Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Alberto Kousuke De la Herrán-Arita.
The intricate mechanisms governing brain health and function have long been subjects of extensive investigation. Recent research has shed light on two pivotal systems, the glymphatic system and the endocannabinoid system, and their profound role within the central nervous system. The glymphatic system is a recently discovered waste clearance system within the brain that facilitates the efficient removal of toxic waste products and metabolites from the central nervous system.
- GS
- endocannabinoid system
- waste clearance
1. Introduction
The intricate and dynamic nature of the central nervous system (CNS) involves a multitude of regulatory systems that contribute to its structural integrity, functional balance, and resilience against pathological insults. Among these, the Glymphatic System (GS) and the Endocannabinoid System (ECS) have emerged as crucial players, each exerting profound influences on neurological processes. The GS, a relatively recent addition to ourthe understanding of CNS physiology, functions as a waste clearance mechanism, facilitating the removal of metabolites and potentially neurotoxic substances. In parallel, the ECS, a complex signaling network, modulates neurotransmission, immune responses, and inflammatory processes throughout the brain.
2. The Glymphatic System
The brain is a highly metabolically active organ that generates substantial waste products that require efficient clearance mechanisms. The GS, initially described by Iliff et al. in 2012, represents a novel waste clearance pathway within the CNS [1]. It involves the exchange of cerebrospinal fluid (CSF) and interstitial fluid (ISF) facilitated by a complex network of interconnected structures within the brain (Figure 1). Key anatomical components include the perivascular spaces surrounding arteries and veins, aquaporin-4 (AQP4) channels on astrocytic endfeet, and the meningeal lymphatic vessels. The perivascular spaces serve as conduits for the convective flow of CSF, while astrocytic AQP4 channels regulate the influx and efflux of fluids [2,3][2][3] (Figure 1, inset A). In addition, the recently discovered meningeal lymphatic vessels provide a route for waste clearance from the CNS to the peripheral lymphatic system [4,5][4][5].