffarini et al. [35] found that a DCMD system with a heat recovery device was more cost-efficient than an AGMD or VMD system. Banat and Jwaied [45] calculated the distillate production cost of compact and large autonomous solar-driven MD systems. The costs were USD 15 USD/m3 and USD 18 USD/m3, respectively, and could be significantly reduced by prolonging the lifetimes of the membranes and units. Moore et al. [46] optimized an SGMD desalination system with solar thermal collectors and PV panels. They found that the cost of water was about USD 85 USD/m3, with the membrane modules and thermal collectors accounting for the majority of the cost. In addition, some simulations and theoretical analyses of solar-powered multi-stage MD have been carried out. It was found that it had better performance than a one-stage system in terms of water production, thermal efficiency, and flux [12][47][48][49].
Although the cost of the distillate produced by a solar-driven MD system is relatively expensive compared with other conventional desalination technologies (e.g., RO, MED, and MSF), solar-powered MD systems remain applicable for potable water production in remote and arid areas [50]. In addition, a solar-powered MD system can be energy self-sufficient and requires little maintenance [6]. Solar-powered MD systems are more commonly applied in small applications. Therefore, more studies should be conducted to assess and evaluate the actual operation of large-scale solar-assisted MD units [27]. In addition, more experiments related to solar-assisted multi-stage MD systems are needed to enhance the performance of the systems.
2.1.2. Salt-Gradient Solar Pond-Powered MD
A salt-gradient solar pond (SGSP) is a stable artificial saltwater pond that is used to absorb and store solar radiation [51][52]. An SGSP consists of three characteristic zones: an upper convective zone (UCZ), a non-convective zone (NCZ), and a lower convective zone (LCZ) [53]. The UCZ is at the top of the pond, including a relatively thin layer of water with very low salinity, and the temperature of the water is close to the ambient temperature [54][55]. The NCZ is under the UCZ, and the saline density and temperature of the water in the NCZ increase gradually with depth. Its function is to suppress the convection process in the pond and act as an insulator between the UCZ and the LCZ [54][55]. The last zone is the LCZ, which is at the bottom of the pond. The saturated brine in the LCZ can absorb and store solar radiation to heat buildings or provide a hot feed for the MD process [56][57]. SGSPs have been widely researched due to their low cost and ability to store solar radiation for a long time [58]. Recently, several studies have been conducted to analyze the performance of an SGSP-powered DCMD system. Suárez et al. [59] developed a model to evaluate the performance of an SGSP-driven DCMD system. The authors found that it was possible for distillate production to reach about 2.7 × 10−3 m3d−1 per m2 of SGSP. Soon after, Suárez et al. [60] conducted the first experimental study of an SGSP-powered DCMD system to produce fresh water. They reached the conclusion that the average distillate flux of the coupled system could obtain 1.0 Lh−1 per m2 of membrane. Nakoa et al. [61] carried out an experiment using an SGSP-assisted DCMD system for fresh water production. They found that the fluxes would decrease due to the significant concentration and temperature polarization induced by laminar flow. Suárez and Urtubia [62] investigated the performance of an SGSP-powered DCMD system. The authors reached the conclusion that the maximum fresh water flow rates were about 3.0 L d−1 per m2 of solar pond.
Membrane distillation powered by a salt-gradient solar pond is an economical and sustainable method for desalination to produce drinkable water. Further research into the economics and practical applications of the systems is needed.
2.2. Geothermal Energy-Assisted MD
Compared to solar energy, geothermal energy has the advantage of continuous reliability, availability, stability, and independence from the weather [63][64][65]. In the process of producing drinkable water, geothermal water can directly or indirectly be used as a feed solution to desalinate or for heating feed water from other sources with heat exchangers, respectively [66][67]. However, it is not suitable for MSF due to the low grade of geothermal heat or MED because of the corrosive chemical species brought by geothermal water [25][68]. In a coupled geothermal and RO system, it is necessary to convert geothermal heat into electricity to power a RO plant, which causes huge energy losses [25][69]. MD can make full use of low-grade geothermal water and resists corrosion. Therefore, it can be coupled effectively to geothermal water for desalination. However, it has not been widely studied. Recently, Sarbatly and Chiam [70] presented an energy evaluation of geothermal water acting as a feed solution for VMD to produce drinkable water. The authors reached the conclusion that the cost of water production was USD 0.5 USD/m3 for a 20,000 m3/d VMD desalination plant operated with geothermal energy, and the cost increased by 144% without using geothermal energy.
Geothermal energy-powered MD desalination is promising. Therefore, more studies should be conducted to address the problems of long-term operation and the corrosion of equipment [68][71].