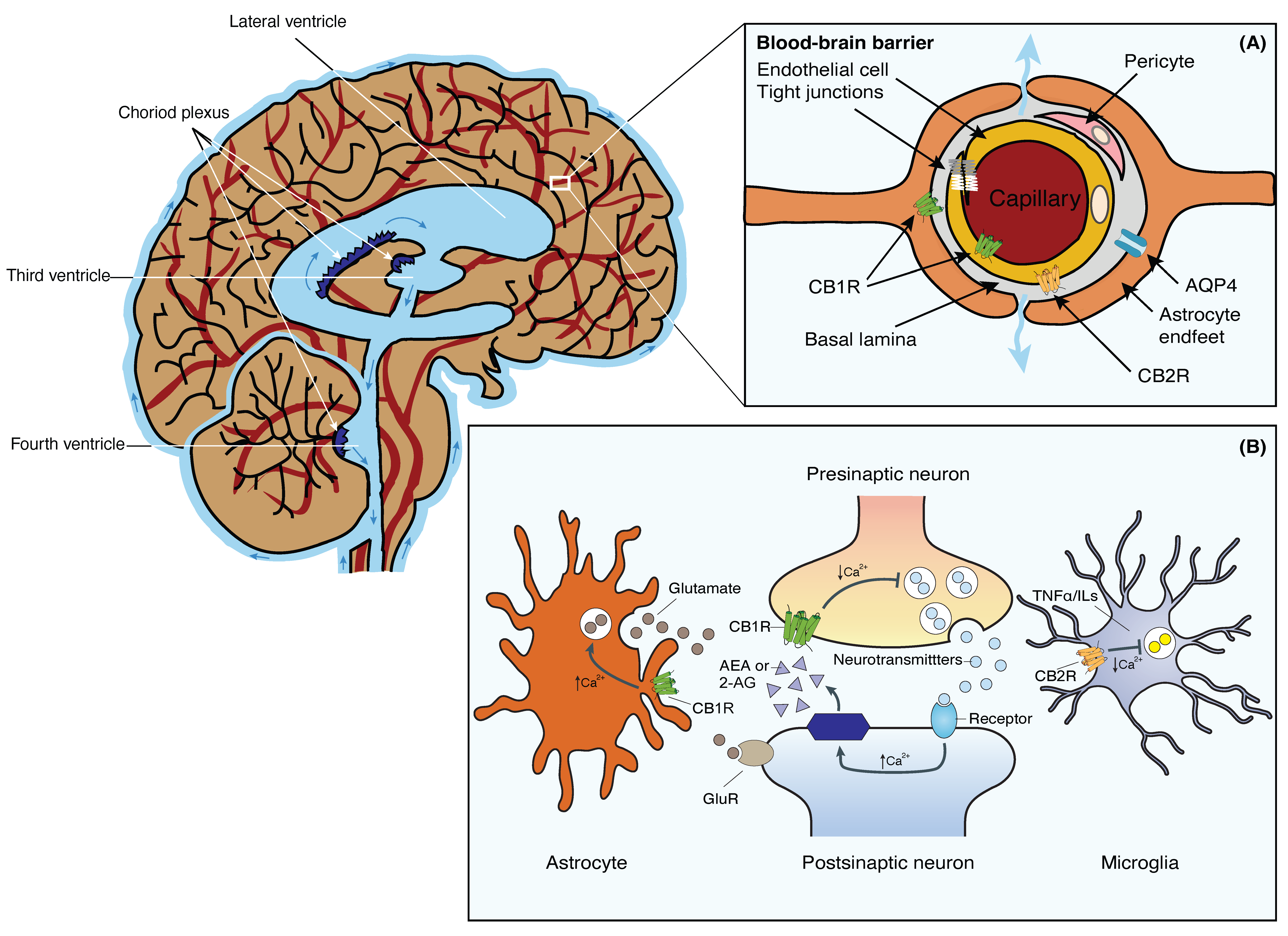
Figure 1. Schematic representation of the brain’s fluid compartments and endocannabinoid receptors in the nervous and glia system. Main: The cerebral ventricles are a system of interconnected, fluid-filled cavities within the brain that contribute to cerebrospinal fluid (CSF) production and circulation. The four ventricles include the paired lateral ventricles, the third ventricle, and the fourth ventricle, connected by the cerebral aqueduct. These ventricles are lined with ependymal cells and house the choroid plexus, a specialized structure responsible for CSF secretion. Inset (A): Tight junctions between the blood endothelial cells constitute the BBB, restricting macromolecules from moving freely from the blood into the brain parenchyma. Fluid and solutes diffuse into the brain parenchyma from the perivascular space located between endothelial cells and astrocytic endfeet that expresses AQP4 and CB1R. CB1Rs are primarily located on the luminal side of the BBB endothelium. CB2Rs, on the other hand, are located on the abluminal side of the BBB. Inset (B): 2-AG or AEA are synthesized from phospholipids on demand. Activation of presynaptic CB1R negatively modulates cell calcium influx and the release of neurotransmitters in neurons. Stimulation of CB1R in astrocytes positively modulates calcium influx and glutamate release. Activation of CB2R in microglia negatively affects the release of TNFα and ILs. Abbreviations: AQP4, Aquaporin-4 water channel; BBB, blood-brain barrier; CB1R, Cannabinoid receptor 1; CB2R, Cannabinoid receptor 2; CSF, cerebrospinal fluid; 2-AG, 2-acylglycerol; AEA, anandamide; GluR, glutamate receptors; ILs, interleukins; TNFα, tumor necrosis factor-α.