2.3. Waste Heat-Powered MD
Waste heat is everywhere in life, especially in industrial production. MD desalination research has been widely conducted to produce high-quality water using waste heat from industrial production. Four different types of waste heat sources were selected for MD studies, including engine waste heat generated by marine vessels, natural gas compressor stations, a recirculating cooling water system, and a gas-fired power station [72][73][74][75]. In their study, Koo et al. [73] optimized the performance of a VMD process driven by waste heat generated from marine vessels. They analyzed the parameters of the operating conditions and found that an increase in the temperature of the feed water would result in an increase in water flux. Lokare et al. [72] evaluated the potential of a DCMD desalination system powered by waste heat from natural gas compressor stations. They developed a mathematical model to predict the flux performance. They came to the conclusion that the flux and heat recovery efficiency increased with the feed temperature. Waste heat from a recirculating cooling water process was utilized for a DCMD system to produce pure water, which reduced the cost of pure water production and maintenance of a pipeline [74]. Dow et al. [75] explored the viability of DCMD driven by waste heat from a gas-fired power station. The authors found that the system had no significant flux change due to a pretreatment or the unique fouling mechanisms in MD.
Studies show that cheap waste heat can be used by MD systems to produce high- quality distillates. It is financially beneficial to use waste heat to power MD desalination systems due to its competitiveness compared to RO and MED [76]. Therefore, more studies related to waste heat-driven MD systems should be conducted.
3. Disposal of Concentrated Brine with MD
3.1. Power Generation
3.1.1. PRO-MD Hybrid
Compared to other desalination processes (such as RO and MED), MD can dispose of a wide range of highly concentrated feed solutions [77][78]. Therefore, MD can act as a complementary process to further concentrate brines from a RO system or a MED system to enhance the recovery of drinkable water and reduce the volume of brines [79][80][81]. Simultaneously, higher-salinity effluent can be supplied to a pressure-retarded osmosis (PRO) system for osmotic power generation [82]. In the RO-MD-PRO process, MD further concentrates the distillate of RO to generate concentrated and pure water, and PRO converts osmotic energy into electricity using a hydro-turbine, which can save energy [83][84][85]. Kim et al. [86] analyzed the feasibility of the RO-MD-PRO hybrid process using numerical approaches. The authors found that the RO-MD-PRO hybrid process reduced the special energy consumption and minimized marine environmental impacts compared to stand-alone RO process. The maximum SEC was ~1.6 kWh/m3 at a brine division ratio of 1.0, which was a 17% reduction compared to the stand-alone RO process. Choi et al. [87] also evaluated the performance and economics of the RO-MD-PRO hybrid system using a theoretical analysis. They obtained results similar to Kim et al. Chae et al. [88] proposed a new dimensionless performance index to compare the energy efficiency between RO-PRO and RO-MD-PRO systems after running several simulations. Using low-grade energy, the simulation results showed that the RO-MD-PRO system had a higher energy efficiency than the RO-PRO system.
3.1.2. MD-RED Hybrid
In the combined membrane distillation and reverse electrodialysis (MD-RED) system, RED converts the Gibbs free energy by mixing the concentrate produced by MD and a diluent, which can convert low-grade heat into electricity [89][90][91]. Several studies related to the hybrid MD-RED system have been conducted by Tufa et al. and Long et al. [92][93]. They reported that a larger NaCl concentration induced greater electrical efficiency. Mercer et al. [91] tested the integration of MD with RED to provide pure water and electrical power and provided the first successful demonstration of a hybrid MD-RED system for energy recovery from waste heat, which was an opportunity for off-grid decentralized sanitation. In another study, Micari et al. [89] provided a detailed description of the behavior of a real RED-MD heat engine and evaluated the performance of the combined system under different operating conditions (such as concentration, velocity, and membrane thickness). Although the integrated MD-RED system outperforms the MD system, the specific costs of the MD and RED equipment are still high, which restricts the development and application of the hybrid system. Therefore, an optimization of the membrane should be performed to enhance the performance of the hybrid process. In addition, a thermo-economic analysis should be conducted for its commercial implementation.
3.2. MD-ZLD Hybrid
In recent years, zero-liquid discharge (ZLD) has attracted renewed interest worldwide because it has the advantages of decreasing environmental pollution and improving water sustainability [78][94]. In order to achieve ZLD, thermal-based technologies, such as brine concentrators and solar ponds, and membrane-based ZLD technologies, such as ED, MD, and FO, can be used [78][94]. Tong et al. [94] highlighted the evolution of ZLD from thermal-based to membrane-based processes by thoroughly analyzing the pros and cons of these technologies. Among these technologies, more and more attention has been paid to MD [95]. Schwantes et al. [95] presented a techno-economic comparison of MD and mechanical vapor compression (MVC) in the same ZLD application. They came to the conclusion that MD could be about 40% cheaper than MVC when the distillate was 100 m3/day and up to 75% cheaper under the condition of a free waste heat-assisted MD system. Tufa et al. [96] proposed a novel approach integrating DCMD with RED systems to dispose of SWRO brines. The coupled systems could not only produce distillate water via an MD process and generate electricity via a RED process but also achieved near-zero-liquid discharge. Nakoa et al. [97] discussed the combination of a DCMD system with a SGSP to achieve ZLD desalination. The authors used the hot concentrated water in the NCZ as a feed solution and injected the discharged saline water into the LCZ of the SGSP. The integrated systems may lead to ZLD desalination, while the treatment capacity of the concentrated solution is limited.
Regarding the further development of using MD to achieve ZLD, more research on scaled-up MD plants should be conducted, and the membrane lifetime should be extended. ZLD will become more and more attractive and promising.
3.3. MD Crystallization
Membrane distillation crystallization (MDC) is an integrated process of MD and crystallization in which pure water is produced via MD and the concentrated brine from the MD system is sent to a crystallizer to recover a valuable crystal product [98][99][100]. A schematic of an MDC system is shown in Figure 42. Edwie and Chung [101] analyzed an MDC system used to treat saturated brine solutions to produce pure water and salt crystals. They came to the conclusion that as the feed temperature increased, the yields of NaCl crystals and pure water would increase, and so would the scaling and membrane wetting. The membrane flux increased first then reduced sharply when the feed temperature was over 60 °C or 70 °C due to the occurrences of membrane scaling and wetting facilitated by salt oversaturation at the boundary layer. Ji et al. [102] conducted an experimental study on the treatment of artificial RO concentrates and natural RO brines using MDC. The authors found that crystallization kinetics were greatly affected by organics in the natural RO retentate. Ali et al. [103] carried out experimental and theoretical research on the treatment of produced water using an integrated MF plus DCMD/membrane crystallization system. By comparing the performances of the integrated processes and MSF in terms of process intensification metrics, the authors reached the conclusion that the coupled system had a better performance than the MSF system according to the productivity/size ratio and productivity/weight ratio metrics.
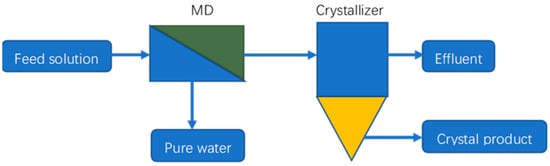
Figure 42. Schematic of an MDC system.
Further applications of MDC have been carried out in the treatment of industrial wastewater to recover lithium chloride, boric acid, sodium sulfate, and ethylene glycol [104][105][106][107]. MDC also serves as a potential method to realize ZLD. Guo et al. [100] and Chen et al. [108] have explored the use of MDC to achieve ZLD.
However, the potential fouling, scaling, and wetting of the membranes caused by organic materials and inorganic scales are serious problems in the treatment of concentrated brine and will cause reductions in permeate quality, permeate flux, module efficiency, and the lifetimes of the membranes [109][110][111][112][113]. Thus, further studies related to membrane fouling control and scaling reduction are needed before the commercialization of MD systems for the disposal of concentrated brine [109][114][115]. Simultaneously, a long-term stable process of concentrated brine treatment using MD is also needed.
References
- Elzahaby, A.M.; Kabeel, A.E.; Bassuoni, M.M.; Elbar, A.R.A. Direct contact membrane water distillation assisted with solar energy. Energy Convers. Manag. 2016, 110, 397–406.
- Baghel, R.; Upadhyaya, S.; Singh, K.; Chaurasia, S.P.; Gupta, A.B.; Dohare, R.K. A review on membrane applications and transport mechanisms in vacuum membrane distillation. Rev. Chem. Eng. 2017, 34, 73–106.
- Banat, F.; Jumah, R.; Garaibeh, M. Exploitation of solar energy collected by solar stills for desalination by membrane distillation. Renew. Energy 2002, 25, 293–305.
- Kim, Y.D.; Thu, K.; Ghaffour, N.; Choon Ng, K. Performance investigation of a solar-assisted direct contact membrane distillation system. J. Memb. Sci. 2013, 427, 345–364.
- Eykens, L.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Van Der Bruggen, B. How to Optimize the Membrane Properties for Membrane Distillation: A Review. Ind. Eng. Chem. Res. 2016, 55, 9333–9343.
- Koschikowski, J.; Wieghaus, M.; Rommel, M.; Ortin, V.S.; Suarez, B.P.; Betancort Rodríguez, J.R. Experimental investigations on solar driven stand-alone membrane distillation systems for remote areas. Desalination 2009, 248, 125–131.
- Abu-Zeid, M.A.E.R.; Zhang, Y.; Dong, H.; Zhang, L.; Chen, H.L.; Hou, L. A comprehensive review of vacuum membrane distillation technique. Desalination 2015, 356, 1–14.
- Chiam, C.K.; Sarbatly, R. Vacuum membrane distillation processes for aqueous solution treatment-A review. Chem. Eng. Process. Process Intensif. 2014, 74, 27–54.
- Summers, E.K.; Lienhard, J.H. Experimental study of thermal performance in air gap membrane distillation systems, including the direct solar heating of membranes. Desalination 2013, 330, 100–111.
- Kujawski, W.; Sobolewska, A.; Jarzynka, K.; Güell, C.; Ferrando, M.; Warczok, J. Application of osmotic membrane distillation process in red grape juice concentration. J. Food Eng. 2013, 116, 801–808.