3. The Blood-Brain Barrier
The BBB is a specialized barrier formed by endothelial cells in brain capillaries. It serves as a physical and functional barrier, tightly regulating the exchange of substances between the blood and the brain. The unique characteristics of the brain endothelium enable a critical limitation through the absence of fenestrations and the presence of tight junctions between cells. The architectural composition of the BBB consists of endothelial cells that line the microvessels of the brain, capillary basement membranes, and specialized structures known as end-feet, which extend from astrocytes to the basement membrane [17,25][17][25]. The selective permeability of the BBB is ensured by intercellular tight junctions that connect brain microvascular endothelial cells. The blood vessels of the BBB are lined with endothelial cells, which are tightly interconnected through tight junctions. Surrounding the basement membrane, astrocyte endfeet and pericytes contribute to the structural support of the BBB (Figure 1, inset A). The main function of tight junctions is to restrict the paracellular pathway for the diffusion of hydrophilic solutes, thereby allowing for the control of chemical substances present in the circulatory system that can access the brain [26,27][26][27]. These features of brain vessels regulate the exchange of molecules and cells between the circulation and the CNS. Additionally, they play crucial roles in preventing the loss of essential substances by controlling transcellular movements of water, ions, oxygen, carbon dioxide, and glucose, all of which are necessary for cerebral cellular metabolism. Consequently, disorders affecting the BBB can disrupt ion regulation and disturb homeostasis, leading to impaired brain function [27,28][27][28]. Given that glymphatic dysfunction can lead to the accumulation of waste products in the brain and contribute to the genesis of neurodegenerative diseases [29], the integrity and functionality of the BBB are crucial for the clearance of these waste products from the brain parenchyma. Moreover, the BBB also controls the entry of nutrients and oxygen into the brain, providing essential energy substrates for neuronal function and maintenance. On the other hand, the GS, by facilitating the exchange of CSF and ISF, ensures the efficient distribution of these nutrients and oxygen to different regions of the brain. Disruptions in either the GS or the BBB can lead to inadequate nutrient supply and subsequent neuronal dysfunction [24,25,28][24][25][28]. In addition, both the GS and the BBB contribute to the regulation of the brain’s immune response. Dysfunction in either system can lead to increased inflammation and impaired clearance of inflammatory molecules, which can negatively impact brain health. Research suggests that an interplay between the GS and the BBB is crucial for the efficient clearance of immune cells and inflammatory mediators from the brain [30,31][30][31]. The relationship between the GS and the BBB is vital for maintaining brain homeostasis. Their coordination ensures the efficient removal of waste products, proper nutrient supply, and regulation of the immune response [24,32][24][32].4. The Endocannabinoid System
The ECS is a complex signaling network present throughout the body. The ECS was first discovered through research on the pharmacological effects of cannabis-derived compounds. It is now recognized as a crucial regulatory system involved in maintaining homeostasis throughout the body. The ECS comprises endogenous cannabinoids (endocannabinoids), cannabinoid receptors (CB1R and CB2R), and enzymes responsible for endocannabinoid synthesis and degradation [33,34][33][34]. The two primary endocannabinoids are anandamide (AEA) and 2-arachidonoylglycerol (2-AG), whereas enzymes involved in endocannabinoid metabolism include fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), which are responsible for the degradation of AEA and 2-AG, respectively [35,36][35][36]. Unlike conventional neurotransmitters, endocannabinoids are not stored in vesicles but are synthesized by neurons on demand, utilizing lipid components of the cell membrane. These endocannabinoids function as retrograde messengers, transmitting intercellular signals from postsynaptic neurons back to presynaptic terminals, where they inhibit the release of neurotransmitters (Figure 1, inset A) [35,36,37][35][36][37]. Initially, their pharmacological properties were thought to be similar, with the assumption that these molecules could be interchangeably and indistinguishably involved in regulating synaptic functions, synaptic plasticity, and behavioral aspects such as learning, memory, reward, addiction, antinociception, and anxiety. However, emerging evidence suggests that AEA and 2-AG possess distinct pharmacological properties, contribute to different forms of synaptic plasticity, and participate in diverse behavioral functions [33,37,38,39][33][37][38][39]. Apart from endocannabinoids, phytocannabinoids constitute a diverse array of compounds with the capacity to activate cannabinoid receptors. Cannabidiol (CBD) and tetrahydrocannabinol (THC) stand as the most extensively studied phytocannabinoids originating from the cannabis plant. They display an extensive spectrum of therapeutic potential, attracting substantial attention due to their interactions with the body’s endocannabinoid receptors. In addition to CBD and THC, alternative phytocannabinoids, like cannabigerol (CBG) and cannabinol (CBN), also engage cannabinoid receptors. These interactions incite a myriad of physiological responses, influencing processes such as nociception, immune modulation, and inflammatory regulation, among others [33,34,35,36,37][33][34][35][36][37]. These endogenous and exogenous cannabinoids bind to and activate cannabinoid receptors, predominantly CB1R and CB2R, which are widely distributed throughout the body. The human body possesses specific binding sites for cannabinoids that are distributed across the surfaces of various cells. These receptors are part of the extensive family of G protein-coupled receptors (GPCRs), which encompasses the majority of commonly found receptors. GPCRs are membrane receptors composed of seven transmembrane domains (7TM), featuring an extracellular amino-terminal and an intracellular carbonyl terminal [36,37][36][37]. Upon ligand binding, the activated cannabinoid receptors interact with and activate specific G proteins located inside the cell. CB1R predominantly activates Gαi/o proteins, while CB2R can couple with Gαi/o, as well as Gαs proteins. The activated G proteins dissociate into their subunits, leading to the modulation of intracellular signaling pathways. In the case of CB1R, Gαi/o inhibits the production of cyclic adenosine monophosphate (cAMP) and reduces calcium ion influx into neurons, thereby modulating neurotransmitter release. CB2R activation can also inhibit cAMP production but stimulates other signaling pathways involved in immune responses and inflammation, like the MAP kinase phosphatase 3 (MKP3) pathway. The primary function of cannabinoid receptors is to regulate the release of other chemical messengers. CB1R modulates the release of specific neurotransmitters, thereby protecting the CNS from excessive stimulation or inhibition caused by other neurotransmitters. On the other hand, CB2R primarily plays a peripheral role via immunomodulatory activities, primarily modulating the release of cytokines, protein molecules responsible for regulating immune function, and inflammatory responses [36] (Figure 1, inset B). Consequently, cannabinoids may impact neurodegenerative diseases through their neuro- and immunomodulatory effects [40]. Cannabinoid receptors exhibit distinct tissue distributions and signaling mechanisms. Among them, CB1Rs are highly abundant and widely distributed within the brain. They are primarily located on neurons in the CNS. In the brain, CB1Rs are particularly prominent in regions associated with motor coordination and movement (e.g., cerebellum, basal ganglia, striatum, substantia nigra), attention, and complex cognitive functions like judgment (e.g., cerebral cortex), learning, memory, and emotions (e.g., amygdala, hippocampus). CB1Rs are also present, albeit to a lesser extent, in select organs and peripheral tissues, including endocrine glands, salivary glands, leukocytes, spleen, heart, and parts of the reproductive, urinary, and gastrointestinal systems. In addition, the activation of CB1R in astrocytes, whether prompted by endogenous or exogenous cannabinoids, initiates intracellular signaling resulting in an elevation of cytosolic calcium. This, in consequence, prompts the release of glutamate from astrocytes (Figure 1, inset B). The released glutamate then activates extra-synaptic NMDA receptors in neurons located at a considerable distance, giving rise to depolarizing currents termed slow inward currents (SICs). Various investigations have demonstrated the involvement of SICs in neuronal synchronization [37]. The distribution of CB1R suggests that endocannabinoids play a physiological role in the regulation of movement and sensory perception, as well as in processes related to learning, memory, and emotional states such as pleasure and aggression. In contrast to CB1Rs, CB2Rs are primarily expressed in immune cells and tissues, such as leukocytes, spleen, tonsils, bone marrow, and to a lesser extent, in the pancreas. Recent findings have also identified CB2Rs in the CNS, specifically on glial and microglial cells, where they induce a reduction of pro-inflammatory factors and microglial migration via MKP-3 (Figure 1, inset B) [37,41][37][41]. The expression of CB1R and CB2R in microglia is contingent upon their activation state and phenotype. Activated microglia in brain tissue express CB2R; however, the specific neuropathology influencing their activation results in varying phenotypes and levels of CB2R expression. Activation of microglial CB2R through cannabinoids plays a regulatory role in their immune-related functions. For instance, CB2R activation enhances microglial cell proliferation and migration, concurrently decreasing the release of harmful factors like TNFα and free radicals. Given the myriad of tissues and cells that express cannabinoid receptors, the ECS plays a vital role in various physiological processes, including pain modulation, immune function, inflammation, appetite regulation, metabolism, neuronal plasticity, and stress responses. It also acts as a homeostatic regulator, maintaining balance within the body [36,37,38,39,40,41][36][37][38][39][40][41]. Dysregulation of the ECS has been implicated in numerous health conditions. Alterations in endocannabinoid signaling have been associated with chronic pain, inflammatory disorders, neurodegenerative diseases, metabolic disorders, mood disorders, and addiction [40,41][40][41].5. The Endocannabinoid System and the Blood-Brain Barrier