- Khayet, M. Membranes and theoretical modeling of membrane distillation: A review. Adv. Colloid Interface Sci. 2011, 164, 56–88.
- Lee, J.-G.; Kim, W.-S.; Choi, J.-S.; Ghaffour, N.; Kim, Y.-D. Dynamic solar-powered multi-stage direct contact membrane distillation system: Concept design, modeling and simulation. Desalination 2017, 435, 278–292.
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18.
- Summers, E.K.; Arafat, H.A.; Lienhard V, J.H. Energy efficiency comparison of single-stage membrane distillation (MD) desalination cycles in different configurations. Desalination 2012, 290, 54–66.
- Shirazi, M.M.A.; Kargari, A.; Ismail, A.F.; Matsuura, T. Computational Fluid Dynamic (CFD) opportunities applied to the membrane distillation process: State-of-the-art and perspectives. Desalination 2016, 377, 73–90.
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Treatment of high salinity solutions: Application of air gap membrane distillation. Desalination 2012, 287, 55–60.
- Alklaibi, A.M.; Lior, N. Membrane-distillation desalination: Status and potential. Desalination 2005, 171, 111–131.
- Francis, L.; Ghaffour, N.; Alsaadi, A.A.; Amy, G.L. Material gap membrane distillation: A new design for water vapor flux enhancement. J. Membr. Sci. 2013, 448, 240–247.
- Khalifa, A.E.; Alawad, S.M. Air gap and water gap multistage membrane distillation for water desalination. Desalination 2018, 437, 175–183.
- Swaminathan, J.; Won, H.; Warsinger, D.M.; Almarzooqi, F.A.; Arafat, H.A.; Lienhard V, J.H. Energy ef fi ciency of permeate gap and novel conductive gap membrane distillation. J. Memb. Sci. 2016, 502, 171–178.
- Gao, L.; Zhang, J.; Gray, S.; Li, J. Experimental study of hollow fiber permeate gap membrane distillation and its performance comparison with DCMD and SGMD. Sep. Purif. Technol. 2017, 188, 11–23.
- Gryta, M. Osmotic MD and other membrane distillation variants. J. Memb. Sci. 2005, 246, 145–156.
- Terki, L.; Kujawski, W.; Kujawa, J.; Kurzawa, M.; Filipiak-Szok, A.; Chrzanowska, E.; Khaled, S.; Madani, K. Implementation of osmotic membrane distillation with various hydrophobic porous membranes for concentration of sugars solutions and preservation of the quality of cactus pear juice. J. Food Eng. 2018, 230, 28–38.
- Wang, L.; Min, J. Modeling and analyses of membrane osmotic distillation using non-equilibrium thermodynamics. J. Memb. Sci. 2011, 378, 462–470.
- Ali, A.; Tufa, R.A.; Macedonio, F.; Curcio, E.; Drioli, E. Membrane technology in renewable-energy-driven desalination. Renew. Sustain. Energy Rev. 2018, 81, 1–21.
- Chafidz, A.; Al-Zahrani, S.; Al-Otaibi, M.N.; Hoong, C.F.; Lai, T.F.; Prabu, M. Portable and integrated solar-driven desalination system using membrane distillation for arid remote areas in Saudi Arabia. Desalination 2014, 345, 36–49.
- González, D.; Amigo, J.; Suárez, F. Membrane distillation: Perspectives for sustainable and improved desalination. Renew. Sustain. Energy Rev. 2017, 80, 238–259.
- Camacho, L.M.; Dumée, L.; Zhang, J.; Li, J.D.; Duke, M.; Gomez, J.; Gray, S. Advances in membrane distillation for water desalination and purification applications. Water 2013, 5, 94–196.
- Chang, H.; Wang, G.B.; Chen, Y.H.; Li, C.C.; Chang, C.L. Modeling and optimization of a solar driven membrane distillation desalination system. Renew. Energy 2010, 35, 2714–2722.
- Chafidz, A.; Kerme, E.D.; Wazeer, I.; Khalid, Y.; Ajbar, A.; Al-Zahrani, S.M. Design and fabrication of a portable and hybrid solar-powered membrane distillation system. J. Clean. Prod. 2016, 133, 631–647.
- Banat, F.; Jwaied, N.; Rommel, M.; Koschikowski, J.; Wieghaus, M. Performance evaluation of the “large SMADES” autonomous desalination solar-driven membrane distillation plant in Aqaba, Jordan. Desalination 2007, 217, 17–28.
- Ben Abdallah, S.; Frikha, N.; Gabsi, S. Simulation of solar vacuum membrane distillation unit. Desalination 2013, 324, 87–92.
- Porrazzo, R.; Cipollina, A.; Galluzzo, M.; Micale, G. A neural network-based optimizing control system for a seawater-desalination solar-powered membrane distillation unit. Comput. Chem. Eng. 2013, 54, 79–96.