Inflammation within the CNS is one of the main mechanisms involved in the development of neurological conditions. This process is initiated as a complex cascade triggered by inflammatory signals arising from infection, injury, or neurodegenerative processes, and it involves the orchestrated migration of leukocytes, such as neutrophils and monocytes, from the bloodstream through BBB to the site of inflammation. Upon reaching the BBB, leukocytes engage in a series of steps, including initial rolling and subsequent firm adhesion to endothelial cells, followed by transmigration through the BBB and into the CNS tissue. Within the CNS tissue, these migrating leukocytes interact with local immune cells, particularly microglia, resulting in the activation of an immune response. This immune response entails the release of pro-inflammatory cytokines, chemokines, and other mediators, aiming to eliminate the source of inflammation. The interplay between circulating leukocytes and resident immune cells can amplify the immune response, leading to an intensified inflammatory process [30,31][30][31]. A balanced resolution of inflammation is crucial to prevent tissue damage and neurodegenerative disorders [42]. Inflammatory conditions and neurodegenerative diseases disrupt the BBB, leading to increased permeability and compromised brain function. The ECS may exert regulatory effects on the BBB, potentially contributing to the maintenance of barrier integrity under pathological conditions. Consequently, there has been a growing interest in cannabinoids for their anti-inflammatory and immunomodulatory properties, as well as their capacity to modulate endothelial and epithelial barriers, making them promising candidates for improving cognitive deficits by protecting the BBB. The ECS components have been identified in the cells of the BBB, including endothelial cells and astrocytes. CB1Rs are primarily located on the luminal side of the BBB endothelium. Expression of CB1R has been observed in astrocytes, microglial cells, and pericytes. CB2Rs, on the other hand, are located on the abluminal side of the BBB (Figure 1, inset A). Activation of CB1R in astrocytes regulates their metabolic activities. For instance, when CB1R on astrocytes is activated, it enhances the rates of both glucose oxidation and ketogenesis, two processes crucial for supplying energy to the brain. Taking into consideration the capacity of astrocytic CB1R to oversee energy metabolism and facilitate neuron-glia interactions, one could conjecture their potential pathological significance. Considering the pivotal role of perivascular astrocytes in delivering energy from the blood to neurons in an activity-dependent fashion, it is plausible that astrocytic CB1R plays a role in regulating the energy supply to neurons [43,44][43][44]. Building on the influence of cannabinoid receptor activation on endothelial cell responses, further insights emerge from investigations into cannabinoid modulation. Through the use of an experimental model for multiple sclerosis known as Theiler’s murine encephalomyelitis virus-induced demyelinating disease, researchers have demonstrated that CBD can modify the detrimental effects of this condition by reducing the infiltration of leukocytes from the systemic circulation. This effect is achieved through down-regulating the expression of chemokines such as C-C motif chemokine ligand 2 (CCL2) and C-C motif chemokine ligand 5 (CCL5), interleukin-1 β, and VCAM-1 [45]. This suggests that the ECS mitigates the observed BBB alterations in these conditions by preventing inflammation in endothelial cells. The authors also found that CBD can prevent cellular margination and reduce the expression of adhesion molecules and chemotaxis in laboratory models of inflammation. In a similar study, CBD administration enhanced the integrity and permeability of the BBB and diminished the protein levels of proinflammatory cytokines (TNFα and IL-1β). Additionally, it substantially elevated the expression of tight junction proteins (claudin-5 and occludin) [46]. Related research has yielded further insights into the potential of cannabinoids in managing neuroinflammatory processes. CB2R activation has been shown to decrease the induction of intercellular adhesion molecule-1 (ICAM-1) and VCAM-1 surface expression and increase the amount of tight junction proteins in human brain microvascular endothelial cells exposed to various proinflammatory mediators [47]. In addition, CB2R activation has also been shown to attenuate BBB damage and neurodegeneration in a murine model of traumatic brain injury through the modulation of macrophage/microglia cell response [48]. The ECS demonstrates immunomodulatory properties that could potentially impact immune responses within the brain, particularly pertinent in the context of neuroinflammatory processes commonly associated with diverse neurological disorders. This interaction gains significance due to its potential consequences for maintaining the integrity of the BBB. Moreover, the ECS’s engagement in neuroprotective functions may indirectly influence BBB performance by counteracting oxidative stress and inflammatory processes, recognized contributors to BBB impairment. In this manner, the ECS might contribute to sustaining BBB integrity and bolstering overall cerebral well-being. Furthermore, the ECS exhibits implications in the intricate phenomenon of neurovascular coupling, governing the intricate interplay between neuronal activity and blood flow regulation that holds pivotal importance in ensuring appropriate perfusion during heightened neural demands. Perturbations in cerebral blood flow can wield effects on BBB permeability, implicating the ECS in the potential coordination of neuronal activity, blood flow dynamics, and BBB functionality [49].References
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012, 4, 147ra111.
- Naganawa, S.; Taoka, T. The Glymphatic System: A Review of the Challenges in Visualizing its Structure and Function with MR Imaging. Magn. Reson. Med. Sci. 2022, 21, 182–194.
- Jessen, N.A.; Munk, A.S.F.; Lundgaard, I.; Nedergaard, M. The Glymphatic System: A Beginner’s Guide. Neurochem. Res. 2015, 40, 2583–2599.
- Tian, Y.; Zhao, M.; Chen, Y.; Yang, M.; Wang, Y. The Underlying Role of the Glymphatic System and Meningeal Lymphatic Vessels in Cerebral Small Vessel Disease. Biomolecules 2022, 12, 748.
- Abbott, N.J.; Pizzo, M.E.; Preston, J.E.; Janigro, D.; Thorne, R.G. The role of brain barriers in fluid movement in the CNS: Is there a ‘glymphatic’ system? Acta Neuropathol. 2018, 135, 387–407.
- Chong, P.L.; Garic, D.; Shen, M.D.; Lundgaard, I.; Schwichtenberg, A.J. Sleep, cerebrospinal fluid, and the glymphatic system: A systematic review. Sleep Med. Rev. 2021, 61, 101572.
- Eide, P.K.; Vinje, V.; Pripp, A.H.; Mardal, K.-A.; Ringstad, G. Sleep deprivation impairs molecular clearance from the human brain. Brain 2021, 144, 863–874.
- Demiral, Ş.B.; Tomasi, D.; Sarlls, J.; Lee, H.; Wiers, C.E.; Zehra, A.; Srivastava, T.; Ke, K.; Shokri-Kojori, E.; Freeman, C.R.; et al. Apparent diffusion coefficient changes in human brain during sleep—Does it inform on the existence of a glymphatic system? NeuroImage 2018, 185, 263–273.
- Sun, B.-L.; Wang, L.-H.; Yang, T.; Sun, J.-Y.; Mao, L.-L.; Yang, M.-F.; Yuan, H.; Colvin, R.A.; Yang, X.-Y. Lymphatic drainage system of the brain: A novel target for intervention of neurological diseases. Prog. Neurobiol. 2018, 163–164, 118–143.
- Taoka, T.; Naganawa, S. Neurofluid Dynamics and the Glymphatic System: A Neuroimaging Perspective. Korean J. Radiol. 2020, 21, 1199–1209.
- Wu, C.; Lirng, J.; Ling, Y.; Wang, Y.; Wu, H.; Fuh, J.; Lin, P.; Wang, S.; Chen, S. Noninvasive Characterization of Human Glymphatics and Meningeal Lymphatics in an in vivo Model of Blood–Brain Barrier Leakage. Ann. Neurol. 2020, 89, 111–124.
- Saade, C.; Bou-Fakhredin, R.; Yousem, D.M.; Asmar, K.; Naffaa, L.; El-Merhi, F. Gadolinium and Multiple Sclerosis: Vessels, Barriers of the Brain, and Glymphatics. Am. J. Neuroradiol. 2018, 39, 2168–2176.
- Lee, M.K.; Cho, S.J.; Bae, Y.J.; Kim, J.-M. MRI-Based Demonstration of the Normal Glymphatic System in a Human Population: A Systematic Review. Front. Neurol. 2022, 13, 827398.