- Cipollina, A.; Di Sparti, M.G.; Tamburini, A.; Micale, G. Development of a Membrane Distillation module for solar energy seawater desalination. Chem. Eng. Res. Des. 2012, 90, 2101–2121.
- Saffarini, R.B.; Summers, E.K.; Arafat, H.A.; Lienhard V, J.H. Economic evaluation of stand-alone solar powered membrane distillation systems. Desalination 2012, 299, 55–62.
- Banat, F.; Jwaied, N. Exergy analysis of desalination by solar-powered membrane distillation units. Desalination 2008, 230, 27–40.
- Guillén-Burrieza, E.; Zaragoza, G.; Miralles-Cuevas, S.; Blanco, J. Experimental evaluation of two pilot-scale membrane distillation modules used for solar desalination. J. Memb. Sci. 2012, 409–410, 264–275.
- Chang, H.; Lyu, S.G.; Tsai, C.M.; Chen, Y.H.; Cheng, T.W.; Chou, Y.H. Experimental and simulation study of a solar thermal driven membrane distillation desalination process. Desalination 2012, 286, 400–411.
- Zaragoza, G.; Ruiz-Aguirre, A.; Guillén-Burrieza, E. Efficiency in the use of solar thermal energy of small membrane desalination systems for decentralized water production. Appl. Energy 2014, 130, 491–499.
- Raluy, R.G.; Schwantes, R.; Subiela, V.J.; Peñate, B.; Melián, G.; Betancort, J.R. Operational experience of a solar membrane distillation demonstration plant in Pozo Izquierdo-Gran Canaria Island (Spain). Desalination 2012, 290, 1–13.
- Gil, J.D.; Roca, L.; Zaragoza, G.; Berenguel, M. A feedback control system with reference governor for a solar membrane distillation pilot facility. Renew. Energy 2018, 120, 536–549.
- Gil, J.D.; Roca, L.; Ruiz-Aguirre, A.; Zaragoza, G.; Berenguel, M. Optimal operation of a Solar Membrane Distillation pilot plant via Nonlinear Model Predictive Control. Comput. Chem. Eng. 2018, 109, 151–165.
- Chen, Y.H.; Li, Y.W.; Chang, H. Optimal design and control of solar driven air gap membrane distillation desalination systems. Appl. Energy 2012, 100, 193–204.
- Ben Abdallah, S.; Frikha, N.; Gabsi, S. Design of an autonomous solar desalination plant using vacuum membrane distillation, the MEDINA project. Chem. Eng. Res. Des. 2013, 91, 2782–2788.
- Banat, F.; Jwaied, N. Economic evaluation of desalination by small-scale autonomous solar-powered membrane distillation units. Desalination 2008, 220, 566–573.
- Moore, S.E.; Mirchandani, S.D.; Karanikola, V.; Nenoff, T.M.; Arnold, R.G.; Eduardo Sáez, A. Process modeling for economic optimization of a solar driven sweeping gas membrane distillation desalination system. Desalination 2018, 437, 108–120.
- Saffarini, R.B.; Summers, E.K.; Arafat, H.A.; Lienhard V, J.H. Technical evaluation of stand-alone solar powered membrane distillation systems. Desalination 2012, 286, 332–341.
- Vega-Beltrán, J.C.; García-Rodríguez, L.; Martín-Mateos, I.; Blanco-Gálvez, J. Solar membrane distillation: Theoretical assessment of multi-stage concept. Desalin. Water Treat. 2010, 18, 133–138.
- Kim, Y.D.; Thu, K.; Choi, S.H. Solar-assisted multi-stage vacuum membrane distillation system with heat recovery unit. Desalination 2015, 367, 161–171.
- Pangarkar, B.L.; Deshmukh, S.K.; Sapkal, V.S.; Sapkal, R.S. Review of membrane distillation process for water purification. Desalin. Water Treat. 2016, 57, 2959–2981.
- Assari, M.R.; Basirat Tabrizi, H.; Parvar, M.; Kavoosi Nejad, A.; Jafar Gholi Beik, A. Experiment and optimization of mixed medium effect on small-scale salt gradient solar pond. Sol. Energy 2017, 151, 102–109.
- Khalilian, M. Exergetic performance analysis of a salinity gradient solar pond. Sol. Energy 2017, 157, 895–904.
- Alcaraz, A.; Montalà, M.; Cortina, J.L.; Akbarzadeh, A.; Aladjem, C.; Farran, A.; Valderrama, C. Design, construction, and operation of the first industrial salinity-gradient solar pond in Europe: An efficiency analysis perspective. Sol. Energy 2018, 164, 316–326.
- Silva, C.; González, D.; Suárez, F. An experimental and numerical study of evaporation reduction in a salt-gradient solar pond using floating discs. Sol. Energy 2017, 142, 204–214.
- Manzoor, K.; Khan, S.J.; Jamal, Y.; Shahzad, M.A. Heat extraction and brine management from salinity gradient solar pond and membrane distillation. Chem. Eng. Res. Des. 2017, 118, 226–237.