- Lee, D.A.; Lee, H.; Park, K.M. Glymphatic dysfunction in isolated REM sleep behavior disorder. Acta Neurol. Scand. 2021, 145, 464–470.
- Liang, T.; Chang, F.; Huang, Z.; Peng, D.; Zhou, X.; Liu, W. Evaluation of glymphatic system activity by diffusion tensor image analysis along the perivascular space (DTI-ALPS) in dementia patients. Br. J. Radiol. 2023, 96, 20220315.
- Park, C.J.; Kim, S.-Y.; Kim, J.H.; Son, N.-H.; Park, J.Y.; Jeong, Y.H.; Kim, H.J.; Park, J.; Kim, W.J. Evaluation of glymphatic system activity using diffusion tensor image analysis along the perivascular space and amyloid PET in older adults with objectively normal cognition: A preliminary study. Front. Aging Neurosci. 2023, 15, 1221667.
- Thomas, J.H. Fluid dynamics of cerebrospinal fluid flow in perivascular spaces. J. R. Soc. Interface 2019, 16, 20190572.
- Stanton, E.H.; Persson, N.D.; Gomolka, R.S.; Lilius, T.; Sigurðsson, B.; Lee, H.; Xavier, A.L.R.; Benveniste, H.; Nedergaard, M.; Mori, Y. Mapping of CSF transport using high spatiotemporal resolution dynamic contrast-enhanced MRI in mice: Effect of anesthesia. Magn. Reson. Med. 2021, 85, 3326–3342.
- Wang, M.; Ding, F.; Deng, S.; Guo, X.; Wang, W.; Iliff, J.J.; Nedergaard, M. Focal Solute Trapping and Global Glymphatic Pathway Impairment in a Murine Model of Multiple Microinfarcts. J. Neurosci. 2017, 37, 2870–2877.
- Jiang, Q. MRI and glymphatic system. Stroke Vasc. Neurol. 2019, 4, 75–77.
- Ahn, J.H.; Cho, H.; Kim, J.-H.; Kim, S.H.; Ham, J.-S.; Park, I.; Suh, S.H.; Hong, S.P.; Song, J.-H.; Hong, Y.-K.; et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 2019, 572, 62–66.
- Iliff, J.J.; Lee, H.; Yu, M.; Feng, T.; Logan, J.; Nedergaard, M.; Benveniste, H. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J. Clin. Investig. 2013, 123, 1299–1309.
- Bohr, T.; Hjorth, P.G.; Holst, S.C.; Hrabětová, S.; Kiviniemi, V.; Lilius, T.; Lundgaard, I.; Mardal, K.-A.; Martens, E.A.; Mori, Y.; et al. The glymphatic system: Current understanding and modeling. iScience 2022, 25, 104987.
- Turtzo, L.C.; Jikaria, N.; Cota, M.R.; Williford, J.P.; Uche, V.; Davis, T.; MacLaren, J.; Moses, A.D.; Parikh, G.; Castro, M.A.; et al. Meningeal blood–brain barrier disruption in acute traumatic brain injury. Brain Commun. 2020, 2, fcaa143.
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596.
- Kadry, H.; Noorani, B.; Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69.
- Langen, U.H.; Ayloo, S.; Gu, C. Development and Cell Biology of the Blood-Brain Barrier. Annu. Rev. Cell Dev. Biol. 2019, 35, 591–613.
- Zhao, Z.; Nelson, A.R.; Betsholtz, C.; Zlokovic, B.V. Establishment and Dysfunction of the Blood-Brain Barrier. Cell 2015, 163, 1064–1078.
- Knox, E.G.; Aburto, M.R.; Clarke, G.; Cryan, J.F.; O’driscoll, C.M. The blood-brain barrier in aging and neurodegeneration. Mol. Psychiatry 2022, 27, 2659–2673.
- Varatharaj, A.; Galea, I. The blood-brain barrier in systemic inflammation. Brain Behav. Immun. 2017, 60, 1–12.
- Huang, X.; Hussain, B.; Chang, J. Peripheral inflammation and blood–brain barrier disruption: Effects and mechanisms. CNS Neurosci. Ther. 2020, 27, 36–47.