- Mericq, J.P.; Laborie, S.; Cabassud, C. Evaluation of systems coupling vacuum membrane distillation and solar energy for seawater desalination. Chem. Eng. J. 2011, 166, 596–606.
- El Mansouri, A.; Hasnaoui, M.; Amahmid, A.; Dahani, Y. Transient theoretical model for the assessment of three heat exchanger designs in a large-scale salt gradient solar pond: Energy and exergy analysis. Energy Convers. Manag. 2018, 167, 45–62.
- Aramesh, M.; Kasaeian, A.; Pourfayaz, F.; Wen, D. Energy analysis and shadow modeling of a rectangular type salt gradient solar pond. Sol. Energy 2017, 146, 161–171.
- Suárez, F.; Tyler, S.W.; Childress, A.E. A theoretical study of a direct contact membrane distillation system coupled to a salt-gradient solar pond for terminal lakes reclamation. Water Res. 2010, 44, 4601–4615.
- Suárez, F.; Ruskowitz, J.A.; Tyler, S.W.; Childress, A.E. Renewable water: Direct contact membrane distillation coupled with solar ponds. Appl. Energy 2015, 158, 532–539.
- Nakoa, K.; Rahaoui, K.; Date, A.; Akbarzadeh, A. An experimental review on coupling of solar pond with membrane distillation. Sol. Energy 2015, 119, 319–331.
- Suárez, F.; Urtubia, R. Tackling the water-energy nexus: An assessment of membrane distillation driven by salt-gradient solar ponds. Clean Technol. Environ. Policy 2016, 18, 1697–1712.
- Wang, K.; Yuan, B.; Ji, G.; Wu, X. A comprehensive review of geothermal energy extraction and utilization in oilfields. J. Pet. Sci. Eng. 2018, 168, 465–477.
- Hou, J.; Cao, M.; Liu, P. Development and utilization of geothermal energy in China: Current practices and future strategies. Renew. Energy 2018, 125, 401–412.
- Nian, Y.L.; Cheng, W.L. Insights into geothermal utilization of abandoned oil and gas wells. Renew. Sustain. Energy Rev. 2018, 87, 44–60.
- Bundschuh, J.; Ghaffour, N.; Mahmoudi, H.; Goosen, M.; Mushtaq, S.; Hoinkis, J. Low-cost low-enthalpy geothermal heat for freshwater production: Innovative applications using thermal desalination processes. Renew. Sustain. Energy Rev. 2015, 43, 196–206.
- Tomaszewska, B.; Pająk, L.; Bundschuh, J.; Bujakowski, W. Low-enthalpy geothermal energy as a source of energy and integrated freshwater production in inland areas: Technological and economic feasibility. Desalination 2018, 435, 35–44.
- Nogara, J.; Zarrouk, S.J. Corrosion in geothermal environment: Part 1: Fluids and their impact. Renew. Sustain. Energy Rev. 2018, 82, 1333–1346.
- Loutatidou, S.; Arafat, H.A. Techno-economic analysis of MED and RO desalination powered by low-enthalpy geothermal energy. Desalination 2015, 365, 277–292.
- Sarbatly, R.; Chiam, C.K. Evaluation of geothermal energy in desalination by vacuum membrane distillation. Appl. Energy 2013, 112, 737–746.
- Nogara, J.; Zarrouk, S.J. Corrosion in geothermal environment Part 2: Metals and alloys. Renew. Sustain. Energy Rev. 2018, 82, 1347–1363.
- Lokare, O.R.; Tavakkoli, S.; Rodriguez, G.; Khanna, V.; Vidic, R.D. Integrating membrane distillation with waste heat from natural gas compressor stations for produced water treatment in Pennsylvania. Desalination 2017, 413, 144–153.
- Koo, J.; Nam, S.-H.; Kim, E.; Hwang, T.-M.; Lee, S. Operation optimization of vacuum membrane distillation using the shipboard waste heat. Desalin. Water Treat. 2017, 77, 57–62.
- Wang, J.; Fan, B.; Luan, Z.; Qu, D.; Peng, X.; Hou, D. Integration of direct contact membrane distillation and recirculating cooling water system for pure water production. J. Clean. Prod. 2008, 16, 1847–1855.
- Dow, N.; Gray, S.; Li, J.D.; Zhang, J.; Ostarcevic, E.; Liubinas, A.; Atherton, P.; Roeszler, G.; Gibbs, A.; Duke, M. Pilot trial of membrane distillation driven by low grade waste heat: Membrane fouling and energy assessment. Desalination 2016, 391, 30–42.
- Jansen, A.E.; Assink, J.W.; Hanemaaijer, J.H.; van Medevoort, J.; van Sonsbeek, E. Development and pilot testing of full-scale membrane distillation modules for deployment of waste heat. Desalination 2013, 323, 55–65.