- Benveniste, H.; Liu, X.; Koundal, S.; Sanggaard, S.; Lee, H.; Wardlaw, J. The Glymphatic System and Waste Clearance with Brain Aging: A Review. Gerontology 2018, 65, 106–119.
- Crocq, M.-A. History of cannabis and the endocannabinoid system. Dialogues Clin. Neurosci. 2020, 22, 223–228.
- Lowe, H.; Toyang, N.; Steele, B.; Bryant, J.; Ngwa, W. The Endocannabinoid System: A Potential Target for the Treatment of Various Diseases. Int. J. Mol. Sci. 2021, 22, 9472.
- Lu, H.-C.; Mackie, K. Review of the Endocannabinoid System. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 607–615.
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833.
- Joshi, N.; Onaivi, E.S. Endocannabinoid System Components: Overview and Tissue Distribution. In Recent Advances in Cannabinoid Physiology and Pathology; Advances in Experimental Medicine and Biology Book Series; Springer: Berlin/Heidelberg, Germany, 2019; Volume 1162, pp. 1–12.
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949.
- Fride, E.; Mechoulam, R. Pharmacological activity of the cannabinoid receptor agonist, anandamide, a brain constituent. Eur. J. Pharmacol. 1993, 231, 313–314.
- Di Iorio, G.; Lupi, M.; Sarchione, F.; Matarazzo, I.; Santacroce, R.; Petruccelli, F.; Martinotti, G.; Di Giannantonio, M. The endocannabinoid system: A putative role in neurodegenerative diseases. Int. J. High Risk Behav. Addict. 2013, 2, 100–106.
- Haspula, D.; Clark, M.A. Cannabinoid Receptors: An Update on Cell Signaling, Pathophysiological Roles and Therapeutic Opportunities in Neurological, Cardiovascular, and Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 7693.
- Kasatkina, L.A.; Rittchen, S.; Sturm, E.M. Neuroprotective and Immunomodulatory Action of the Endocannabinoid System under Neuroinflammation. Int. J. Mol. Sci. 2021, 22, 5431.
- Golech, S.A.; McCarron, R.M.; Chen, Y.; Bembry, J.; Lenz, F.; Mechoulam, R.; Shohami, E.; Spatz, M. Human brain endothelium: Coexpression and function of vanilloid and endocannabinoid receptors. Mol. Brain Res. 2004, 132, 87–92.
- Papa, A.; Pasquini, S.; Contri, C.; Gemma, S.; Campiani, G.; Butini, S.; Varani, K.; Vincenzi, F. Polypharmacological Approaches for CNS Diseases: Focus on Endocannabinoid Degradation Inhibition. Cells 2022, 11, 471.
- Patel, D.C.; Wallis, G.; Fujinami, R.S.; Wilcox, K.S.; Smith, M.D. Cannabidiol reduces seizures following CNS infection with Theiler’s murine encephalomyelitis virus. Epilepsia Open 2019, 4, 431–442.
- Jiang, H.; Li, H.; Cao, Y.; Zhang, R.; Zhou, L.; Zhou, Y.; Zeng, X.; Wu, J.; Wu, D.; Wu, D.; et al. Effects of cannabinoid (CBD) on blood brain barrier permeability after brain injury in rats. Brain Res. 2021, 1768, 147586.
- Ramirez, S.H.; Haskó, J.; Skuba, A.; Fan, S.; Dykstra, H.; McCormick, R.; Reichenbach, N.; Krizbai, I.; Mahadevan, A.; Zhang, M.; et al. Activation of Cannabinoid Receptor 2 Attenuates Leukocyte–Endothelial Cell Interactions and Blood–Brain Barrier Dysfunction under Inflammatory Conditions. J. Neurosci. 2012, 32, 4004–4016.
- Amenta, P.S.; Jallo, J.I.; Tuma, R.F.; Elliott, M.B. A cannabinoid type 2 receptor agonist attenuates blood–brain barrier damage and neurodegeneration in a murine model of traumatic brain injury. J. Neurosci. Res. 2012, 90, 2293–2305.
- Calapai, F.; Cardia, L.; Sorbara, E.E.; Navarra, M.; Gangemi, S.; Calapai, G.; Mannucci, C. Cannabinoids, Blood–Brain Barrier, and Brain Disposition. Pharmaceutics 2020, 12, 265.
More