- Martinetti, C.R.; Childress, A.E.; Cath, T.Y. High recovery of concentrated RO brines using forward osmosis and membrane distillation. J. Memb. Sci. 2009, 331, 31–39.
- Tsai, J.H.; Macedonio, F.; Drioli, E.; Giorno, L.; Chou, C.Y.; Hu, F.C.; Li, C.L.; Chuang, C.J.; Tung, K.L. Membrane-based zero liquid discharge: Myth or reality? J. Taiwan Inst. Chem. Eng. 2017, 80, 192–202.
- Mericq, J.P.; Laborie, S.; Cabassud, C. Vacuum membrane distillation of seawater reverse osmosis brines. Water Res. 2010, 44, 5260–5273.
- Bamufleh, H.; Abdelhady, F.; Baaqeel, H.M.; El-Halwagi, M.M. Optimization of multi-effect distillation with brine treatment via membrane distillation and process heat integration. Desalination 2017, 408, 110–118.
- Naidu, G.; Jeong, S.; Choi, Y.; Vigneswaran, S. Membrane distillation for wastewater reverse osmosis concentrate treatment with water reuse potential. J. Memb. Sci. 2017, 524, 565–575.
- Han, G.; Zuo, J.; Wan, C.; Chung, T.S. Hybrid pressure retarded osmosis–membrane distillation (PRO–MD) process for osmotic power and clean water generation. Environ. Sci. Water Res. Technol. 2015, 1, 507–515.
- Bogler, A.; Lin, S.; Bar-Zeev, E. Biofouling of membrane distillation, forward osmosis and pressure retarded osmosis: Principles, impacts and future directions. J. Memb. Sci. 2017, 542, 378–398.
- Lin, S.; Yip, N.Y.; Cath, T.Y.; Osuji, C.O.; Elimelech, M. Hybrid Pressure Retarded Osmosis—Membrane Distillation System for Power Generation from Low-Grade Heat: Thermodynamic Analysis and Energy Efficiency. Environ. Sci. Technol. 2014, 48, 5306–5313.
- Park, K.; Kim, D.Y.; Yang, D.R. Theoretical Analysis of Pressure Retarded Membrane Distillation (PRMD) Process for Simultaneous Production of Water and Electricity. Ind. Eng. Chem. Res. 2017, 56, 14888–14901.
- Kim, J.; Park, M.; Shon, H.K.; Kim, J.H. Performance analysis of reverse osmosis, membrane distillation, and pressure-retarded osmosis hybrid processes. Desalination 2016, 380, 85–92.
- Choi, Y.; Kim, S.-H.; Lee, S. Comparison of performance and economics of reverse osmosis, membrane distillation, and pressure retarded osmosis hybrid systems. Desalin. Water Treat. 2017, 77, 19–29.
- Ho, S.; Seo, J.; Kim, J.; Mi, Y.; Ha, J. A simulation study with a new performance index for pressure-retarded osmosis processes hybridized with seawater reverse osmosis and membrane distillation. Desalination 2018, 444, 118–128.
- Micari, M.; Cipollina, A.; Giacalone, F.; Kosmadakis, G.; Papapetrou, M.; Zaragoza, G.; Micale, G.; Tamburini, A. Towards the first proof of the concept of a Reverse ElectroDialysis—Membrane Distillation Heat Engine. Desalination 2019, 453, 77–88.
- Avci, A.H.; Santoro, S.; Politano, A.; Propato, M.; Micieli, M.; Aquino, M.; Wenjuan, Z.; Curcio, E. Photothermal Sweeping Gas Membrane Distillation and Reverse Electrodialysis for light-to-heat-to-power conversion. Chem. Eng. Process. 2021, 164, 108382.
- Mercer, E.; Davey, C.J.; Azzini, D.; Eusebi, A.L.; Tierney, R.; Williams, L.; Jiang, Y.; Parker, A.; Kolios, A.; Tyrrel, S.; et al. Hybrid membrane distillation reverse electrodialysis configuration for water and energy recovery from human urine: An opportunity for off-grid decentralised sanitation. J. Membr. Sci. 2019, 584, 343–352.
- Long, R.; Li, B.; Liu, Z.; Liu, W. Hybrid membrane distillation-reverse electrodialysis electricity generation system to harvest low-grade thermal energy. J. Memb. Sci. 2017, 525, 107–115.
- Tufa, R.A.; Noviello, Y.; Profio, G.D.; Macedonio, F.; Ali, A.; Drioli, E.; Fontananova, E.; Bouzek, K.; Curcio, E. Integrated membrane distillation-reverse electrodialysis system for energy-efficient seawater desalination. Appl. Energy 2019, 253, 113551.
- Tong, T.; Elimelech, M. The Global Rise of Zero Liquid Discharge for Wastewater Management: Drivers, Technologies, and Future Directions. Environ. Sci. Technol. 2016, 50, 6846–6855.
- Schwantes, R.; Chavan, K.; Winter, D.; Felsmann, C.; Pfafferott, J. Techno-economic comparison of membrane distillation and MVC in a zero liquid discharge application. Desalination 2018, 428, 50–68.
- Tufa, R.A.; Curcio, E.; Brauns, E.; van Baak, W.; Fontananova, E.; Di Profio, G. Membrane Distillation and Reverse Electrodialysis for Near-Zero Liquid Discharge and low energy seawater desalination. J. Memb. Sci. 2015, 496, 325–333.
- Nakoa, K.; Rahaoui, K.; Date, A.; Akbarzadeh, A. Sustainable zero liquid discharge desalination (SZLDD). Sol. Energy 2016, 135, 337–347.
- Jiang, X.; Tuo, L.; Lu, D.; Hou, B.; Chen, W.; He, G. Progress in membrane distillation crystallization: Process models, crystallization control and innovative applications. Front. Chem. Sci. Eng. 2017, 11, 647–662.
- Creusen, R.; van Medevoort, J.; Roelands, M.; van Renesse van Duivenbode, A.; Hanemaaijer, J.H.; van Leerdam, R. Integrated membrane distillation-crystallization: Process design and cost estimations for seawater treatment and fluxes of single salt solutions. Desalination 2013, 323, 8–16.
- Guo, H.; Ali, H.M.; Hassanzadeh, A. Simulation study of flat-sheet air gap membrane distillation modules coupled with an evaporative crystallizer for zero liquid discharge water desalination. Appl. Therm. Eng. 2016, 108, 486–501.
- Edwie, F.; Chung, T.S. Development of simultaneous membrane distillation-crystallization (SMDC) technology for treatment of saturated brine. Chem. Eng. Sci. 2013, 98, 160–172.
- Ji, X.; Curcio, E.; Al Obaidani, S.; Di Profio, G.; Fontananova, E.; Drioli, E. Membrane distillation-crystallization of seawater reverse osmosis brines. Sep. Purif. Technol. 2010, 71, 76–82.
- Ali, A.; Quist-Jensen, C.A.; Drioli, E.; Macedonio, F. Evaluation of integrated microfiltration and membrane distillation/crystallization processes for produced water treatment. Desalination 2018, 434, 161–168.
- Quist-Jensen, C.A.; Ali, A.; Mondal, S.; Macedonio, F.; Drioli, E. A study of membrane distillation and crystallization for lithium recovery from high-concentrated aqueous solutions. J. Memb. Sci. 2016, 505, 167–173.
- Quist-Jensen, C.A.; Macedonio, F.; Horbez, D.; Drioli, E. Reclamation of sodium sulfate from industrial wastewater by using membrane distillation and membrane crystallization. Desalination 2017, 401, 112–119.
- Jia, F.; Li, J.; Wang, J. Recovery of boric acid from the simulated radioactive wastewater by vacuum membrane distillation crystallization. Ann. Nucl. Energy 2017, 110, 1148–1155.
- Lu, D.; Li, P.; Xiao, W.; He, G.; Jiang, X. Simultaneous recovery and crystallization control of saline organic wastewater by membrane distillation crystallization. AIChE J. 2017, 63, 2187–2197.
- Chen, G.; Lu, Y.; Krantz, W.B.; Wang, R.; Fane, A.G. Optimization of operating conditions for a continuous membrane distillation crystallization process with zero salty water discharge. J. Memb. Sci. 2014, 450, 1–11.
- Nguyen, Q.M.; Jeong, S.; Lee, S. Characteristics of membrane foulants at different degrees of SWRO brine concentration by membrane distillation. Desalination 2017, 409, 7–20.
- Sanmartino, J.A.; Khayet, M.; García-Payo, M.C.; El Bakouri, H.; Riaza, A. Desalination and concentration of saline aqueous solutions up to supersaturation by air gap membrane distillation and crystallization fouling. Desalination 2016, 393, 39–51.
- Kim, J.; Kwon, H.; Lee, S.; Lee, S.; Hong, S. Membrane distillation (MD) integrated with crystallization (MDC) for shale gas produced water (SGPW) treatment. Desalination 2017, 403, 172–178.
- Duong, H.C.; Gray, S.; Duke, M.; Cath, T.Y.; Nghiem, L.D. Scaling control during membrane distillation of coal seam gas reverse osmosis brine. J. Memb. Sci. 2015, 493, 673–682.
- Qu, D.; Wang, J.; Fan, B.; Luan, Z.; Hou, D. Study on concentrating primary reverse osmosis retentate by direct contact membrane distillation. Desalination 2009, 247, 540–550.
- Wu, Y.; Kang, Y.; Zhang, L.; Qu, D.; Cheng, X.; Feng, L. Performance and fouling mechanism of direct contact membrane distillation (DCMD) treating fermentation wastewater with high organic concentrations. J. Environ. Sci. 2018, 65, 253–261.
- Zhang, P.; Knötig, P.; Gray, S.; Duke, M. Scale reduction and cleaning techniques during direct contact membrane distillation of seawater reverse osmosis brine. Desalination 2015, 374, 20–30.
More
